Abstract
Objectives:
To evaluate the efficacy of Amla in patients with type II hyperlipidemia and compare its hypolipidemic effects with those of simvastatin.
Materials and Methods:
Sixty type II hyperlipidemic patients of both sexes with plasma total cholesterol and low density lipoprotein level more than 240 mg% and 130 mg%, respectively, were selected for the trial. Out of total 60 selected patients, 40 were treated with Amla capsule (500 mg) daily for 42 days and 20 patients were given simvastatin capsule (20 mg) daily for 42 days. After the day of enrolment, all patients were followed up twice during the 42-day period. Blood samples were analyzed for various biochemical parameters and the values of Total Cholesterol (TC), Low Density Lipoprotein (LDL), High Density Lipoprotein (HDL), and Very Low Density Lipoprotein (VLDL) were measured before and after completion of the treatment with Amla and simvastatin. Cardiovascular parameters were recorded before and after completion of treatment.
Results:
Treatment with Amla produced significant reduction of TC (P<0.0001), LDL (P<0.0001), triglyceride (TG) and VLDL (P<0.0002), and a significant increase in HDL levels (P<0.0002). Similarly, treatment with simvastatin produced significant reduction of TC (P<0.0001), LDL (P<0.0009), TG and VLDL (P<0.017), and a significant increase in HDL levels (P<0.0001). Both treatments produced significant reduction in blood pressure; however, this beneficial effect was more marked in patients receiving Amla.
Conclusion:
In view of the above findings, it is suggested that Amla produced significant hypolipidemic effect along with a reduction in blood pressure. Addition of Amla to the currently available hypolipidemic therapy would offer significant protection against atherosclerosis and coronary artery disease, with reduction in the dose and adverse effects of the hypolipidemic agents.
KEY WORDS: Amla, clinical study, hyperlipidemia, lipid profile, simvastatin
Introduction
Hyperlipidemia is one of the most frequently implicated risk factors for development of atherosclerosis.[1] A strong association exists between hyperlipidemia and coronary artery disease (CAD), cerebrovascular stroke, and peripheral vascular disease.[2] In the peripheral circulation, it can cause intermittent claudication and gangrene and can jeopardize limb viability.[3] The hypolipidemic drugs have attracted considerable attention because of their potential to prevent cardiovascular disease by retarding the accelerated atherosclerosis in hyperlipidemic individuals. The drugs used in the management of hyperlipidemia are bile acid sequestrants, nicotinic acid, fibric acid derivatives, and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG Co-A) reductase inhibitors. Of these, statins and fibrates have shown greater promise.[4]
Bile acid sequestrants in a dose-dependent manner lower the total cholesterol (TC) and low density lipoprotein (LDL).[5,6] At a higher dose, they cause bloating and dyspepsia, which limit their compliance. A new potent drug colesevelam hydrochloride is associated with a low incidence of gastrointestinal symptoms.[7]
Both regular (crystalline) niacin and sustained release niacin, which were developed to reduce flushing and itching, have been reported to cause severe liver toxicity. Sustained-release niacin can cause fulminant hepatic failure.[8] The new fibrates are contraindicated in renal failure.[9] Moreover, when used in combination with statins, these cause rhabdomyolysis.[10]
Considering the above-mentioned facts, HMG Co-A reductase inhibitors are the only drugs that are well tolerated and produce better improvement in lipid profile either as monotherapy or in combination with other drugs such as fibric acid derivates. But even these can increase serum transaminase level in 1% cases.[11] In rare cases, these cause hepatopathy[12] and, in some cases, renal failure.[13] Thus, attempts to lower lipids with allopathic medicines have not been successful in most patients.
There is immense potential for medicinal plants used in various traditional systems. Among the several herbs, Emblica officinalis (Amla or Indian gooseberry) is one of the herbs described to possess multiple therapeutic activities. Its use has been described in Ayurveda[14] and Unani medicines.[15] Mostly, all parts of E. officinalis are used in clinical practice: e.g, root bark in stomach ulcer; bark in gonorrhea, jaundice, and diarrhea;[16] leaves in conjunctivitis and inflammation;[17] fruits as digestive, laxative, and antipyretics.[18] In addition, it has been shown to reduce cholesterol level in experimental animals[19] and in clinical studies.[20]
In the present study, the efficacy of Amla was evaluated in patients with type II hyperlipidemia and its hypolipidemic effects compared with those of the commonly used HMG Co-A reductase inhibitor – simvastatin.
