Abstract
Background:
Soil-transmitted intestinal helminth infection is prevalent in rural communities of Malaysia. Risk factors contributing to helminth infections are largely unknown in the country.
Aim:
To determine the prevalence and risk factors of intestinal helminth infections among children in Beris Lalang, a rural Muslim community of Malaysia.
Settings and Design
In this cross-sectional study, children aged 7-9 years were recruited during the mass Friday prayer at Beris Lalang mosque by trained imams (religious leaders). A standardized questionnaire was used to obtain information on socio-demographic profile, daily hygienic practices, and history of helminth infection.
Results:
Out of 79 samples, 29 (37%) were positive for helminthic ova, of which 24 were ova of Trichuris trichiura. Poor education of the mother (primary education or less) (P=0.015), eating raw salad (P=0.03), and no physical activities (P=0.03) were found independent risk factors for the child's helminth infections in univariate analysis. A higher proportion of children with helminth infections complained of tiredness and fatigue compared to those without such infections (36% vs. 12%, P=0.019). In a multivariate analysis of predictors of helminth infection, poor education of the mother (P=0.02) and eating raw salad (P=0.04) remained statistically significant, after controlling for several other potential risk factors.
Conclusions
T. trichiura was the most prevalent intestinal helminth infection in children in rural Malaysia. Risk factors of helminth infection included mother's poor education and eating raw salad and vegetables.
Keywords: Children, Intestinal helminth, Malaysia, Prevalence, Risk factors
INTRODUCTION
Intestinal helminth infections are widespread among children in the tropics and subtropics. These infections are rarely fatal but they may impair growth, physical fitness, cognition, and reduce school attendance and performance.[1,2] Children between 5–14 years of age in developing countries are especially at risk of soil-transmitted helminth (STH) infections.[3] More than 500 million people are infected with trichuriasis, ascariasis, or hookworm infections globally. These infections are classified among the seven of the most common neglected tropical infectious diseases that afflict the bottom billion because of their high prevalence and amenability to control.[4] Because it is uncommon for STHs to kill their human host, citing mortality figures provides only a small window on their health impact. Instead, measurements of disease burden using disability-adjusted life years (DALYs) and similar tools portray a more accurate picture for helminthic disease burden. Based on an earlier estimate, the DALY lost due to helminthes is approximately 19.8 million.[3]
In Malaysia, numerous reports revealed that helminth infections among rural children are still widespread.[5–10] Poor literacy, lack of awareness, lack of people's participation, and lack of personal hygiene are among the common barriers of a successful intervention program. However, risk factors for intestinal helminths have been rarely reported and awareness programs are scanty. In a study in Pos Betau, Kuala Lipis, Pahang, age >10 years, no toilet in the house, working mother, low household income, and large family size were identified as risk factors for ascariasis.[6] Reports of risk factors for other helminth infections are almost nonexistent in this country. This study aimed to identify risk factors for STH infection in a rural community of Beris Lalang, Bachok, Malaysia.
MATERIALS AND METHODS
Study subjects and design
Beris Lalang is a typical mukim (sector) of the Telung sub-district of Bachok, Malaysia. This agriculture-based mukim is situated near the coastline of South-China Sea in Kelantan, a north-eastern state of Peninsular Malaysia.
This cross-sectional study was based on purposive sampling of subjects. First, the village headman of Beris Lalang mukim was contacted and discussed about the project objectives. The villagers were Muslim Malays, who performed their mass Friday prayer at mosques near their homes. The investigators identified the imam (Muslim religious leader) of Beris Lalang mosque as the key person to recruit subjects. The imam was given a formal two-week training, which included 1) knowledge about common intestinal helminths; 2) importance of screening children's stool samples for intestinal helminthes; 3) the consent form; 4) recruitment; and 5) referral for treatment. On two consecutive Friday sermons, the mosque imam disseminated the information to the villagers. The imam first distributed the informed consent forms to parents. Once they gave consent to participate, the imam distributed the questionnaire and stool containers to parents. The questionnaire and stool samples were returned to the imam on the next three successive mornings thereafter, depending on the availability of the children's stool samples. Inclusion criteria were children aged 7–9 years, with permanent addresses in Beris Lalang. The reason for selecting children between 7 and 9 years was that these children are classified as Level 1 children, and the children at this stage in Malaysia are the key focus for educating and practicing basic personal hygiene. The fecal container distributed contained 5 ml of 4% formalin as a preservative. A standardized questionnaire was used to obtain information on socio-demographic profile, daily hygienic practices, and history of helminth infection.
