Abstract
Chronic periodontitis is a complex infection initiated by gram-negative bacteria which destroy the supporting structures of the tooth. Recently, it has been recognized that it is the host response to bacterial infection which causes greater destruction of the connective tissue elements, periodontal ligament and alveolar bone in periodontitis. This has led to the development of various host modulating approaches to target cells and their destructive mediators involved in tissue degradation. Chemically modified tetracyclines (CMTs) are derivatives of tetracycline group of drugs which lack antimicrobial action but have potent host modulating affects. They inhibit pathologically elevated matrix metal loproteinases, pro-inflammtory cytokines and other destructive mediators. Bone resorption is also suppressed due to their combined anti-proteinase and apoptotic affects on osteoblasts and osteoclasts, respectively. Development of resistant bacteria and gastrointestinal toxicity seen with parent tetracyclines is not produced by CMTs. Hence, CMTs are viewed as potential therapeutic agents in the management of chronic diseases like periodontitis that involve destruction of connective tissue and bone.
KEY WORDS: Chemically modified tetracyclines, host modulation, host response, periodontitis
Introduction
Chronic periodontitis is a complex immune inflammatory disease instigated by anerobic, gram-negative and micro aerophilic bacteria.[1] These periodontopathogens secrete enzymes that destroy the extracellular matrix and bone. Prior to the recognition of host factors responsible for tissue destruction, various antimicrobial agents were used to control the bacterial infection. Tetra cyclines were one such group of drugs that were traditionally used as adjuncts to periodontal therapy. The perceived advantages of this group of antibiotics were: 1) effectiveness against anerobic gram-negative periodontopathogens in the subgingival plaque; 2) increased concentration in gingival crevicular fluid at levels much above the serum; 3) the substantivity property which enabled them to bind to the biological tissues and get released over a period of time, resulting in prolonged efficacy; 4) anti collagenase property.[2]
Presently, it is accepted that destruction of supporting periodontal tissues is primarily related to host derived enzymes, cytokines and inflammatory mediators. This has led to increased interest in the development of agents capable of modulating the host response.[3]
Chemically modified tetracyclines (CMTs) are one such group of drugs which have been viewed as potential host modulating agents. Literature shows that the major side effects of tetracyclines are development of resistant bacteria and gastrointestinal upset which were related to their antimicrobial properties.[2] Golub et al., in 1987 recognized that the antimicrobial and anti collagenase properties of tetracyclines resided in different parts of the four ringed structure. He altered the structure of tetracyclines which led to the development of the CMTs.[4]
Since that time several CMTs have been developed. Among them CMT-1, CMT-3 and CMT-8 have been tested for periodontal applications. The present review focuses on the current status of these novel agents for host modulation in periodontitis.
Host Response in Periodontitis
The overgrowth of the dental plaque biofilm in sub gingival area is the primary etiology for periodontitis.[1] Bacterial species in plaque biofilm including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Fusobacterium nucleatum and Tannerella forsythia have been strongly associated with periodontitis.[5] They destroy the periodontal tissues by both direct and indirect mechanisms. Once the bacterial virulence factors (lipopolysaccharide cell wall and endotoxins) have overwhelmed the local defense mechanisms, they stimulate a myriad of reactions in the host [Figure 1], resulting in loss of soft connective tissue elements and bone resorption as follows:
Figure 1.
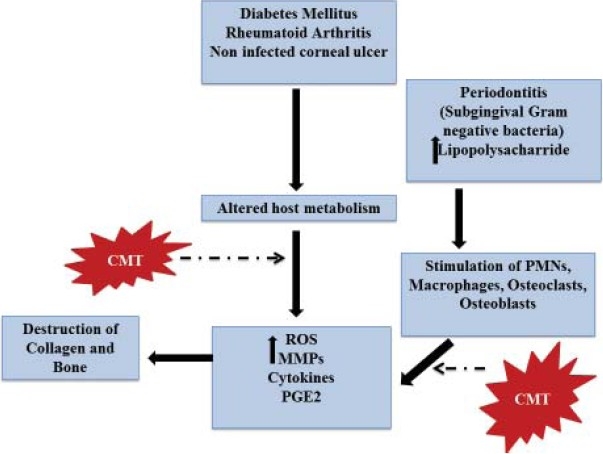
Host response in various chronic inflammatory conditions
a) Release of reactive oxygen species (ROS) and reactive nitrogen species (RNS)
The initial host response is the release of ROS and RNS via the metabolic process of “respiratory burst” in the Polymorpho nuclear neutrophils (PMNs), macrophages and monocytes.[6] Excessive production of these reactive species creates oxidative stress in the body. The ROS include oxygen-derived free radicals (e.g., superoxides, hydroxyl, peroxyl, alkoxyl) and non radical compounds (e.g., hypochlorus acid, ozone, peroxynitrite, singlet oxygen and hydrogen peroxide).[7] RNS include nitric oxide (NO), peroxynitrite, nitrogen dioxide radicals and products arising from the reaction of NO with oxygen-free radicals.[7] The NO-free radical is formed by the oxidation of L-arginine to L-citrulline in the presence of inducible NO synthase (iNOS).[8] NO reacts with superoxide to produce peroxynitrite which stimulates cyclooxygenase activity and prostaglandin (PGE2) synthesis.[9]
Increased levels of ROS or RNS causes alteration of DNA and proteins, oxidation of enzymes (eg: α-1 antitrypsin), stimulation of proinflammatory cytokine release and peroxidation of lipid membranes. These reactions damage the gingival tissue, periodontal ligament and alveolar bone resulting in further progression of periodontitis.[10]
b) Release of proinflammatory cytokines
The dental plaque biofilm stimulates host immune cells to produce proinflammatory cytokines including interleukin (IL) - 1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α. They provide molecular signals to other cells and induce connective tissue and alveolar bone destruction. They are present in increased concentration in diseased periodontal tissues and gingival crevicular fluid. The role of proinflammatory cytokines in chronic periodontitis is summarized in Table 1.