Materials and Methods
Study Design
Present comparative trial was carried out in the medical wards of Shri Sayaji General (S S G) Hospital, Vadodara, on 60 patients (45 male and 15 female) between 35 and 65 years of age selected on the basis of the following criteria:
Inclusion Criteria
Presence of type II hypercholesterolemia (i.e., serum Total Cholesterol (TC)>240 mg/dl and serum Low Density Lipoprotein-cholesterol (LDL)>130 mg/dl) and presence of at least two of the following risk factors of Coronary Heart Disease (CHD): Family history of CHD, male >45 years or female >55 years (not on estrogen replacement therapy), smoking, hypertension, serum high density lipoprotein (HDL) <35 mg/dl, obesity (Body Mass Index >27), and those consenting to participate in the study were enrolled. The patients were evaluated clinically and subjected to routine hematological and biochemical investigations to rule out associated medical ailments.
Exclusion Criteria
Patients with history of recent (within past 6 months) serious cardiovascular diseases or any endocrine or concomitant serious disorder of the liver, kidney, heart, lung, and/or other organs and receiving any drug treatment for the same were excluded from the study. Also, patients with secondary hyperlipidemia (except diabetes mellitus) and pregnant and lactating women were not included in the study.
Ethical Consideration
Permission and approval for conducting proposed clinical trial was obtained (wide no.: G-1/Ethics committee/1639-43/01 dated 11/29-01-2001) from the Institutional Human Research Ethics Committee, Medical College, Vadodara.
Drugs Used
Amla capsule (500 mg) (dried Amla fruit juice powder) (Pharma Intel Co, Kolkata).
Simvastatin capsule each containing 20 mg (Ranbaxy Laboratories, Gurgaon, Haryana).
Study Design
A total of 100 patients were screened and 60 patients were finally selected for the study as per inclusion and exclusion criteria. Of these, 40 patients (Group-A) were given one capsule of Amla (500 mg) daily at night for 42 days, while the other 20 patients (Group-B) received one capsule of Simvastatin (20 mg) daily at night for 42 days.
Schedule of Investigations
The patients were evaluated for physical and cardiovascular parameters; further, all routine biochemical investigations including lipid profile were performed before starting the treatment as well as after completion of the treatment (i.e., at the end of 42 days). All signs and symptoms previously identified or present and new signs and symptoms or adverse effects, if any, were assessed during as well as after completion of the treatment. Patients having systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg were considered as hypertensive. Control of hypertension or improvement of blood pressure was considered when systolic blood pressure was <140 mm Hg and/or diastolic blood pressure <90 mm Hg, while further increase in the existing blood pressure in the same hypertensive patient was considered as worsening of blood pressure.
Lipid Profile Measurement
For lipid profile, venous blood samples of 10 ml were collected in sterilized bulbs after at least 12 hours of overnight fasting. The samples were centrifuged at 3,000 rpm for 10 minutes and serum was separated from formed cellular elements. Samples were analyzed and values of TC[21] and triglycerides[22] were estimated as described previously, while HDL was calculated using the HDL kit and LDL and Very Low Density Lipoprotein (VLDL) were calculated by using Friedewald's formula.
Adverse Effects
Adverse effects were monitored throughout the study and assessments followed up to two weeks post treatment.
Statistical Analysis
Student's t test was employed for comparison between two means. One-way Analysis Of Variance (ANOVA) test with post-test significance was employed for comparison among all the means. P<0.05 was considered as statistically significant.
Data Analysis and Results
The study sample included 60 hyperlipidemic patients. Of these, 40 patients were treated with Amla and 20 with Simvastatin in a parallel study.
Characteristics of Patients
Out of 60 patients, two had a family history of heart disease, 38 had hypertension, 30 were smokers, 13 had HDL <35 mg/dl, and 16 patients had BMI >27.
Age and Sex Distribution
Ages of patients were in the range of 35-65 years. A majority of patients (45) were in the age group of 40-60 years and 6 were in the age group of 60-65 years. Fifteen patients were female and 45 were male. A majority of males (33) and females (12) were in the age range of 40-60 years.