The study was approved by Universiti Sains Malaysia Research Ethics Committee. Approval was also obtained from the headman of Beris Lalang to engage the mosque imam in encouraging parental support for recruitment of the children. Verbal consent was also obtained from the children.
Stool examination
Stool samples were screened for helminth ova based on the conventional saline wet mounting technique.[11] One inner and one outer portions of each formed stool sample were used to prepare two microscopy slide replicates to detect eggs of intestinal helminths. For loose watery stool sample, any two portions were used to prepare the two slide replicates. Subsequently, the microscopy results were categorized qualitatively as either positive or negative for each category of infection. Names of all infected children were later disclosed to the local health officer for anthelminthic treatment. Treatment for helminthic infection was provided by the nurses in the community clinic, based on the advices of an assigned medical officer in the district health clinic.
Anthropometric measurements
Anthropometric variables were measured according to standard anthropometric techniques.[12] Height was measured to the nearest of 0.5 cm without shoes. Weight was determined to the nearest of 0.1 kg with the participant in light clothes and without shoes using pre-calibrated weighing scale (Seca, UK). Body mass index (BMI) was calculated based on the following formula: weight (kg) divided by height (m2).
Statistical analysis
The minimum sample size of 74 was estimated based on the formula n = (ȥ/d)2 p(1 – p), with a confidence level of 95% (ȥ=1.96) and margin error of 10% (d = 0.1).[13] SPSS version 17.0 was used to analyze the distributions of helminths. Student's t-test and Chi-square or Fisher's exact test were used to analyze statistical significant difference of the potential risk factors between the two groups of children: those who had helminths present and those did not have helminths isolated in their stool samples. A multiple logistic regression test and 95% confidence intervals (CI) of odds ratio were calculated to compare the risk factors among the two groups. A probability level of 5% was used as statistical significance.
RESULTS
In the community there were 275 eligible children, of which 195 (71%) received the research materials during the second Friday sermon. Eighty-seven (45%) of 195 villagers who received the research materials gave consent for the children to be recruited in the study. Eight were excluded as the children's stool samples were not submitted along with the questionnaires and consent forms. A total of 79 children (48 boys and 31 girls) who fulfilled the inclusion criteria were recruited.
Prevalence
Twenty-nine of 79 samples (37%) were positive for ova of helminths. A total of 30.4% of children were infected with Trichuris trichiura, of which 12 had mixed infections with Ascaris lumbricoides. Ova of A. lumbricoides were detected in three boys and one girl. A 9-year-old boy was positive for ova of Enterobius vermicularis. Interestingly, hookworm infection was not detected in any of these children [Figure 1].
Figure 1.
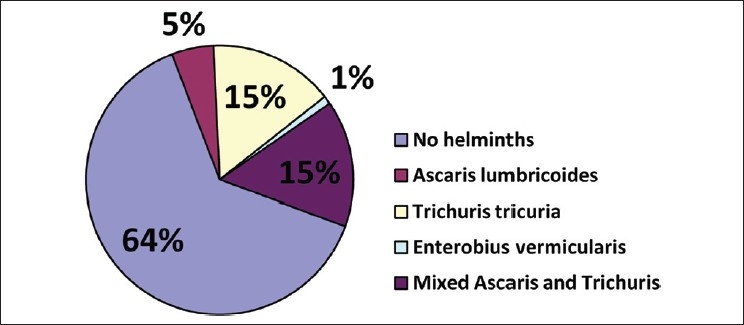
Distribution of rural Malay children with intestinal helminth ova isolated from stool samples
Bivariate association of factors with helminth infection
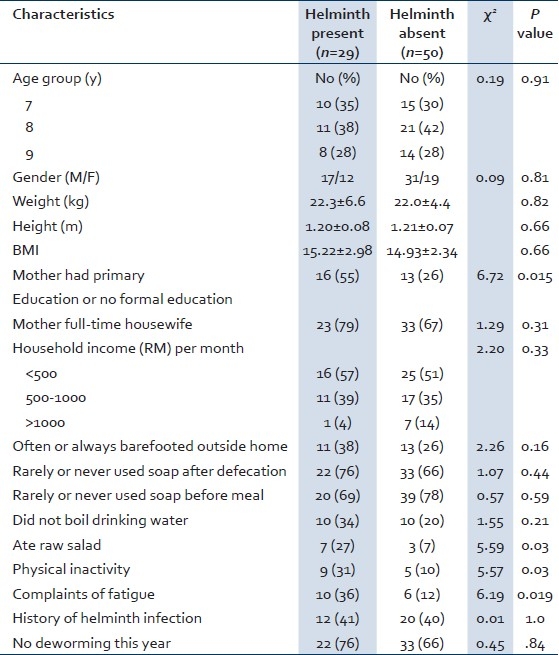
The children who had helminths isolated and those who did not have any helminths isolated were compared to identify risk factors of infection. The two groups did not differ significantly by age group, gender, weight, height, and body mass index (BMI) [Table 1]. However, there were significantly more infected children whose mothers had either primary education or no formal education, as compared to the healthy ones (P=0.015). In addition, a higher proportion of infected children lived with mothers who were full-time housewives, often or always barefooted outside home, rarely or never used soap after defecation, and did not boil water for drinking, although the variables were not statistically significant [Table 1]. Conversely, infected children ate raw salad and vegetables (P=0.03), had physical inactivity (P=0.03) and complained of fatigue (P=0.019) more often than the noninfected children.
