Abstract
In recent years, many Ayurvedic formulations are being researched to provide an effective antidepressant and anxiolytic drug in the field of psycho-pharmacology. The present study was planned to evaluate the anti-depressant and anxiolytic activity of Rasayana Ghana Tablet comprising three herbs Guduchi (Tinospora cordifolia Miers), Aamalaki (Emblica officinalis Garten) (RGT) and Gokshura (Tribulus terrestris Linn). Swiss albino mice were divided into four groups of six animals each, comprising of both male and female in each group. Group I received water served as normal control (WC), group II received vehicle and served as vehicle control (VC), group III received Rasayana Ghana tablet and group IV received standard drug diazepam (2 mg/kg) for anxiolytic study in elevated plus maze and standard antidepressant imipramine (5 mg/kg) for anti-depressant activity in behavior despair test. Rasayana Ghana tablet along with ghee and honey as vehicle is found to be having antidepressant and anxiolytic activity in experimental animals. Thus, this formulation can be used in prevention and treatment of depression and anxiety.
Keywords: Aamalaki (Emblica officinalis Garten), Anti-depressant, anxiolytic, Gokshura (Tribulus terrestris Linn), Guduchi (Tinospora cordifolia Miers), Rasayana Ghana
Introduction
Depression and anxiety are the two most prevalent psychiatric disorders challenging medical science. Stress has significant impact on causation of these two diseases. Modern medical science is always in search of better treatment options with minimum side effects. Ayurveda also bears the responsibility to provide an effective psycho-active drug to combat with disease. There are ample references for psycho-neuro-pharmacological effects of herbal medicines, and factors affecting the psyche in the classics[1] which are to be revealed scientifically.
In Ayurvedic classical texts, potent psycho active and rejuvenator formulations are quoted which can be utilized for improving the quality of life in modern distressed society. Rasayana churna is one among the most commonly used classical formulations comprising three potent well established rejuvenator herbs viz., Guduchi (Tinospora cordifolia Miers), Aamalaki (Emblica officinalis Garten), and Gokshura (Tribulus terrestris Linn) advocated along with unequal quantity of ghee and honey as Anupana (vehicle). As per classics, ghee and honey mixed in equal quantity are Matra viruddha (incompatible in same quantity).[2] Therefore, the quantity of vehicle, i.e. ghee and honey was calculated unequal as 2:1 Classics quote that this Rasayana formulation with anupana of ghee and honey is beneficial in maintenance of sexual vigor (Vrishah), physical and mental stability and steadiness (Sthiratva), peacefulness and eradication of diseases (Shanta vikara-dukkham), balance in mind and body (Samah) and longevity (Shatam Jeevati) with restriction graying of hair.[3] In this study, to overcome the disadvantages of Churna (Powder) form in terms of palatability, storage etc. for clinical use, Rasayana Churna was converted to Ghana (solid form prepared from decoction) tablets, which is a more concentrated and palatable form of drug than that of Churna. The tablet form (Rasayana Ghana Tablet) also provides an accurately measured dosage of the active ingredient in a convenient portable package.[4] Furthermore, as per clinical experience of author, it is observed that patients, especially those with psychological disorders (here stress related disorders), prefer to take tablet form due to convenience and palatability rather than any other forms like powder, decoction etc. Thus prepared Rasayana Ghana Tablet was subjected to experimental study to evaluate antidepressant and anxiolytic activities.
Materials and Methods
Test formulation
The genuine and authenticated raw materials [Table 1] of the test formulation were procured from pharmacy attached to Gujarat Ayurved University, Jamnagar. They were made to coarse powder form and mixed in equal proportions. The decoction was prepared by following the classical method.[5] The decoction was then filtered and further heated up to a concentrated form, i.e. Ghana was formed and then tablets (each of 500 mg) were made and coded as RGT. The honey and ghee of standard brands were purchased from local market.
Table 1.