Table 1.
Role of proinflammtory cytokines in periodontitis
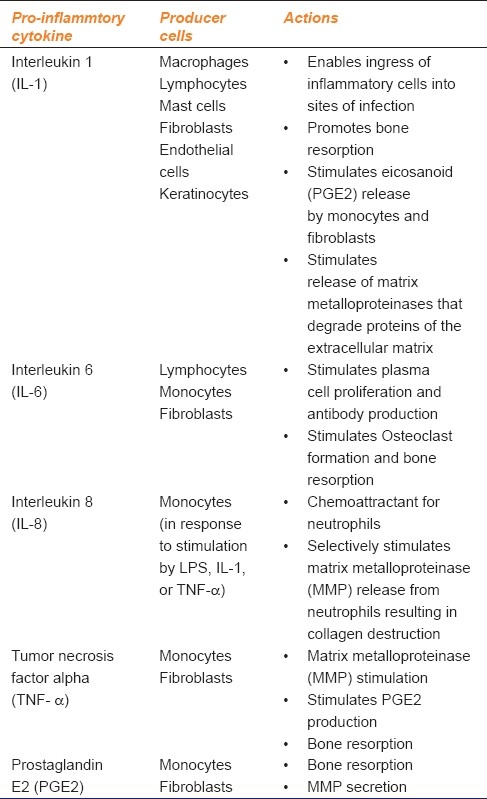
The catabolic activities of these cytokines are controlled by endogenous inhibitors like IL-1 and TNF receptor antagonists and anti-inflammtory cytokines like IL-4, IL-10, IL-11, and TGF-β. The host modulation therapy in the treatment of periodontitis should target the activated immune cells to inhibit the release of IL-1, TNF-α, ROS/RNS and enhance the action of anti-inflammtory cytokines.
c) Release of matrix metalloproteinases
The matrix metalloproteinases (MMPs) are zinc- and calcium-dependent endopeptidases secreted by PMNs, macrophages, fibroblasts, epithelial cells, osteoblasts and osteoclasts. They destroy extracellular matrix components like collagen, gelatin, laminin, fibronectin and proteoglycans.[11,12] Increased activity of MMPs is seen in chronic inflammatory conditions including periodontitis, rheumatoid arthritis and cancer.
The host cells stimulated directly or indirectly by components of the plaque biofilm secrete MMPs which are associated with altered connective tissue remodeling and alveolar bone resorption. periodontopathogens like P. gingivalis and A. actinomycetemcomitans also produce MMPs but it is the endogenous MMPs that are primarily responsible for tissue destruction.[3]
The collagen matrix is degraded by MMP-8 (collagenase) and MMP-9 (gelatinase) secreted from PMNs.[13] The MMP-13 (collagenase -3) destroys bone and cartilage.[13]
MMPs are inhibited by endogenous tissue inhibitors of MMP (TIMP) and α2-macroglobulins. In healthy tissues there is a balance between MMPs and TIMPs. Disruption of this balance results in tissue degradation.
d) Production of arachidionic acid metabolites
The damage to phospholipids in cellular plasma membrane initiated by bacterial and host factors activates the arachidionic acid (AA) metabolism. Phospholipase A2, a proinflammatory enzyme hydrolyses phospholipids to produce AA which is further metabolized by the cyclooxygenase (CO) or lipoxygenase (LO) pathways.[9] There are two isoforms of cyclooxygenase: Cyclooxygenase 1 (COX-1) and Cyclooxygenase 2 (COX-2). The latter is inducible, up regulated by proinflammatory cytokines, and more active during inflammation. PGE2, prostacyclin, and thromboxane A2 are released via the CO pathway while LO pathway produces leukotrienes and other hydroxy-eicosatetraenoic acids.[14] Elevated levels of PGE2 in periodontitis cause bone resorption.