Table 1.
Factors associated with helminth infection in Malay children
Multivariate analysis of predictors of helminth infection
A multiple logistic regression analysis was done using the stepwise forward selection method. The dependent variable was isolation of helminths in the stool. The independent variables found statistically significant at bivariate analysis were kept in the model. BMI, although not statistically significant at bivariate level, was kept in the model to assess the contribution of nutritional status of the children. Parents’ education and occupation being interrelated, only education was included in the model. Similarly, household income being dependent primarily on father's education, income was not included in the model. Two variables, physical inactivity and fatigue, although found significant at bivariate level, were first included to assess their relationship with helminth infection. However, these two variables were removed from the final model [Table 2] because they were considered physical symptoms, rather than risk factors of helminth infection.
Table 2.
Multivariate logistic regression analysis to predict helminth infection in Malay children

Table 2 shows that poor education of the mother (primary education or no formal education) (P=0.03) and eating raw salad (P=0.04) remained significant predictors of the child's helminth infections, after controlling for a number of other potential risk factors. The variables that were not statistically significant but included in the model were: BMI, father's education, bare footed outside home, use of soap before meal, use of soap after defecation, boiling drinking water, and deworming.
DISCUSSION
The overall helminth infection rate in the study population was 37%. The significant factors associated with helminth infections included: poor education (primary education or less) of the mother, use of raw salads and vegetables, physical inactivity, and complaints of fatigue. In multivariate analyses, poor education of the mother and eating raw salad were significant predictors of the child's helminth infection.
The most common isolation was T. trichiura in 24/79 (30%) of the children in this study. This finding was consistent with a previous study in Bachok in which 66.8% of the children studied had trichuriasis.[5] In the later study, a modified Stoll's volumetric dilution method was used in contrast to the conventional saline wet mounting technique[11] used in our study. The observed difference in isolation rate could be partially due to the different techniques used in the two studies.
The prevalence and intensities of intestinal helminth infection vary according to geographical location. In Nigerian schoolchildren, the prevalence of any species of intestinal helminth was 30%, with the prevalence being significantly higher among the rural subjects than among the urban children (36% vs. 24%, P<0.001).[14] Among the different species, A. lumbricoides was the most common one, followed by hookworm and T. trichiura infection. In southern Indians, hookworm was the commonest helminth infection seen in 48/78 (61.5%).[15] Hookworm infections are normally correlated with habitual barefooted children.[16,17] More than one-quarter of our study children did not use shoes or slippers while they walked or played outside. However, the proportion of the barefooted children did not differ significantly among those who had and those who did not have helminths isolated, and none had hookworm infection.