Ingredients of Rasayana Ghana tablet and their Ayurvedic pharmacological properties

Animals and husbandry conditions
Swiss albino mice (24±04 g) of either sex were procured from animal house (Registration No. 548/2002/Committee for the purpose of control and supervision on experiments on animals) attached to the pharmacology laboratory, Institute for Post Graduate Teaching and Research in Ayurveda, Gujarat Ayurved University, Jamnagar. They were housed in the groups of six under the standard laboratory conditions (Temperature 23±2°C, relative humidity 50-60%) with food (Amrut brand) and tap water. Tests were performed only after the animals had acclimated to the laboratory conditions for at least 7 days and the experiments were performed during morning hours (08.00-10.00 hours). The experimental protocol was approved by Institutional Animal Ethics Committee (IAEC 05/09-10/Ph.D.03) and the care of animals was taken as per the Committee for the purpose of control and supervision on experiments on animals (CPCSEA) guidelines.
Dose selection and schedule
The dose of Rasayana Ghana tablet in human beings is 2 g/day in two divided doses. This dose was calculated considering the Roga bala (disease) and applicability of the modified drug dosage form. The dose for experimental animals was calculated by extrapolating the human dose to animals (260 mg/kg) based on the body surface area ratio by referring to the standard table of Paget and Barnes (1964).[6] The vehicle for this drug advocated in classical text is ghee and honey in unequal quantity (2:1). Hence, the drug solution was made by suspending the fine powder of Rasayana Ghana in a triturated mixture of unequal quantity of ghee (1040 mg/kg) and honey (520 mg/kg). This was administered to animals orally with the help of gastric catheter sleeved to syringe. The drugs were administered to overnight fasted animals.
Study design
Animals were divided in to four groups of six animals each, comprising of both male and female in each group. Group I received water served as normal water control (WC), group II received vehicle and served as vehicle control (VC), group III received Rasayana Ghana and group IV received standard drug diazepam (2 mg/kg) for anxiolytic study and standard antidepressant imipramine (5 mg/kg) for anti-depressant activity.
Elevated plus maze
The plus-maze apparatus, consisting of two open arms (16 × 5 cm) and two closed arms (16 × 5 × 12 cm) having an open roof, with the plus-maze elevated (25 cm) from the floor, was used to observe anxiolytic behaviour in mice. Mice were given a single oral dose of the vehicle, test drug and standard drug one hour before their placement on the Elevated plus maze (EPM). Dose administration schedule was adjusted so that each mouse took its turn on the elevated plus-maze apparatus one hour after administration of the dose. To begin a test session, mice were placed on the open arm facing the center of the maze. An entry into an arm was defined as the animal placing all four paws over the line marking that area. The number of entries and the time spent in the open and closed arms were recorded during a 5-minute test period. During the entire experiment, mice were allowed to socialize. Every precaution was taken to ensure that no external stimuli, other than the height of the plus-maze could invoke maze anxiety.[7]
Behavioral despair test
Mice were forced to swim individually in glass jar (25 × 12 × 25 cm3) containing fresh water to a height of 15 cm and maintained at 25°±2°C. After an initial two minutes period of vigorous activity each animal assumed a typical immobile posture. A mouse was considered to be immobile when it remained floating in the water without struggling, making only minimum movements of its limbs necessary to keep its head above water. The total duration of immobility was recorded during the next 4 minutes of a total 6 minute test. The changes in immobility periods were studied after administering drugs in separate groups of animals.[8] Each animal was used only once.
Statistical analysis
Results from the pharmacological screening were expressed as Mean ± standard error of the mean (SEM). Differences between the control and treatment groups in the experiments’ were tested for significance using unpaired student's ‘t’ test. values of P<0.05 were considered as statistically significant.
Results
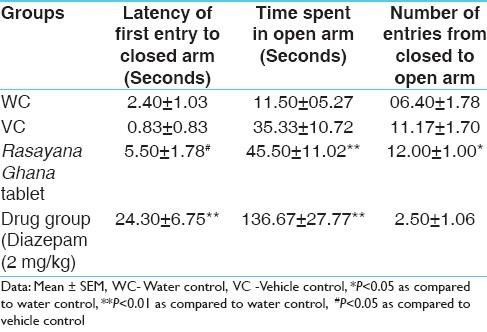
Diazepam which is a well established anti-anxiety drug significantly increased the latency of first entry to closed arm and highly significantly increased the time spent in open arm in comparison to control group. Pre-treatment with Rasayana Ghana tablet (RGT) also significantly increased the latency of first entry to closed arm and significantly increased the time spent in open arm. Further it also increased the number of entries from closed to open arm which was found to be not affected by administration of diazepam. However, in comparison to vehicle control only, latency of first entry to closed arm is found to be statistically significant in RGT treated group [Table 2].