As a non-lipid factor for coronary heart disease, hypertension was found to be more prevalent in patients whose LDL level was more than 160 mg% as compared to those having LDL level within 130-160 mg% [Table 1].
Table 1.
Correlation between hypertension and LDL cholesterol in patients

Effect of Amla and Simvastatin on Control of Hypertension
Of the 40 patients receiving Amla, 28 were hypertensive while of the 20 patients receiving simvastatin, 10 were hypertensive. At the end of the treatment, a total of 27 patients showed improvement in their blood pressure. Of these, 21 patients were on Amla and 6 on simvastatin therapy [Table 2].
Table 2.
Effects of Amla and Simvastatin treatments on blood pressure of hypertensive patients
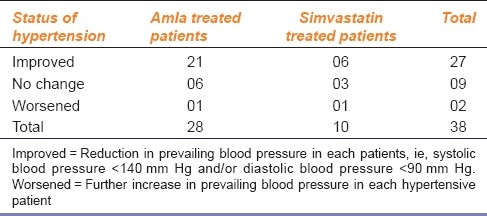
Effects of Amla and Simvastatin Treatments on Lipid Profile
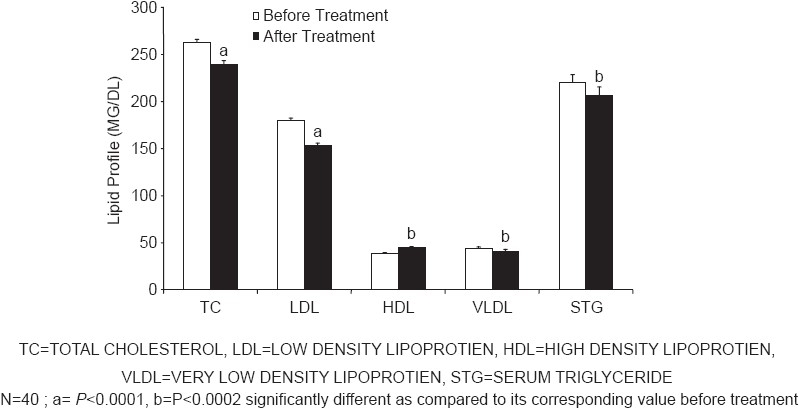
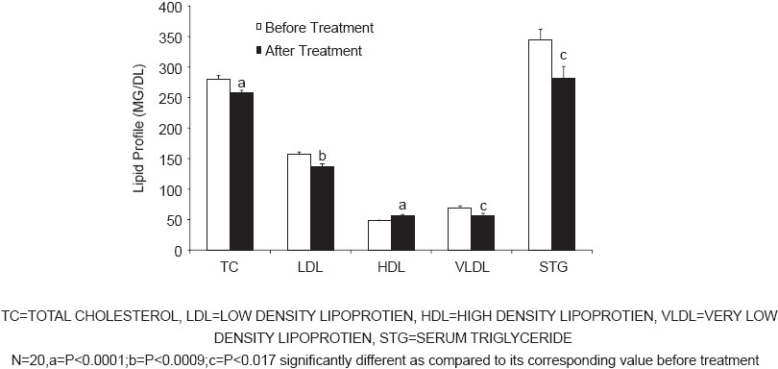
Treatment with Amla for 42 days produced significant decrease in the mean levels of TC (P<0.0001), LDL (P<0.0001), VLDL, and triglycerides (P<0.0002), and a significant increase in HDL (P<0.0002) levels [Figure 1]. Similarly, treatment with simvastatin for 42 days produced a significant decrease in the mean levels of TC (P<0.0001), LDL (P<0.0009), VLDL, and triglycerides (P<0.017) and a significant increase in HDL (P<0.0001) levels [Figure 2]. Both treatments produced nearly equivalent alterations in lipid levels except that simvastatin treatment produced significantly (P<0.005) more reduction in VLDL and triglyceride levels as compared to that produced by Amla treatment [Figure 3].
Figure 1.
Effect of amla treatment on lipid profile
Figure 2.
Effects of simvastatin treatment on lipid profile
Figure 3.