Interestingly, there were significantly more infected children whose mothers had neither formal education nor finished primary schooling, as compared to the healthy ones (P=0.015). This is in congruent with the study reported from fishing families, in which children with mothers of low levels of education had the highest intensity of A. lumbricoides infection.[18] Moreover, a higher proportion of infected children lived with mothers who were full-time housewives, compared to noninfected children (79% vs. 67%), although not statistically significant. Mothers who had the traditional homemaker role and spent more time with the children presumably should have more opportunity to educate their children. That was not the case in our study. A similar observation was made by Curtale et al.[19] A high percentage of these rural, less educated mothers were likely to be still ignorant about proper hygienic habits and health-seeking behaviors.
In our study, there was no significant correlation between the two groups of children in relation to handwashing before meals or after defecation. This was in accordance with the review on the ambivalent relationship between these two hygienic practices and reduction of ascariasis.[20] However, it is important to note that nearly 70% of the people did not use soap before meal or after defecation, although no significant differences were observed between the two study groups. Twenty percent or more people did not boil water for drinking, probably because either they did not know the importance of boiling water or they could not afford it. This indicates importance of a comprehensive health education program for the rural community of Bachok. Conversely, significantly more infected children were found to eat raw salad (P=0.03). Presence of helminth eggs in raw vegetables have been reported.[21] Many infected children often offered excuses to avoid physical activities (P=0.03) and complained of fatigue (P=0.019). Similar complaints of infected children were observed in earlier studies.[22] Intestinal helminth infection can reduce nutrient absorption, which subsequently cause children too lethargic to perform physical activities. Parents in rural areas should be educated to observe for these negative behaviors among their children, which may be a warning sign to seek medical attention for the children. Also, parents, especially the mothers, need to know the preventive and control measures in order for them to be actively involved in reducing the infections among their children.
One of the limitations of this study was that data were collected by convenience sampling which yielded a small sample size. Parents either failed to respond or were reluctant to deal with collecting their children's stool samples. Furthermore, attendances of parents and adults during health-related programs organized by schools were often poor. This emphasizes the importance of involving parents and guardians in health education programs, in addition to educating school children in healthy living.
One uniqueness of this study was the involvement of imams (Muslim religious leaders) in recruiting children and educating parents. Beris Lalang being a Muslim-predominant community, involvement of an imam in health services was a culturally appropriate and acceptable approach by the community. Success stories are accumulating in using community lay people in health interventions. Religious leaders, who have day-to-day contact with people, were found useful in disseminating health education programs in Egypt.[23]
CONCLUSION
Based on this study, the prevalence rate of helminth infection was 37% among children in rural Malaysia, with T. trichiura being the predominant helminth isolated. The independent risk factors for helminth infection were poor education of the mother and consumption of raw salad and vegetables. Future studies should focus on the efficacy of educating mothers in preventing intestinal helminth infections among their children.
ACKNOWLEDGMENTS
The study was funded by the Universiti Sains Malaysia, University Research Grant (1001/PPSK/812022). The authors are thankful to Nik Mohmed Zaipul Mahmud, Mohamad Noor M. Roze and Chan Siok Gim for their technical assistance, and Fauziah Mohd Nor for offering free treatment to the study children.
Footnotes
Source of Support: The study was funded by the Universiti Sains Malaysia, University Research Grant (1001/PPSK/812022)
Conflict of Interest: None declared.