Table 2.
Results of elevated plus maize test in different groups of Swiss albino mice during the experimental study
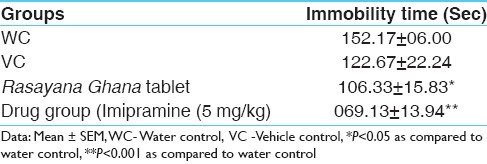
Pre-treatment with RGT and imipramine significantly reduced the immobility duration of mice in comparison to control group. However, in comparison to vehicle control, RGT non-significantly reduced the immobility duration [Table 3].
Table 3.
Results of behavior despair test in different groups of Swiss albino mice during the experimental study
Discussion
Psychopharmacology is the study of drug-induced changes in mood, sensation, thinking, and behavior.[9] It encompasses a wide range of substances with various types of psychoactive properties. Psychoactive drugs interact at particular target sites or receptors found in the nervous system to induce widespread changes in physiological or psychological functions. Researchers are interested in any substance that crosses the blood-brain barrier, and thus has an effect on behavior, mood or cognition. Drugs are researched for their physicochemical properties, physical side effects, and psychological side effects.[10] Therefore, the effects are evaluated in experimental animals. Recent researches suggest that animal models are valid for studying the antidepressant effects of drug because of strong resemblances between stressed animals and depressed humans in terms of changes in various body functions, such as, the regulation of endocrine systems, learning and memory, histology of certain parts of the brain, behavior and others. Therefore, stress-induced brain changes in animal models of depression may validly represent the brain changes in depressed humans, and antidepressant-induced changes in animal models may validly represent antidepressant mechanisms in depressed humans.[11]
Elevated plus maze is highly sensitive to the influence of both anxiolytic and anxiogenic drugs acting at the gamma-aminobutyric acid (GABA)-benzodiazepine complex.[12] Rodents have a natural aversion for high and open spaces and prefer closed arms, which have a burrow like ambience and therefore, spend greater amount of time in the closed arm. When exposed to the novel maze allay, the animals experienced an approach-avoidance conflict, which was stronger in the open arms as compared to closed arms. The decrease aversion to the open arms was the result of an anxiolytic effect expressed by an increased number of open arm entries, and time spent in the elevated plus maze, and the decreased time spent on the central platform was another indication of a reduced decision making behavior. Pre-treatment with RGT along with ghee and honey significantly increased the latency of first entry to closed arm and significantly increased the time spent in open arm. Further, it also increased the number of entries from closed to open arm which was found to be not affected by administration of diazepam. This indicates anxiolytic effect of ghee and honey potentiated by formulation. Benzodiazepines act by facilitating inhibitory GABAergic transmission.[13] RGT with the vehicle of ghee and honey may modulate the GABAergic system.
Antidepressant effect on forced swimming model of depression provides a rapid and reliable behavior screening test for anti-depressants. The model is valid for a broad spectrum of antidepressants mainly including tricyclics and Mono-amine oxidase (MAO) inhibitors, which significantly decrease immobility time in forced swimming test (FST).[14] Immobility is thought to reflect either a failure to persist in escape directed behavior after persistent stress, or the development of passive behavior that disengages the animal from active forms of coping with stressful stimuli.[15] Several antidepressants reduce the immobility after forced swimming.[16] In the present study, RGT significantly decreased the immobility time of mice in FST and was comparable with standard anti-depressant drug imipramine. The observed effect may be attributed to blockage of 5–HT reuptake or MAO inhibition.
As stated in introductory part, RGT is a poly herbal compound comprising of Tinospora cordifolia, Emblica officinalis and Tribulus terrestris. Among these, Tinospora cordifolia is with proven stress-attenuating activity.[17] In addition, Tinospora cordifolia has several properties generally associated with adaptogenic[18,19] antioxidant[20,21] properties. It is also known to possess beneficial effects on learning, stress and memory.[22] Emblica officinalis is having immunomodulatory activity, adoptogenic and antioxidant activity.[23–30] Earlier studies on formulation containing Guduchi (Guduchyadi ghrita) and Aamalaki (Bhringarajadi rasayana) showed potent antidepressant, anti stress, anxiolytic activity.[31] The studies on Tribulus terrestris (Gokshura) suggest that the harmine content of Tribulus acts as Monoamine Oxidase (MAO) inhibitor, leading to higher levels of dopamine in the brain. Due to higher levels of dopamine, the mood is elevated slowly and the stronger and better is the feeling.[32] Further honey[33–35] and ghee[36] are also proven for their anti-oxidant and adaptogenic activities. Thus, the observed and anxiolytic and anti-depressant profile of RGT may be attributed to one or more bioactive principles present in these drugs. There may be synergistic herb-herb interactions enhancing the total efficacy of the formulation. The exact mechanism of action of the drug needs to be evaluated by further extensive studies.