e) Resorption of alveolar bone
Bone resorption in periodontitis occurs as a consequence to activation of osteoblasts and osteoclasts by MMPs, proinflammatory cytokines (IL-1β, IL-6 and TNF-α) and AA metabolites (PGE2 and leukotrienes). The osteoblasts synthesize osteoid which is composed of Type I collagen. They also initiate bone resorption by synthesizing MMPs (e.g., collagenase) which degrade the bone matrix.[15] The release of collagen degradation fragments and biologic mediators (e.g., osteocalcin and cytokines) attracts the osteoclasts onto the mineralized bone surface and initiate its resorption.[16] Matrix degrading collagenases (MMP-1 and MMP-13), gelatinases (MMP-2 and MMP-9), metalloelastases (MMP-12) and membrane- type MMPs (MMP-14) are produced by osteoclasts.[17]
The bacterial LPS stimulate monocytes and other proinflammatory cells by reacting with the CD14 membrane receptors on their surface.[18] This causes increased production of IL-lβ, IL-6, granulocyte macrophage-colony stimulating factor, TNF-α, IL-8, PGE2, and leukotrienes. These cytokines are potent activators of osteoclasts and promote bone resorption by stimulating expression of MMPs.
Various therapeutic agents have been developed in recent years to regulate the host reactions and hence the periodontal destruction. These include the ROS/RNS inhibitors, anti-cytokine agents, nonsteroidal anti-inflammatory agents and MMP inhibitors. The most common MMP inhibitors studied are the recombinant tissue inhibitors of MMP and synthetic MMP inhibitors like tetracycline group of drugs.
The Structure of CMT
Golub et al., discovered that the carbon-4 position side-chain was responsible for the antimicrobial activity of tetracyclines. In a series of experiments conducted later, the chemically modified tetracyclines (CMTs) were produced by removing the dimethylamino group from the carbon-4 position of the A ring of the four ringed (A, B, C, D) structure. The resulting compound, 4-de-dimethyl amino tetracycline (CMT-1) did not have antimicrobial property but the anti collagenase activity was retained both in vitro and in vivo.[4]
Further modifications in the central structure of tetracyclines by addition or deletion of functional groups resulted in the formation of eight CMTs. Currently about ten CMTs including CMT-1 (4 dedimethylaminotetracycline), CMT -2 (tetracyclinonitrile), CMT-3 (6-deoxy-6-demethyl-4-de-dimethylaminotetracycline) and CMT-4 (7-chloro-4-de- dimethylaminotetracycline), CMT-5 (tetracycline pyrazole), CMT-6 (4-dedimethyl amino. 4-hydroxytetracycline), CMT-7 (12_-deoxy-4-dedimethyl amino tetracycline) and CMT-8 (4-dedimethylamino doxycycline) have been developed [Figure 2].
Figure 2.
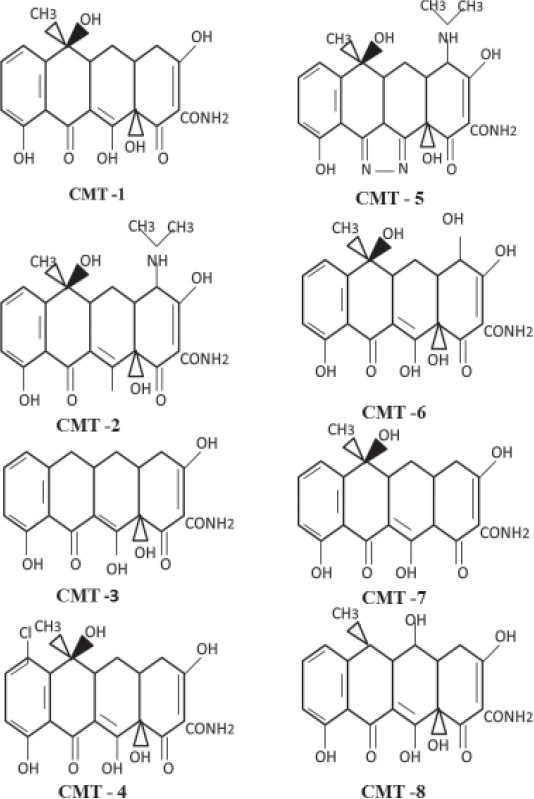
Chemical structure of chemically modified tetracyclines
The Ca2+ and Zn2+ binding sites at the carbonyl oxygen and the hydroxyl groups of carbon-11 and carbon-12 positions are responsible for the anti-collagenase action of the CMTs. The CMT-5 is a pyrazole analog of tetracycline, formed by the replacement of carbonyl oxygen at C-11 and the hydroxyl group at C-12 by nitrogen atoms. It does not have metal binding site and therefore it is inactive against the MMPs.[19]
The main advantage of CMTs over the conventional tetracyclines is that long-term systemic administration does not cause gastrointestinal toxicity and higher plasma concentrations can be obtained with less frequent administration regimens.