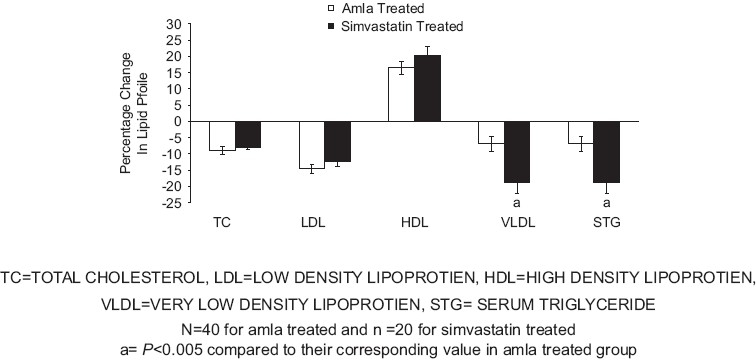
Percentage change in various parameters of lipid profile in amla and simvastatin treated patients at visit III
Other Biochemical Parameters
There was no significant alteration in any of the other laboratory biochemical parameters in patients treated with either Amla or simvastatin.
Adverse Drug Reaction Due to the Treatments
None of the patients receiving either Amla or simvastatin therapy reported any adverse effects during the treatment or till two weeks later.
Discussion
Hyperlipedemia, an abnormal elevation of plasma lipids, is associated with a higher rate of cardiovascular, cerebrovascular, and peripheral vessel involvement, and hence increased morbidity and mortality.
The Multiple Risk Intervention Trial (MRFIT) reported that there is a curvilinear relationship between TC and CAD. There is a small but definite increase in the risk for subjects having TC level of 150-200 mg/ml. The study showed that the CAD-related mortality rate escalated more dramatically with cholesterol levels exceeding 280 mg/ml.[23]
In the primary prevention trials, decrements in total cholesterol regardless of the therapeutic modality were associated with significantly decreased CAD events.[4] In the Framingham study, it was reported that low level of HDL cholesterol is an independent risk factor for CAD.[24] For each 4 mg/ml decrease in HDL, there was a 10% increase in risk for CAD.
Elevated TC and LDL cholesterol levels should be controlled in all patients with CAD or at risk of CAD. The therapeutic options that proved to be beneficial include diet, regular exercise, controlling other risk factors, and appropriate use of statins and new fibrates for a long duration.
The statin therapy in high-risk asymptomatic individuals for primary prevention strategy has been found to be safe, beneficial, and cost-effective.[5] In the secondary prevention trials with simvastatin,[25] it has been reported that in patients with CAD having TC 250 mg/ml, therapy for 5.4 years with simvastatin produced reduction of TC by 25%, LDL levels by 35%, and risk for CAD by 42%. Both primary and secondary prevention trials demonstrate that each 1 mg/ml decrease in plasma cholesterol is associated with a 2% decrease in CAD.[26]
In our study, simvastatin treatment for 42 days produced significant reduction in the TC, LDL, and triglyceride (TG) levels as compared to that reported by Scandinavians Simvastatin Survival Study[25] and the study by Down et al.[11] However, the discrepancy in the lipid lowering capacity of statin in our study and that reported in the literature could be attributed to the shorter duration of treatment employed by us.
In Ayurveda, Amla is reported to be beneficial in the treatment of respiratory, cardiovascular, and rheumatic diseases as well as in diabetes. Various experimental studies also have suggested antioxidant[27] and hypolipidemic effects[19] of Amla. In a clinical study, lipid lowering action of Amla has been reported in patients aged 35-55 years when raw Amla was administered for 28 days.[20] In our study, Amla showed a reduction of TC, LDL and TG and an increase in HDL. The drug was administered as 500 mg capsule per day for 42 days. It is also interesting to note that both Amla and simvastatin produced similar changes in the lipid profile; however, simvastatin treatment produced greater reduction in the TG level as compared to that produced by Amla.
A clinical study, has reported that 8.5-14% reduction in serum TC is associated with a statistically significant reduction in CAD.[4] Thus, in our study, the reductions in lipid profile observed after a short-term treatment with simvastatin and Amla may offer significant protection against CAD.
