Abstract
Objective:
To analyse the behavioral effects of Melissa officinalis extract in rats following acute or subacute treatment.
Materials and Methods:
The behavioral effects of an acute or subacute (10-day course) orally administered M. officinalis (MO; 0, 30, 100 or 300 mg/kg) ethanol extract were evaluated in male and female Wistar rats in elevated plus-maze (EPM), forced swimming (FS) and open field (OF) tests. The effects of diazepam (DZP; 1 mg/kg) and fluoxetine (FXT; 10 mg/kg) were also assessed.
Results:
In the EPM test, the percentage of open arm entries and open arm times of both males and females given the subacute M. officinalis ethanol extract were significantly higher than those of the vehicle-treated animals but were at levels similar to those observed in the DZP group, regardless of the treatment length. In the FS test, immobility duration was significantly lower in both males and females treated with the plant extract when compared to vehicle-treated counterparts. A 10-day treatment with FXT induced the same antidepressant response, regardless of gender, and was more effective than the M. officinalis extract. Male and female rats demonstrated distinct gender profiles, and treatment × gender interactions were observed. Locomotion in the EPM and OF tests was not significantly altered by treatments.
Conclusion:
The potential psychoactive properties of M. officinalis may provide a unique pharmacological alternative for certain psychiatric disorders; however, the efficacy appears to be dependent on both gender and administration length.
KEY WORDS: Anxiety, depression, gender, locomotion, Melissa officinalis
Introduction
Melissa officinalis L., commonly known as lemon balm, is a perennial shrub of the Lamiaceae family. Although this scented herb was most commonly found in Europe during the middle ages, it is now cultivated worldwide.[1,2] Recently, substances with biological activity have been isolated from this plant. HPLC analyses demonstrate that rosmarinic acid, an ester of caffeic acid and 3,4-dihydroxyphenyllactic, is the major compound present in extracts and may drive the observed pharmacological effects.[3,4]
Investigations in healthy volunteers found that it was able to modulate mood.[5] However, data concerning the psychotropic effects of M. officinalis are limited, and a few comparative studies with putative anxiolytics and antidepressants as positive controls have been conducted. Additionally, the potential influences of gender and length of administration (e.g., acute versus subacute) have not been assessed.
The present study analyzed the behavioral effects of an M. officinalis extract in male and female rats during elevated plus maze, forced swimming and open field tests following acute or subacute treatment.
Materials and Methods
Animals
Male and female Wistar rats (2 months old; 250-350 g) were housed in groups of five in macrolon cages, with ad libitum access to food and water. The animals were kept under a reversed 12 h light/dark cycle (lights on at 6:00 a.m.) at a constant room temperature (23 ± 1°C). This study complied with the Brazilian Principles of Laboratory Animal Use (COBEA) and was approved by the Animal Ethics Committee of the Institute of Biology from our University.
Preparation of the Plant Extract
Dried M. officinalis leaves were supplied by the Centroflora Group (Botucatu, Brazil). The same group also provided a certification of the plant's identity and quality. Powdered plant material (1900 g) was macerated and extracted in ethanol at room temperature (24 ± 3°C). The solvent was removed under vacuum conditions at temperatures below 40°C. The subsequent crude ethanol extract (EE; 13% yield) was stored at –18°C.
Drugs and Pharmacological Procedures
M. officinalis EE (30, 100 and 300 mg/kg) was dissolved in a solution containing the following: 150 μL of Tween 80, 150 μL of alcohol and 150 μL of DMSO. Each preparation was then suspended in 0.9% (NaCl) saline. An equivalent preparation of Tween/alcohol/DMSO/saline was used as a vehicle control. Solutions were prepared 24 h in advance and stored at 4°C prior to administration via oral gavage (1 mL/kg). Diazepam (DZP; 1 mg/kg; Roche, Brazil) and fluoxetine (FXT; 10 mg/kg; Bluepharma, Brazil) were suspended in distilled water containing 2% Tween 80 and 0.9% saline. Both drugs were prepared the day of administration via oral gavage (1 mL/kg).
Experimental Procedures
Animals were randomly assigned to one of two administration regimes: Acute or subacute. Within each treatment length, animals were further divided into six treatment groups (n=10/treatment): 0, 30, 100 or 300 mg/kg M. officinalis EE, DZP or FXT. For the acute treatment groups, a single dose was administered 1 h before testing. Animals in the subacute groups, received daily treatments for 10 days and were tested 1 h following the final dose. Regardless of the length or type of treatment, subjects were transferred in their cages to the experimental room and allowed to habituate for at least 1 h before beginning the behavioral tests. Testing was performed in a randomized order during the animals’ active period (8:00 am – 2 pm).