Host Modulation with CMTs in the Management of Chronic Periodontitis
It is well established that the destruction of periodontal tissues in chronic periodontitis occurs subsequent to activation of bacterially induced host-mediated processes. The bacterial LPS activation of immune cells and subsequent production of destructive mediators is the major pathway for connective tissue and bone loss in periodontitis.
The desirable non antimicrobial properties of tetracyclines in CMTs which would prevent progression of periodontitis are 1) prevention of connective tissue breakdown through inhibition of metal-dependant MMPs; 2) suppression of PMNs and inhibition of the generation of AA metabolites by blocking the phospholipase A2 and PGE2 synthesis; 3) scavenge the ROS/RNS; and 4) enhancement of the attachment of fibroblasts and connective tissues to the tooth surface and hence regeneration of lost periodontium.
Among all these actions the anti-MMP effect of CMTs has been widely discussed in the management of periodontitis.
I) Inhibition of MMPs
The CMTs have been shown to modulate integrin expression on endothelial cells in early stages of inflammation. They counteract the effects of transforming growth factor β-1 (TGF-β1) which is chemotactic for mast cells, monocytes, neutrophils and fibroblasts. They inhibit the actions of TGF-β 1 induced expression of MMPs, secretion of proinflammatory cytokines and expression of FcγRIII which enhances phagocytosis. CMTs also stimulate the production of matrix molecules and protease inhibitors like tissue inhibitor of metalloproteinase-1 (TIMP-1) from fibroblasts.[20]
The host MMP is inactivated by interaction of CMTs with its metal ion constituent, Zn2+ at the active site and Ca2+ as a cofactor[21] [Figure 3]. It has been suggested that the PMNs provide the major source of collagenases that mediate the connective tissue breakdown during inflammatory periodontal disease, while the fibroblasts contribute the collagenase required for connective tissue remodeling in normal gingiva. The anti-collagenase activity of CMTs is specific against the collagenase produced from neutrophils but not the fibroblasts. This non antimicrobial action of CMTs is important as it would help in the reduction of pathologic concentrations of collagenases without affecting the normal collagen turnover required to maintain the tissue integrity.[21]
Figure 3.
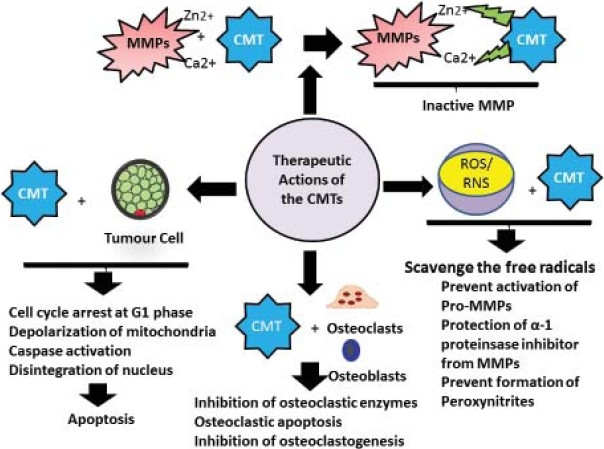
Therapeutic actions of CMTs
The initial in vitro and in vivo investigations with CMTs in diabetic rats showed that at a concentration of 2 μg/ml, both human and animal collagenases were inhibited.[4] It was reported that in diabetic rats weighing 300 g, an optimum dose of 5 mg/day produced a peak plasma concentration of 4 μg/ml.[22,23] A daily dose of 10 mg of CMT-1 for 4 weeks reduced the host collagenases in the gingival tissues without the development of resistant bacteria as opposed to parent tetracyclines.[21]
When administered in germ-free animals infected with P. gingivalis, CMT-1 inhibited the host collagenases to the same extent as doxycycline. However, it was not effective in reducing the bacterial concentration.[21,24]
The CMT-3 is specifically active against MMP-2, MMP-9 and MMP-14 isozymes due to its pleiotropic action toward MMPs. It exerts an inhibitory effect on MMPs in micro molar concentrations by decreasing trypsinogen-2 and inducible nitric oxide (iNOS) production which is discussed later.[25]
A comparative evaluation of six different CMTs in inhibition of MMPs showed that the CMT-8 was most effective inhibitor of periodontal breakdown. CMT-8,-1, -3, -4, -7 and doxycycline inhibited TNF-α, IL-1, IL-6 and MMPs in descending order. There in vivo efficacy was related to serum concentration.[26]
In a rat model of P. gingivalis-induced chronic periodontitis it was found that CMT-1 and doxycyline reduced the inhibitory effect on the expression of type-I and type XII collagen m-RNA expression from cells. This would enhance the production of collagen in periodontitis.[27]
The CMT 1 and 3 also inhibit gingipains (tissue destructive cysteine proteinases) produced by P. gingivalis. They protect periodontal tissues from gingipain-induced inactivation of host proteinase inhibitors (α1-macroglobulin, TIMP and α2-macroglobulin), activation of MMPs, and disruption of integrins as well as immunoglobulins.[28]
The anti-MMP actions of CMTs can be summarized as follows:[9]
Direct inhibition of the active MMPs by the virtue of Ca2+ and Zn2+ binding sites.