REFERENCES
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. Parasitic helminth infection and cognitive function in school children. Proc Biol Sci. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- 3.World development report 1993. New York: Oxford University Press; 1993. World Bank; pp. 25–9. [Google Scholar]
- 4.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–5. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 5.Anees AH, Zulkifli A, Azmi A, Syukri M. Helminthiasis among primary rural schoolchildren in Bachok, Kelantan. Malays J Pub Health Med. 2003;3:19–22. [Google Scholar]
- 6.Hesham Al-Mekhlafi MS, Atiya AS, Lim YA, Mohammed Mahdy AK, Wan Ariffin WA, Che Abdullah H, et al. An unceasing problem: Soil-transmitted helminthiases in rural Malaysia communities. Southeast Asian J Trop Med Public Health. 2007;38:998–1007. [PubMed] [Google Scholar]
- 7.Hakim SL, Gan CC, Malkit K, Azian MN, Chong CK, Shaari N, et al. Parasitic infections among Orang Asli (aborigine) in the Cameron Highlands, Malaysia. Southeast Asian J Trop Med Public Health. 2007;38:415–9. [PubMed] [Google Scholar]
- 8.Al-Mekhlafi MH, Surin J, Atiya AS, Ariffin WA, Mahdy AK, Abdullah HC. Anaemia and iron deficiency anaemia among aboriginal school children in rural Peninsular Malaysia: An update on a continuing problem. Trans R Soc Trop Med Hyg. 2008;102:1046–52. doi: 10.1016/j.trstmh.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Hesham Al-Mekhlafi M, Surin J, Atiya AS, Ariffin WA, Mohammed Mahdy AK, Che Abdullah H. Pattern and predictors of soil-transmitted helminth reinfection among aboriginal school children in rural Peninsular Malaysia. Acta Trop. 2008;107:200–4. doi: 10.1016/j.actatropica.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Lim YA, Romano N, Colin N, Chow SC, Smith HV. Intestinal parasitic infections amongst Orang Asli (indigenous) in Malaysia: Has socioeconomic development alleviated the problem? Trop Biomed. 2009;26:110–22. [PubMed] [Google Scholar]
- 11.Basic laboratory methods in medical parasitology. Geneva: WHO; 1991. World Health Organization; pp. 9–31. [Google Scholar]
- 12.Physical status: The use and interpretation of anthropometry. WHO Technical Report Series 854. Geneva: 1995. World Health Organization. [PubMed] [Google Scholar]
- 13.Sample size determination in health sciences: A practical manual. Geneva: WHO; 1991. World Health Organization. [Google Scholar]
- 14.Oninla SO, Owa JA, Onayade AA, Taiwo O. Intestinal helminthiases among rural and urban schoolchildren in south-western Nigeria. Ann Trop Med Parasitol. 2007;101:705–13. doi: 10.1179/136485907X241406. [DOI] [PubMed] [Google Scholar]
- 15.Kang G, Mathew MS, Rajan DP, Daniel JD, Mathan MM, Mathan VI, et al. Prevalence of intestinal parasites in rural Southern Indians. Trop Med Int Health. 1998;3:70–5. doi: 10.1046/j.1365-3156.1998.00175.x. [DOI] [PubMed] [Google Scholar]
- 16.Nmor JC, Onojafe JO, Omu BA. Anthropogenic indices of soil-transmitted helminthiasis among children in Delta State, Southern Nigeria. Iran J Public Health. 2009;38:31–8. [Google Scholar]
- 17.Tadesse G. The prevalence of intestinal helminthic infections and associated risk factors among school children in Babile town, eastern Ethiopia. Ethiop J Health Dev. 2005;19:140–7. [Google Scholar]
- 18.Naish S, McCarthy J, Williams GM. Prevalence, intensity and risk factors for soil-transmitted helminth infection in a South Indian fishing village. Acta Trop. 2004;91:177–87. doi: 10.1016/j.actatropica.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Curtale F, Pezzotti P, Sharbini AL, al Maadat H, Ingrosso P, Saad YS, et al. Knowledge, perceptions and behaviour of mothers toward intestinal helminths in Upper Egypt: Implications for control. Health Policy Plan. 1998;13:423–32. doi: 10.1093/heapol/13.4.423. [DOI] [PubMed] [Google Scholar]
- 20.Fung IC, Cairncross S. Ascariasis and hand washing. Trans R Soc Trop Med Hyg. 2009;103:215–22. doi: 10.1016/j.trstmh.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Kozan E, Gonenc B, Sarimehmetoglu O, Aycicek H. Prevalence of helminth eggs on raw vegetables used for salads. Food Control. 2005;16:239–42. [Google Scholar]
- 22.Mohammed KA, Haji HJ, Gabrielli AF, Mubila L, Biswas G, Chitsulo L, et al. Triple co-administration of ivermectin, albendazole and praziquantel in Zanzibar: A safety study. PLoS Negl Trop Dis. 2008;2:e171. doi: 10.1371/journal.pntd.0000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Gibaly O, Hemeyda K. Are imams in Egypt prepared to help stop the spread of HIV/AIDS? [Last accessed on 2010 Dec 08]. Available from: http://www.prb.org/Articles/2010/imams.aspx .