In Ayurvedic parlance, both anxiety and depression can be stemmed at the vitiation of Vata dosha, imbalance of Kapha dosha and Pitta dosha at the somatic level. Therefore, to prevent these pathological events, the dosha states must be kept in balance. RGT may act as to maintain the homeostasis of dosha in either of following ways. RGT has predominantly Madhura rasa (sweet taste), Madhura vipaka (sweet after digestion) Sheeta veerya (cold potency), Snigdha (unctuous), Guru guna (heavy) and Vata-Pitta dosha shamaka properties. Rasayana Ghana tablets possess Madhura rasa and Madhura vipaka, which means that it is finally converted in Madhura rasa after metabolism by Agni.[37] As per the classics, Madhura vipaka promotes the formation of normal Kapha, and helps in proper elimination of stool and urine.[38] Madhura also can cause Aalhada, i.e. soothening and delightfulness.[39] Sushruta states that Madhura can produce Soumansya (sense of well being), Bala (power), Utsaha (enthusiasm), Harshana (pleasure) and Sukham (happiness).[40] These are important and symbiotic qualities to enhance the anti-depressant, anxiolytic and adaptogenic activities of the formulation. With reference to the Sheeta Virya (cold potency), it produces Alhadana (delightening of mind) at the psychological level,[41] while at physical level it can aggravate Vata and Kapha due to similarity in qualities.[42] Sheeta Virya has soothening and calming effect, which is necessary in adapting to the given situations in stress prone patients’ for relaxation. Its sedative property may be helpful in agitated and anxious patients. Furthermore, Pitta is pacified by Sheeta, which is of great significance in correcting balance of dosha. Rest of the work is done by the synergistic activity of ghee and honey which are having adaptogenic activity.
Conclusion
From the present study, it can be concluded that Rasayana Ghana tablet along with ghee and honey as vehicle is found to be having antidepressant and anxiolytic activity in experimental animals. Thus, this formulation can be used in prevention and treatment of depression and anxiety. The present study opens new windows for further researches on this classical combination at different dose levels, as adjuvant with and in comparison with various anti-depressant and anxiolytic drugs. This may lead to a better integrative management of these psychiatric disorders in future.
References
- 1.Acharya Vaidya Jadavaji Trikamaji., editor. Sutra Sthana. 40. Vol. 25. Gopal Mandir Lane, Varanasi: Krishnadas Academy; 2000. Agnivesha, ‘Charaka Samhita’, revised by Charaka and Dridhbala with ‘Ayurveda Dipika’ commentary by Chakrapanidatta; p. 131. [Google Scholar]
- 2.Ibid. Charaka Samhita Sutra sthana. 26(90):151. [Google Scholar]
- 3.Vaidya Pt. Harishastri Paradakar., editor. 159. Vol. 39. Varanasi: Krishanadas Academy; 2000. Vagbhata, Ashtanga Hridayam, with the commentaries, ‘Sarvangasundara’ of Arunadatta and ‘Ayurvedarasayana’ of Hemadri collated by Dr. Anna Moreshvara Kunte, and Krishna Ramachandra Shastri Navre; p. 937. Gopal Mandir Lane, Uttara stana chapter. [Google Scholar]
- 4. [Last accessed on 2010 Nov 12]. Available from: http://www.en.wikipedia.org/wiki/Tablet .