Inhibition of ROS mediated activation of Pro-MMPs
Proteolysis of Pro-MMPs into enzymatically inactive fragments
Protection of α-1 proteinsase inhibitor from MMPs
Reduction in the activity of serine proteinases
II) Other Anti-inflammatory Actions of CMTs
a) Inhibition of inducible nitric oxide synthase (iNOS)
CMTs may decrease the ROS burden by inhibiting neutrophils, directly scavenging free radicals and inhibiting reactions that lead to free radical generation. They inhibit the expression of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) activity. The peroxynitrite radical formed by the reaction of NO is highly cytotoxic, inhibits collagen and proteoglycan synthesis and up regulates the MMP expression. Inhibition of iNOS production causes reduction in the peroxynitrite levels, thus preventing denatuartion of proteins. CMT-3 and CMT-8 have shown maximum inhibitory effect on the iNOS, CMT-1 and-2 had intermediary effect while CMT-5 was ineffective.[29]
b) Inhibition of proinflammatory mediators
CMTs inhibit release of IL-1β, IL-6, IL-8, TNF-α and PGE2 from LPS stimulated host immune cells by suppressing phosphorylation of the nuclear factor κ–B cell signalling pathway. The CMT-3 inhibits COX-2-mediated PGE-2 production.[9]
In an ex vivo human whole blood model stimulated with P. gingivalis LPS, doxycycline and CMT-3 were investigated for their efficacy in suppressing the production of proinflammatory mediators and MMPs. There was a significant reduction in the secretion of proinflammatory cytokines but the levels of MMPs were not affected.[30]
Studies have shown that CMT -3 inhibited intracellular accumulation and synthesis of TNF-α in activated mast cells. It also inhibited IL-8 and protein kinase – C production. Protein kinase – C is an important mediator of transcription of MMPs, therefore inhibition of this mediator may produce an anti-inflammatory effect.[31]
c) Inhibition of bone resorption
In the standard organ culture systems, the nonantimicrobial tetracyclines were found to inhibit bone resorption induced by parathyroid hormone, PGE2, and bacterial endotoxin. They suppress peroxynitrite levels and cyclo-oxygenase activity which reduces PGE2 synthesis.
In vivo experiments showed that the CMTs produced a 90% reduction in the activity of osteoblastic collagenase at concentrations much below that required for minocycline.[18] CMT-1, CMT-3, CMT-6,-7 and -8 were effective inhibitors of osteoblastic collagenase in culture. CMT-8 was the most potent among these.[32]
A combination of CMT-8 with Clodronate reduced endotoxin induced alveolar bone loss in rats. This was attributed to their ability to reduce the production of IL-1β, IL-6, TNF-α and convert latent MMPs into active MMPs. CMT-8 also binds to the bone surface and gets released over a period of time. It induces morphological and functional changes in osteoclasts leading to their apoptosis.[33]
CMTs inhibit the bone resorption by altering both the osteoblastic and osteoclastic function. The osteoclastic enzymes like tartrate resistant acid phosphstase and Cathepsin –L which degrade the organic components of bone are suppressed. They alter the ruffled border (site of active bone resorption) and increase the size of “clear zone” (site of no bone resorption) in the rat osteoclasts. They also decrease the number of osteoclasts by inhibiting their development and inducing apoptosis. CMT-3 and -8 have been shown to produce a maximum reduction the number of multinucleated osteoclasts.[34] They inhibit the osteoclastogenesis from peripheral blood monocytes in response to macrophage colony stimulating factor and RANK at a concentration of 250 ng/ml while apoptosis occurred at a concentration of 5-20 μg/ml.[35]
Thus CMTs shift the local microenvironment in the periodontal tissues toward more anabolic conditions. They promote matrix and collagen deposition and inhibit bone resorption through anti-MMP and pro-TIMP actions and reduced activity of inflammatory cytokines (e.g., IL-1, IL-6, TNF-α) and PGE2. These pleiotropic mechanisms of CMT provide significant therapeutic potential for treating periodontitis and various other chronic inflammatory conditions.