Amla contains high amounts of vitamin C in the natural form as well as cytokine-like substances identified as zeatin, Z-riboside, Z-nucleotide, flavonoids pectin, and 30% tannins. Tannins present in Amla retard the oxidation of vitamin C, while pectin has been reported to decrease serum cholesterol levels in human beings.[27] The flavonoid content of Amla was analyzed for its biological activity and found to possess a potent hypolipidemic effect.[28]
Though the exact mechanism of hypolipidemic action of Amla is not known, it is likely that the Amla-induced favourable changes in the lipid profile may be due to several mechanisms such as an interference with cholesterol absorption,[19] inhibition of HMG Co-A reductase activity, and increase in Lecithin-Cholesterol Acyltransferase (LCAT) activity.[29]
In our study, both Amla and simvastatin therapy produced a reduction in blood pressure in hypertensive patients. However, this effect was more prevalent in the Amla-treated group as compared to the simvastatin-treated group. Amla has been reported to reduce oxidative stress, prevent development and progression of hypertension, as well as cardiac and renal hypertrophy in desoxycorticosterone acetate (DOCA)-salt-induced hypertension in rat via modulation of activated Endothelial Nitric Oxide Synthase (eNOS), endogenous antioxidants, serum Nitric Oxide (NO), and electrolyte levels.[30] Therefore, it is likely that a reduction in blood pressure observed in the present study by Amla treatment may be mediated through the above mechanisms.
In view of the above action of Amla, it is suggested that Amla produced significant hypolipidemic as well as antihypertensive effects. Addition of Amla to the currently available hypolipidemic therapy would offer significant protection against atherosclerosis and CAD, with a reduction in the dose and adverse effects of the hypolipidemic agents. Extensive clinical studies in a larger population are required to establish the role of Amla in management of hyperlipidemia along with hypertension.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Assman G, Schulte H. Relation of high density cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (The PROCAM experience) Am J Cardiol. 1992;70:733–7. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 2.Miller GJ, Miller NE. Plasma high density lipoprotein concentration and development of ischaemic heart disease. Lancet. 1975;1:16–9. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 3.Davey-Smith G, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors and mortality. The White Hall Study. Circulation. 1990;82:1925–31. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee S. Therapeutic management of hyperlipidemia – Rationale and outcome. Assoc Physicians India. 2002:315–30. [Google Scholar]
- 5.Casdorph HR. The single dose method of administering cholestyramine. Angiology. 1975;26:671–82. doi: 10.1177/000331977502600905. [DOI] [PubMed] [Google Scholar]
- 6.Huninghake DB, Probstfeild JL, Crow LO, Isaacson SO. Effect of colestipol and clofibrate on plasma lipid and lipoprotein in type IIa hyperlipoproteinemia. Metabolism. 1981;30:605–9. doi: 10.1016/0026-0495(81)90140-2. [DOI] [PubMed] [Google Scholar]
- 7.Davidson MH, Dillon MA, Gordon B, Jones P, Samuels J, Weiss S, et al. Colesevelam hydrochloride (cholestogel): A new side effect. Arch Intern Med. 1999;159:1893–900. doi: 10.1001/archinte.159.16.1893. [DOI] [PubMed] [Google Scholar]
- 8.Mullin GE, Greenson JK, Mitchell MC. Fulminant hepatic failure after ingestion of sustained-release nicotinic acid. Ann Intern Med. 1989;111:253–5. doi: 10.7326/0003-4819-111-3-253. [DOI] [PubMed] [Google Scholar]
- 9.Rubin HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemifibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. Veterans Affairs High Density Lipoprotein Cholesterols Intervention Trial Study Group. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 10.Alexandridis G, Pappas GA, Elisaf MS. Rhabdomyolysis due to combination therapy with cerivastatin and gemfibrozil. Am J Med. 2000;109:261–2. doi: 10.1016/s0002-9343(00)00514-3. [DOI] [PubMed] [Google Scholar]