- 5.Kwatha Kalpana. 4th ed. 1. Vol. 2. Nagpur: Baidyanath Ayurveda Bhavan Ltd; 1994. Sharangadhara, Sharangadhara Samhita, commentary by Radhakrishna parashar; p. 189. chapter. [Google Scholar]
- 6.Paget GE, Barnes JM. Evaluation of drug activities. In: Lawrence DR, Bacharach AL, editors. Pharmacometrics. Vol. 1. New York: Academic Press; 1964. p. 161. [Google Scholar]
- 7.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 8.Porsolt RD, Bertin A, Jalfre M. Behaviour despair in mice: A primary screening test for anti-depressants. Arch. Int. Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 9.Meyer JS, Quenzer LS. Psychopharmacology: Drugs, the Brain and Behavior. Sinauer Associates. 2004. [Last accessed on 2008 Feb 27]. ISBN 0-87893-534-7. Available from: http://www.en.wikipedia/Psychopharmacology/
- 10. [Last accessed on 2008 Feb 27]. Available from: http://www.en.wikipedia/psychoactive substances/htm .
- 11.Andrade C, Rao NS. How antidepressant drugs act: A primer on neuroplasticity as the eventual mediator of antidepressant efficacy. [Last cited 2011 Feb 12];Indian J Psychiatry. 2010 52:378–86. doi: 10.4103/0019-5545.74318. Available from: http://www.indianjpsychiatry.org/text.asp?2010/52/4/378/74318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhonnchadha BA, Bourin M, Hascoet M. Anxiolytic-like effects of 5-HT2 ligands on three mouse models of Anxiety. Behav Brain Res. 2003;140:203–14. doi: 10.1016/s0166-4328(02)00311-x. [DOI] [PubMed] [Google Scholar]
- 13.Tripathi KD. Essentials of Medical Pharmacology. 4th ed. Delhi: Jaypee Brothers Medical Publishers (p) Ltd; 1999. p. 7. (28,415). [Google Scholar]
- 14.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiat. 1967;122:509. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 15.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacol. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 16.Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindolacetic acid in the rat. J Pharmacol Exp Ther. 1997;282:967–76. [PubMed] [Google Scholar]
- 17.Rawal AK, Muddeshwar MG, Biswas SK. Rubia cordifolia, Fagonia cretica and Tinospora cordifolia exert neuroprotection by modulating the antioxidant system in rat hippocampal slices subjected to oxygen glucose deprivation.London WC1X 8HB United Kingdom. (11).BMC Complementary and Alternative Medicine. 2004:4. doi: 10.1186/1472-6882-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six Rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275–91. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Bafna PA, Balaraman R. Anti-ulcer and antioxidants activity of Pepticare - a herbo-mineral formulation. Phytomedicine. 2005;12:264–70. doi: 10.1016/j.phymed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Goel HC, Prem Kumar I, Rana SV. Free radical scavenging and metal chelation by Tinospora cordifolia, a possible role in radioprotection. Indian J Exp Biol. 2002;40:727–34. [PubMed] [Google Scholar]
- 21.Subramanian M, Chintalwar GJ, Chattopadhyay S. Antioxidant properties of a Tinospora cordifolia polysaccharide against iron mediated lipid damage and gamma-ray induced protein damage. Redox Rep. 2002;7:137–43. doi: 10.1179/135100002125000370. [DOI] [PubMed] [Google Scholar]
- 22.Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies. [Last cited 2011 Feb 13];Int J Ayurveda Res. 2010 1:122–21. doi: 10.4103/0974-7788.64405. Available from: http://www.ijaronline.com/text.asp?2010/1/2/112/64405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–91. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- 24.Babu PS, Mainzen Stanely, Prince P. Antihyperglycaemic and antioxidant effect of hyponidd, an ayurvedic herbomineral formulation in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2004;56:1435–42. doi: 10.1211/0022357044607. [DOI] [PubMed] [Google Scholar]