Other Uses of CMT
CMTs can be used in the management of various medical conditions associated with abnormal concentration and activity of collagenase. Some of these conditions are as follows:
1) Noninfected corneal ulcers
The sterile corneal ulcerations are due to an excessive host collagenase activity. Patients with noninfected corneal ulcers treated with tetracycline (250 mg q.i.d.) showed improvement in the condition within 48 hours of therapy with no recurrence over a period of 14 months. Histologically, reduced stromal loss seen after the administration of tetracyclines was attributed to faster epithelialization rate and reduced collagenase activity induced by anticollagenase action of tetracyclines.[36] Thus, CMTs can be used as potential agents for the treatment of noninfected corneal ulcers without the risk of development of antibiotic resistance.
2) Rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory disease primarily related to excessive collagenase and PGE2 production causing bone, joint or tissue destruction.[21] The PGE-2 increase local blood flow and potentiates the action of mediators such as bradykinin. It affects the cellular functions causing activation of MMPs, induction of apoptosis, inhibition of chondrocytic growth, activation of osteoclastic bone resorption, upregulation of IL-1 transcription factor and cAMP levels.
Both in vitro and in vivo studies have demonstrated the beneficial role of tetracyclines and CMT-1 in suppressing the collagenase activity in the cultured synovial tissue. However, a combination therapy using CMT-1 and flurbiprofen (nonsteroidal anti-inflammatory drug) produced a greater suppression of the clinical inflammation along with radiographic improvement of the joint condition. This was due to the synergistic effects of anti-inflammatory action of flurbiprofen along with anticollagenase action of CMT-1.[37] Besides CMT-1, CMT-3 and -8 have also shown inhibitory effect on COX-2-induced PGE2 production. Additional animal and human trials are however required to evaluate the efficacy of these agents in treatment of arthritis.
3) Diabetes mellitus
Experiments in diabetic rats showed that daily oral administration of CMT for 21-37 days reduced levels of pathologically excessive collagenase in gingival tissues and skin. The CMTs also increased the skin collagen production as revealed by increased concentrations of hydroxyproline. There was increased osteoblastic activity and bone formation.[4]
Both Type I and Type II diabetes mellitus have been related to periodontitis. In vitro and in vivo studies in rats with both the types of diabetes showed that the CMTs inhibited MMP activity, enzyme expression and alveolar bone loss. Administration of CMT-8 in type II diabetic rats with nephropathy or retinopathy showed a reduction in the incidence of cataract development, proteinuria, and tooth loss. The results were better with CMTs as compared to the commercial tetracyclines.[38]
4) Tumor metastasis
Recently, it has been reported that the CMTs are effective in treating metastasis which accounts for more than 90% of cancer mortality. Excessive production of matrix-degrading metallo-, cysteine-, and serine proteinase enzymes generated by tumor cells creates an imbalance between the destructive enzymes and their inhibitors. Tetracyclines, especially the minocycline and CMTs have been investigated for their antimetastatic actions in the tumors of prostate, breast and melanomas. They not only inhibit the invasive potential and MMP activity, but also cell proliferation by inducing cell cycle arrest and apoptosis.[39] The CMTs kill tumor cells by generation of hydroxyl free radicals which permeate and depolarize mitochondria. They also activate caspase-mediated apoptosis and reduce the rate of angiogenesis. Inhibition of the type IV collagenase prevents the tumor cells from invading the basement membrane barriers and hence metastasis.[39]
In a phase II trial of CMT-3 in the treatment of Kaposi's sarcoma in HIV patients, a significant decrease in serum MMP-2 and MMP-9 levels was seen. It was suggested that high doses of CMT-3 (50–150 mgqd) may be beneficial in the Kaposis's sarcoma patients who did not respond to highly active antiretroviral therapy alone. This may be attributed to its ability to inhibit neutrophil elastase.[40]
5) Other uses
CMT-3 has been shown to have antifungal properties.[41] Lower oral doses of CMT-3 are effective in decreasing the severity of acne.[42] They have also been used in the treatment of life threatening conditions like epidermolysis bullosa and acute respiratory distress syndrome associated with excessive collagenase activity.[43,44]
Conclusions