- 11.Downis JR, Clearfield M, Weis S, Whitney E, Shaprio DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. Results of AFCAPS/Tex CAPS. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Nakad A, Bataille L, Hamior V, Sempoux C, Horsmans V. Atorvastatin induced acute hepatitis with absence of cross toxicity with simvastatin. Lancet. 1999;353:1763–4. doi: 10.1016/S0140-6736(99)00569-3. [DOI] [PubMed] [Google Scholar]
- 13.Pogson GW, Kindred LH, Carper BG. Rhabdomyolysis and renal failure associated with cerivastatin, gemfibrozil combination therapy. Am J Cardiol. 1999;83:1146. doi: 10.1016/s0002-9149(99)00034-x. [DOI] [PubMed] [Google Scholar]
- 14.Rani G, Bala S, Grover I S. Antimutagenic studies of diethyl ether extract and tannin fractions of Emblica myroblan (Emblica officinalis) in Ames assay. J Plant Sci Res. 1994;10:1–4. [Google Scholar]
- 15.Arora BB. 8.7 mg of vitamin C from amla is equivalent to 100 mg of vitamin C from synthetic sources. Dev Unani Drugs Herb Sour. 1985:234. [Google Scholar]
- 16.Gulati R, Agrawal S, Agarwal SS. Hepatoprotective studies on phyllanthus emblica and querectin. Indian J Exp Biol. 1995;33:261–8. [PubMed] [Google Scholar]
- 17.Kumar KC, Muller K. Medicinal plants from Nepal II. Evaluation as inhibitors of lipid peroxidation in biological membranes. J Ethnopharmacol. 1999;64:135–9. doi: 10.1016/s0378-8741(98)00117-2. [DOI] [PubMed] [Google Scholar]
- 18.Biswas S, Talukdar G, Sharma A. Protection against cytotoxic effects of arsenic by dietary supplementation with crude extract of Emblica officinalis fruit. Phytother Res. 1999;13:513–6. doi: 10.1002/(sici)1099-1573(199909)13:6<513::aid-ptr525>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Mathur R, Sharma A, Dixit VP, Varma M. Hypolipidemic effect of fruit juice of Emblica officinalis in cholesterol – fed rabbits. J Ethnopharmacol. 1996;50:61–8. doi: 10.1016/0378-8741(95)01308-3. [DOI] [PubMed] [Google Scholar]
- 20.Jacob A, Pandey M, Kapoor S, Saroja R. Effect of the Indian gooseberry (amla) on serum cholesterol level in men aged 35-55 years. Eur J Clin Nutr. 1988;42:939–44. [PubMed] [Google Scholar]
- 21.Alloin CC, Poom LS, Chan CS. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470. [PubMed] [Google Scholar]
- 22.Bucolo G, David H. Quantitative determination of serum triglyceride by the use of enzymes. Clin Chem. 1973;19:476. [PubMed] [Google Scholar]
- 23.Stamler M, Wenworth D, Neaton JD. For the MRFIT. Is the relationship between serum cholesterol and risk of premature death from coronary artery disease continuous and graded? Finding in trial. (MRFIT) JAMA. 1986;256:2823–8. [PubMed] [Google Scholar]
- 24.Kuirtrervich PO., Jr The antiartherogenic role of high density lipoprotein cholesterol. Am J Cardiol. 1998;82:13Q–21. doi: 10.1016/s0002-9149(98)00808-x. [DOI] [PubMed] [Google Scholar]
- 25.Scandinavian Simvastatin Survival Study group. Randomized trial of cholesterol lowering in 4444 patients with coronary artery disease: The SSSS (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 26.Lipid Research Clinic Program: The lipid research clinic. Coronary Primary Prevention Trial Results II. The relationship of reduction in incidences of coronary artery disease to cholesterol lowering. JAMA. 1984;251:365–74. [PubMed] [Google Scholar]
- 27.Bhattacharya A, Chatterjee A, Ghoshal S, Bhattacharya SK. Antioxidant activity of active tannoid principle of Emblica offinalis (Amla) Indian J Exp Biol. 2000;37:676–80. [PubMed] [Google Scholar]
- 28.Anila L, Vijyalaxmi NR. Beneficial effects of flavanoids from Seasamum indicum, Emblica offinalis and Momordica charantia. Phytother Res. 2000;14:592–5. doi: 10.1002/1099-1573(200012)14:8<592::aid-ptr772>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Anila L, Vijyalaxmi NR. Flavanoids from Emblica offinalis and Magnifera indica: Effectiveness for dyslipidemia. J Ethnopharmacol. 2002;79:81–7. doi: 10.1016/s0378-8741(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia J, Tabassum F, Sharma AK, Bharati S, Golechha M, Joshi S, et al. Emblica officinalis exerts antihypertensive effect in a rat model of DOCA-salt-induced hypertension: Role of (p) eNOS, NO and oxidative stress. Cardiovasc Toxicol. 2011;11:272–9. doi: 10.1007/s12012-011-9122-2. [DOI] [PubMed] [Google Scholar]