Elevated plus maze test
Measures of anxiety were obtained in the elevated plus maze (EPM) test. This test comprises two opposing open arms (50 × 10 cm) and two opposing closed arms (50 × 10 × 40 cm), connected by a common central platform (10 × 10 cm). The entire apparatus is elevated 50 cm above floor level. To prevent falls, both open arms were fitted with a 1 cm Plexiglass edge. At the beginning of the test, the animal was placed on the central platform facing an enclosed arm and allowed to explore the maze freely for 5 min. The following parameters were scored: Number of open and enclosed arm entries and time spent on the central platform and in the open and enclosed arms. These data were used to calculate the percentage of open arm entries (%OAE) and open arm time (%OAT). Arm entry occurred when an animal placed all four paws in a specific arm.[6] After each trial, the EPM was cleaned with alcohol (10% v/v).
Forced swimming test
A modified version of the forced swimming (FS) test described by Porsolt et al.[7] was used. The animal was placed in a glass cylinder (30 cm in diameter and 50-cm high) containing 40 cm of water at 23 ± 1°C for 5 min, forcing the rat to either swim or float. The time spent immobile during the last 3 min of the test was recorded. Immobility occurred when the animal stopped swimming and floated, making only small limb movements necessary to keep its head above water. After 5 min, the animal was removed from the apparatus and dried. The water was changed after each trial.
Open field test
To evaluated effects on locomotor activity, animals were subjected to an open field (OF) test. The apparatus consisted of a wooden square box (60 × 60 × 35 cm) divided into nine equal squares (20 × 20 cm). Each animal was placed in the centre of the field and allowed to explore the area freely for 5 min. Locomotion was measured by counting the number of quadrants each animal crossed with all four paws. After each trial, the apparatus was cleaned with alcohol (10% v/v).
Statistical Analysis
The data were analyzed using two-way analysis of variance (ANOVA). Gender (male/female) and treatment (M. officinalis EE, DZP or FXT) were used as factors. Whenever significant, within gender, comparisons were performed using the Tukey's test with the appropriate error variance terms from the respective ANOVA summary tables. Statistical significance was set at P<0.05, and the data are expressed as the mean±S.E.M.
Results
Acute administration of M. officinalis EE (30, 100 or 300 mg/kg) or DZP did not significantly affect indicators of anxiety as measured by the EPM test. Only DZP showed a significant effect when compared to the control group [Figure 1]. Subacute treatment with M. officinalis EE or DZP, however, was effective at reducing behavioral indicators of anxiety during in the EPM test in both male and female rats [Figure 2]. Relative to the vehicle group, %OAE was significantly higher in males treated with 300 mg/kg M. officinalis EE or DZP. Similarly, females exhibited higher %OAE after treatment with all three doses of the plant extract or DZP [F(4,99) =7.28, P<0.001]. A significantly higher %OAT was also observed following subacute exposure to 100 or 300 mg/kg M. officinalis EE or DZP in males. Females treated with all three doses of the plant extract or DZP also exhibited significantly higher %OAT scores [F(4,99) =7.84, P<0.001]. Importantly, certain treatment effects were dependent on gender. Statistical analyses indicated significant interactions between treatment and gender for both the %OAE [F(4,99) =2.21, P<0.05] and %OAT [F(4,99) =2.07, P<0.05] scores, but not for the number of enclosed arm entries [F(4,99) =1.96, P=0.11] [Figures 1 and 2].
Figure 1.
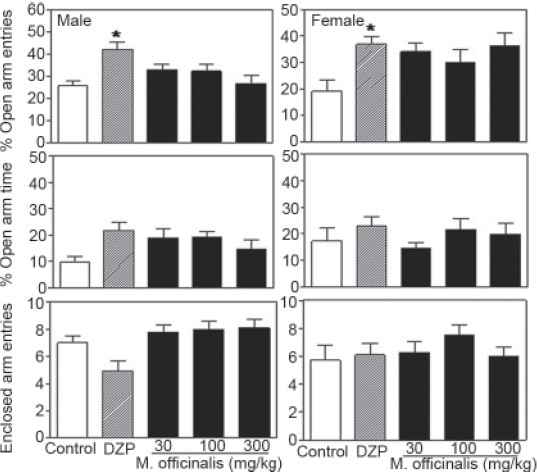
Percentage of open arm entries (top) and open arm time (middle) as well as the number of nclosed arm entries (bottom) for male and female rats tested in the elevated plus maze following acute treatment with Melissa officinalis ethanol extract (0, 30, 100 or 300 mg/kg) or diazepam (DZP; 1 mg/kg); n=10/group; *P≤0.05 vs. control group
Figure 2.