The CMTs are still in their infancy with regard to use in humans as they have not been approved due to concerns like excessive suppression of MMPs which may hinder the normal physiologic turnover of collagen. Further studies on CMTs which may be useful in suppressing not only the extracellular MMPs but also the intracellular targets are warranted. The CMT-3 has been shown to be the most promising agent among all the CMTs. If approved the CMTs might be a boon for patients as multiple conditions could be treated with a single drug.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Page C, Kornman S. The pathogenesis of human periodontitis: An introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 2.Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 3.Oringer RJ. Research, Science, and Therapy Committee of the American Academy of Periodontology. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73:460–70. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- 4.Golub LM, McNamara TF, D’Angelo G, Greenwald RA, Ramamurthy NS. A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dent Res. 1987;66:1310–4. doi: 10.1177/00220345870660080401. [DOI] [PubMed] [Google Scholar]
- 5.Consensus report. Periodontal diseases: Pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–32. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 6.Katsuragi H, Ohtake M, Kurasawa I, Saito K. Intracellular production and extracellular release of oxygen radicals by PMNs and oxidative stress on PMNs during phagocytosis of periodontopathic bacteria. Odontology. 2003;91:13–8. doi: 10.1007/s10266-003-0022-1. [DOI] [PubMed] [Google Scholar]
- 7.Houde V, Grenier D, Chandad F. Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J Periodontol. 2006;77:1371–9. doi: 10.1902/jop.2006.050419. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–8. [PubMed] [Google Scholar]
- 9.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 10.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–96. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryan ME, Golub LM. Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy. Periodontol 2000. 2000;24:226–38. doi: 10.1034/j.1600-0757.2000.2240111.x. [DOI] [PubMed] [Google Scholar]
- 12.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal disease. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 13.Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–9. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt DL, Meade EA, Smith WL. PGH synthase isoenzyme selectivity: The potential safer anti-inflammatory drugs. Am J Med. 1993;95:40S–4. doi: 10.1016/0002-9343(93)90396-7. [DOI] [PubMed] [Google Scholar]
- 15.Bord S, Horner A, Hembry RM, Reynolds JJ, Compston JE. Production of collagenase by human osteoblasts and osteoclasts in vivo. Bone. 1996;19:35–40. doi: 10.1016/8756-3282(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL. Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem. 1997;272:22053–8. doi: 10.1074/jbc.272.35.22053. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, del Carmen Ovejero M, Hou P, Heegaard AM, Kumegawa M, Foged NT, et al. Identification of the membrane-type matrix metalloproteinase MT1-MMP in osteoclasts. J Cell Sci. 1997;110:589–96. doi: 10.1242/jcs.110.5.589. [DOI] [PubMed] [Google Scholar]
- 18.Amano S, Kawakami K, Iwahashi H, Kitano S, Hanazawa S. Functional role of endogenous CD14 in lipopolysaccharide- stimulated bone resorption. J Cell Physiol. 1997;173:301–9. doi: 10.1002/(SICI)1097-4652(199712)173:3<301::AID-JCP1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes: Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–26. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 20.Steinsvoll S. Periodontal disease, matrix metalloproteinases and chemically modified tetracyclines. Microb Ecol Health Dis. 2004;16:1–7. [Google Scholar]
- 21.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: New therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Leung MK, Ramamurthy NS, McNamara TF, Golub LM. HPLC determination of a chemically modified nonantimicrobial tetracycline: Biological implications. Biochem Med Metab Biol. 1992;47:10–20. doi: 10.1016/0885-4505(92)90003-h. [DOI] [PubMed] [Google Scholar]
- 23.Yu Z, Ramamurthy NS, Leung M, Chang KM, McNamara TF, Golub LM. Chemically-modified tetracycline normalizes collagen metabolism in diabetic rats: A dose-response study. J Periodontal Res. 1993;28:420–8. [PubMed] [Google Scholar]