- 25.Rao TP, Sakaguchi N, Juneja LR, Wada E, Yokozawa T. Amla (Emblica officinalis Gaertn) extracts reduce oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2005;8:362–8. doi: 10.1089/jmf.2005.8.362. [DOI] [PubMed] [Google Scholar]
- 26.Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, et al. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res. 2005;19:582–6. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 27.Naik GH, Priyadarsini KI, Mohan H. Evaluating the antioxidant activity of different plant extracts and herbal formulations. Res Chem Intermed. 2005;31:145–51. [Google Scholar]
- 28.Al-Rehaily AJ, Al-Howiriny TA, Al-Sohaibani MO, Rafatullah S. Gastroprotective effects of ‘Amla’ Emblica officinalis on in vivo test models in rats. Phytomedicine. 2002;9:515–22. doi: 10.1078/09447110260573146. [DOI] [PubMed] [Google Scholar]
- 29.Sairam K, Rao ChV, Babu MD, Kumar KV, Agrawal VK, Goel RK. Antiulcerogenic effect of methanolic extract of Emblica officinalis: An experimental study. J Ethnopharmacol. 2002;82:1–9. doi: 10.1016/s0378-8741(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 30.Bandyopadhyay SK, Pakrashi SC, Pakrashi A. The role of antioxidant activity of Phyllanthus emblica fruits on prevention from indomethacin induced gastric ulcer. J Ethnopharmacol. 2000;70:171–6. doi: 10.1016/s0378-8741(99)00146-4. [DOI] [PubMed] [Google Scholar]
- 31.Shukla DN, Chandola HM, Ravishankar B. A Comparative Psycho-Neuro-Pharmacological Study on Guduchyadi Ghrita Rasayana & Bhringarajadi Ghrita Rasayana. AYU. 2007;27:9–18. [Google Scholar]
- 32.Sabnis M. Chemistry and pharmacology of ayurvedic medicinal plants. Varanasi: Choukhamba orientalia; 2007. p. 349. [Google Scholar]
- 33.Marcucci MC. Propolis; chemical composition, biological properties and therapeutical utility. Apidologie. 1995;26:83–9. [Google Scholar]
- 34.Viuda Martos M, Ruiz-Navajas Y, Fern´Andez-L´Opez J, P´Erez-´Alvarez JA. Functional Properties of Honey, Propolis and Royal Jelly. J Food Sci. 2008;73:116–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 35.Aonan A, AL-Mazrooa, Mansour I. Sulaiman, Effects of honey on stress-induced ulcers in rats. J Kau Med Sci. 1999;7:115–22. [Google Scholar]
- 36.India: Swami Ramanand Teerth Marathwada University; 2006. Shriram Sheshgir Savrikar. ‘Comparative Study of Physico0chemical characteristics of ‘Accha Sneha’ and ‘Siddha Sneha’ with reference to ‘Guduchi Ghrita’, the butter fat, medicated with Tinospora cordifolia (Willd) Miers. PhD thesis submitted to Nanded, Maharashtra. [Google Scholar]
- 37.Vaidya Harishastri Paradakar., Pt., editor. Sutra Sthana. 20. Vol. 9. Gopal Mandir Lane, Varanasi: Krishanadas Academy; 2000. Vagbhata, Ashtanga Hridayam, with the commentaries, ‘Sarvangasundara’ of Arunadatta and ‘Ayurvedarasayana’ of Hemadri collated by Dr. Anna Moreshvara Kunte, and Krishna Ramachandra Shastri Navre; p. 169. [Google Scholar]
- 38.Sharma Ram Karan, Dr., Dash Vaidya Bhagvan. Sutra Sthana. seventh edition. Vol. 1. Varanasi: Chowkhamba Sanskrit Series Office; 2002. Agnivesha, ‘Charaka Samhita’ Text with English Translation and Critical Exposition Based on Chakrapanidatta's ‘Ayurveda Dipika’; p. 473. 26/61. [Google Scholar]
- 39.Ibid. Charaka Samhita, Sutra Sthana. 1:480. 26/74. [Google Scholar]
- 40.Acharya Vaidya Jadavaji Trikamaji, Aachrya Narayana Rama. Sutra Sthana. eighth edition. 481. Vol. 46. Gopal Mandir Lane, Varanasi: Chaukhamba Orientalia; 2005. Sushruta, ‘Sushruta Samhita’ with ‘Nibandha Sangrha’ commentary by Dallhanacharya; p. 249. [Google Scholar]
- 41.Ibid. Sushruta Samhita, Sutra Sthana. 46(515):252. [Google Scholar]
- 42.Vaidya Harishastri Paradakar., Pt., editor. Sutra Sthana. 11-12. Vol. 1. Varanasi: Krishanadas Academy; 2000. Vagbhata, Ashtanga Hridayam, with the commentaries, ‘Sarvangasundara’ of Arunadatta and ‘Ayurvedarasayana’ of Hemadri collated by Dr. Anna Moreshvara Kunte, and Krishna Ramachandra Shastri Navre; p. 9. Gopal Mandir Lane. [Google Scholar]