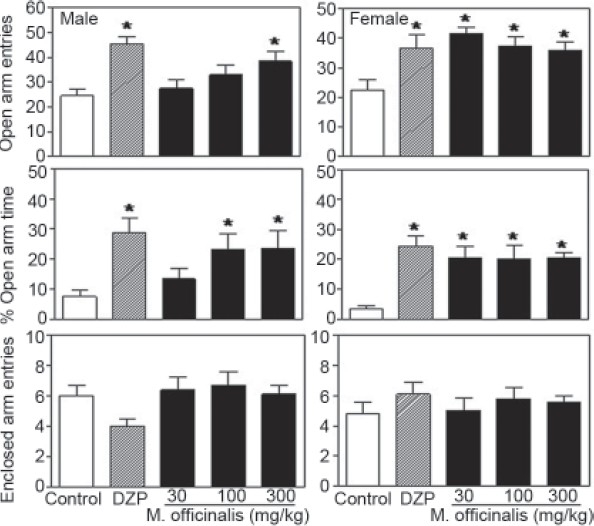
Percentage of open arm entries (top) and open arm time (middle) and the number of enclosed arm entries (bottom) for male and female rats tested in the elevated plus maze following the 10-day subacute treatment with Melissa officinalis ethanol extract (0, 30, 100 or 300 mg/kg) or diazepam (DZP; 1 mg/kg); n=10/group; *P≤0.05 vs. control group
No significant effects of treatment, gender or treatment × gender interactions were observed in the FS test following a single administration [Figure 3]. When male rats were treated subacutely, however, M. officinalis EE (100 or 300 mg/kg) induced a significant reduction in the amount of time spent immobile during the last 3 min of the trial. This reduction in time was greater in animals in the FXT group [F(4,99) =26.48, P<0.001]. Immobility time was also decreased significantly in females that were administered any of the three doses of the M. officinalis EE or FXT. Again, the plant extracts were not as effective as FXT. A significant between-gender difference was found for the time spent immobile in the FS test [F(1,99) =9.18, P<0.01], but there was no treatment × gender interaction [F(4,99) =0.19, P=0.94]. Animals acutely or subacutely treated with any of the three doses of the M. officinalis EE or DZP did not exhibit altered rates of locomotion [Figure 4]. No between-gender or treatment × gender interaction effects were observed following either the acute or subacute treatments [Figures 3 and 4].
Figure 3.
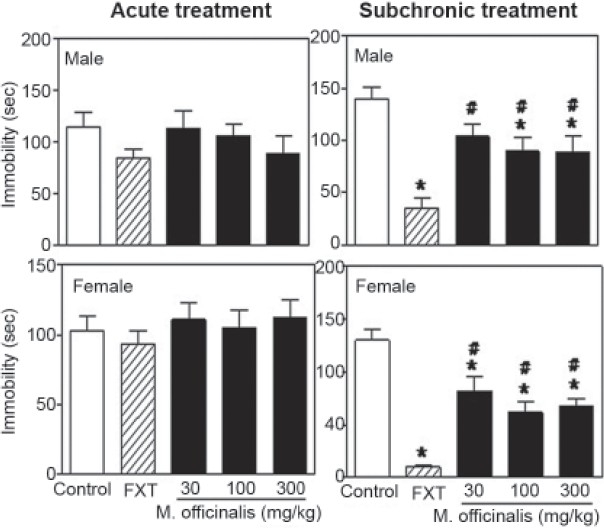
Time spent immobile (seconds) by male and female rats during the last 3 min of a 5-min forced swimming test following acute (left side) or 10-day subacute (right side) treatment with Melissa officinalis ethanol extract (30, 100 or 300 mg/kg), vehicle control (VEH) or fluoxetine (FXT; 10 mg/kg); n=10/group; *P≤0.05 vs. control group; #P≤0.05 vs. FXT group
Figure 4.