- 24.Chang KM, Ramamurthy NS, McNamara TF, Evans RT, Klausen B, Murray PA, et al. Tetracyclines inhibit Porphyromonas gingivalis-induced alveolar bone loss in rats by a non-antimicrobial mechanism. J Periodontal Res. 1994;29:242–9. doi: 10.1111/j.1600-0765.1994.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 25.Roy SK, Kendrick D, Sadowitz BD, Gatto L, Snyder K, Satalin JM, et al. Jack of all trades: Pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS) Pharmacol Res. 2011;64:580–9. doi: 10.1016/j.phrs.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramamurthy NS, Rifkin BR, Greenwald RA, Xu JW, Liu Y, Turner G, et al. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: A comparison of 6 chemically modified tetracyclines. J Periodontol. 2002;73:726–34. doi: 10.1902/jop.2002.73.7.726. [DOI] [PubMed] [Google Scholar]
- 27.Karimbux NY, Ramamurthy NS, Golub LM, Nishimura I. The expression of collagen I and XII mRNAs in Porphyromonas gingivalis-induced periodontitis in rats: The effect of doxycycline and chemically modified tetracycline. J Periodontol. 1998;69:34–40. doi: 10.1902/jop.1998.69.1.34. [DOI] [PubMed] [Google Scholar]
- 28.Grenier D, Plamondon P, Sorsa T, Lee HM, McNamara T, Ramamurthy NS, et al. Inhibition of proteolytic, serpinolytic, and progelatinase-b activation activities of periodontopathogens by doxycycline and the non-antimicrobial chemically modified tetracycline derivatives. J Periodontol. 2002;73:79–85. doi: 10.1902/jop.2002.73.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Trachtman H, Futterweit S, Greenwald R, Moak S, Singhal P, Franki N, et al. Chemically modified tetracyclines inhibit inducible nitric oxide synthase expression and nitric oxide production in cultured rat mesangial cells. Biochem Biophys Res Commun. 1996;229:243–8. doi: 10.1006/bbrc.1996.1787. [DOI] [PubMed] [Google Scholar]
- 30.Cazalis J, Tanabe S, Gagnon G, Sorsa T, Grenier D. Tetracyclines and chemically modified tetracycline-3 (CMT-3) modulate cytokine secretion by lipopolysaccharide-stimulated whole blood. Inflammation. 2009;32:130–7. doi: 10.1007/s10753-009-9111-9. [DOI] [PubMed] [Google Scholar]
- 31.Sandler C, Ekokoski E, Lindstedt KA, Vainio PJ, Finel M, Sorsa T, et al. Chemically modified tetracycline (CMT)-3 inhibits histamine release and cytokine production in mast cells: Possible involvement of protein kinase C. Inflamm Res. 2005;54:304–12. doi: 10.1007/s00011-005-1358-5. [DOI] [PubMed] [Google Scholar]
- 32.Rifkin BR, Vernillo A, Golub L, Ramamurthy N. Modulation of bone resorption by tetracyclines. Ann N Y Acad Sci. 1994;732:165–80. doi: 10.1111/j.1749-6632.1994.tb24733.x. [DOI] [PubMed] [Google Scholar]
- 33.Llavaneras A, Ramamurthy NS, Heikkilä P, Teronen O, Salo T, Rifkin BR, et al. A combination of a chemically modified doxycycline and a bisphosphonate synergistically inhibits endotoxin-induced periodontal breakdown in rats. J Periodontol. 2001;72:1069–77. doi: 10.1902/jop.2001.72.8.1069. [DOI] [PubMed] [Google Scholar]
- 34.Vernillo AT, Rifkin BR. Effects of tetracyclines on bone metabolism. Adv Dent Res. 1998;12:56–62. doi: 10.1177/08959374980120012101. [DOI] [PubMed] [Google Scholar]
- 35.Holmes SG, Still K, Buttle DJ, Bishop NJ, Grabowski PS. Chemically modified tetracyclines act through multiple mechanisms directly on osteoclast precursors. Bone. 2004;35:471–8. doi: 10.1016/j.bone.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Perry HD, Golub LM. Systemic tetracyclines in the treatment of noninfected corneal ulcers: A case report and proposed new mechanism of action. Ann Ophthalmol. 1985;17:742–4. [PubMed] [Google Scholar]
- 37.Greenwald RA, Moak SA, Ramamurthy NS, Golub LM. Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J Rheumatol. 1992;19:927–38. [PubMed] [Google Scholar]
- 38.Ryan ME, Ramamurthy NS, Sorsa T, Golub LM. MMP-mediated events in diabetes. Ann N Y Acad Sci. 1999;878:311–34. doi: 10.1111/j.1749-6632.1999.tb07692.x. [DOI] [PubMed] [Google Scholar]
- 39.Lokeshwar BL. Chemically modified non-antimicrobial tetracyclines are multifunctional drugs against advanced cancers. Pharmacol Res. 2011;63:146–50. doi: 10.1016/j.phrs.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dezube BJ, Krown SE, Lee JY, Bauer KS, Aboulafia DM. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi's sarcoma: An AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24:1389–94. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Ryan ME, Lee HM, Simon S, Tortora G, Lauzon C, et al. A chemically modified tetracycline (CMT-3) is a new antifungal agent. Antimicrob Agents Chemother. 2002;46:1447–54. doi: 10.1128/AAC.46.5.1447-1454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golub LM. Introduction and background. Pharmacol Res. 2011;63:99–101. doi: 10.1016/j.phrs.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg J, Halter J, Schiller H, Gatto L, Carney D, Lee HM, et al. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock. 2005;24:348–56. doi: 10.1097/01.shk.0000180619.06317.2c. [DOI] [PubMed] [Google Scholar]
- 44.White JE. Minocycline for dystrophic epidermolysis bullosa. Lancet. 1989;1:966. doi: 10.1016/s0140-6736(89)92555-5. [DOI] [PubMed] [Google Scholar]