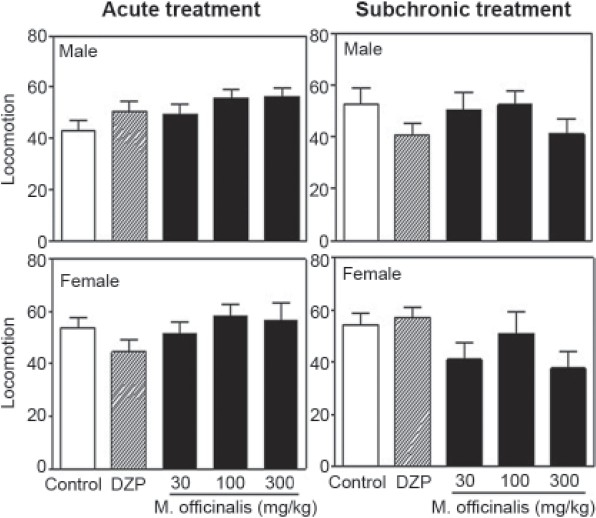
Number of quadrants crossed by male and female rats during 5 min in a rectangular open field following acute (left side) or 10-day subacute (right side) treatment with Melissa officinalis ethanol extract (0, 30, 100 or 300 mg/kg) or diazepam (DZP; 1 mg/kg); n=10/group
Discussion
The results of the current study indicate that oral ingestion of M. officinalis EE has anxiolytic and antidepressant-like properties. Importantly, the length of administration (acute vs. subacute) and gender (male vs. female) were found to significantly affect the observed behavioral profiles.
In the present study, ingestion of M. officinalis induced anxiolytic-like effects in rodents that were similar to those observed with DZP. However, male-female differences were observed, particularly with regard to the effective dose for each exposure length.
While all three subacute doses of M. officinalis EE resulted in a significantly higher %OAE and %OAT in females, the same effect was observed only in males that were administered the highest dose (300 mg/kg). Females demonstrate pharmacokinetic and pharmacodynamic profiles for anxiolytic compounds that are distinct from males[8,9] and have a higher prevalence of anxiety disorders.[10,11] Accordingly, specific features, particularly gender, may play an important role in the anxiolytic potential of the lemon balm plant.
Recently, a 15-day treatment with M. officinalis extract in male mice reduced anxiety-like response during an EPM test. These findings may be related to the effects that components of the extract have on γ-aminobutyric acid transaminase (GABA-t) activity. They are hypothesized to increase in brain GABA levels, thus reducing anxious behavior.[12] Acute treatment with a methanol extract or dried leaves has been reported to increase self ratings of calmness, modulate mood and reduce laboratory-induced stress in healthy young volunteers.[5,13]
The antidepressant-like effects of the M. officinalis EE detected in the FS test was highly dependent on the treatment length and gender. Contrary to the “mood altering” properties reported with a similar dose range in healthy young volunteers,[5,13] a single administration of the plant extract did not alter the time spent immobile in the present study. Acute treatment with the widely used antidepressant FXT (10 mg/kg) was also ineffective in the present study. FXT did, however, significantly lower immobility rates in subacutely treated males and females, in congruence with numerous preclinical and clinical trials.[14]
Although the magnitude of the effect was not as pronounced as that observed with FXT, male and female rats that received the M. officinalis EE also spent significantly less time immobile in the FS test compared to vehicle-treated animals, indicating an antidepressant effect. However, the lowest dose of the M. officinalis EE was ineffective in the male rats, and distinct response profiles were observed between males and females following subacute treatment.
Gender differences are also an important factor to consider when analyzing the potential antidepressant effect of lemon balm. Females demonstrate more depressive-like behaviors,[15] respond more intensely to the FS test[16] and react differently than males to antidepressant treatments.[17,18] Given that this preclinical study is the first to consider such effects in both genders, that different preparations derived from the same plant species may exhibit distinct properties and that not all active compounds and mechanisms of action have been fully elucidated, the potential antidepressant actions of M. officinalis presently observed require further investigation.
There is evidence to indicate that gender influences the neurotransmitter system and that this factor must be considered during behavioral and pharmacological investigations.[19–21] In rodents, gender differences related to emotionally linked behavior have been described in distinct experimental conditions.[22,23] Imhof et al.[24] showed that male and female rats exhibit increased fear-related behavior in the EPM as a function of age but not gender. In the present study, treatment with M. officinalis EE showed better results in females in a manner that was dependent on the experimental model.
Regardless of the active component or mechanisms of action, the extract presently investigated supports previous studies that have reported anxiolytic-like properties of M. officinalis comparable to those of DZP. Furthermore, these findings suggest a potential antidepressant effect in rodents, which needs to be confirmed by further studies. These psychoactive properties, along with lemon balm's safety profile over a wide dose range, may provide a unique pharmacological alternative for specific psychiatric disorders. Because the response profiles observed were dependent on both the administration length and gender, further studies are also needed to investigate the difference between male and female rats in the behavioral study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Koch-Heitzmann I, Schultze W. 2000 Jahre Melissa officinalis. Z Phytother. 1988;9:77–85. [Google Scholar]
- 2.Obulesu M, Rao DM. Effect of plant extracts on Alzheimer's disease: An insight into therapeutic avenues. J Neurosci Rural Pract. 2011;2:56–61. doi: 10.4103/0976-3147.80102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guginski G, Luiz AP, Silva MD, Massaro M, Martins DF, Chaves J, et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 2009;93:10–6. doi: 10.1016/j.pbb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Pereira P, Tysca D, Oliveira P, Brum LF, Picada JN, Ardenghi P. Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacol Res. 2005;52:199–203. doi: 10.1016/j.phrs.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy DO, Wake G, Savelev S, Tildesley NT, Perry EK, Wesnes KA, et al. Modulation of mood and cognitive performance following administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor binding properties. Neuropsychopharmacology. 2003;28:1871–81. doi: 10.1038/sj.npp.1300230. [DOI] [PubMed] [Google Scholar]
- 6.Pellow S, Chopin P, File SE, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 7.Porsolt RD, Lepichon M, Jalfre M. Depression–New animal-model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 8.Palanza P. Animal models of anxiety and depression: How are females different? Neurosci Biobehav Rev. 2001;25:219–33. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 9.Yonkers KA, Kando JC, Cole JO, Blummenthal S. Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. Am J Psychiatry. 1992;149:587–95. doi: 10.1176/ajp.149.5.587. [DOI] [PubMed] [Google Scholar]
- 10.Doering LV, Eastwood JA. A literature review of depression, anxiety, and cardiovascular disease in women. J Obstet Gynecol Neonatal Nurs. 2011;40:348–61. doi: 10.1111/j.1552-6909.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 11.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings – Report from the World Health Organization collaborative study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 12.Ibarra A, Feuillere N, Roller M, Lesburgere E, Beracochea D. Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine. 2010;17:397–403. doi: 10.1016/j.phymed.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy DO, Little W, Haskell CF, Scholey AB. Anxiolytic effects of a combination of Melissa officinalis and Valeriana officinalis during laboratory induced stress. Phytother Res. 2006;20:96–102. doi: 10.1002/ptr.1787. [DOI] [PubMed] [Google Scholar]
- 14.Wong DT, Perry KW, Bymaster FP. The discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–74. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 15.Negrigo A, Medeiros M, Guinsburg R, Covolan L. Long-term gender behavioral vulnerability after nociceptive neonatal formalin stimulation in rats. Neurosci Lett. 2011;490:196–9. doi: 10.1016/j.neulet.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 16.Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the Forced Swim Test in rats. Neuroscience. 2004;126:849–57. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int Rev Psychiatry. 2010;22:485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- 18.Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–70. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–94. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain SK, Zelena D. Gender specific influence of endogenous glutamate release on stress-induced fear in rats. Endocr Regul. 2011;45:13–21. [PubMed] [Google Scholar]
- 21.Sutcliffe JS. Female rats are smarter than males: Influence of test, oestrogen receptor subtypes and glutamate. Curr Top Behav Neurosci. 2011;8:37–56. doi: 10.1007/7854_2011_120. [DOI] [PubMed] [Google Scholar]
- 22.Basso AM, Gallagher KB, Mikusa JP, Rueter LE. Vogel conflict test: Sex differences and pharmacological validation of the model. Behav Brain Res. 2011;218:174–83. doi: 10.1016/j.bbr.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 23.García-Cáceres C, Lagunas N, Calmarza-Font I, Azcoitia I, Diz-Chaves Y, García-Segura LM, et al. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology. 2010;35:1525–35. doi: 10.1016/j.psyneuen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56:177–80. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]


