Abstract
Objectives:
To determine the rates of device-associated healthcare-associated infections (DA-HAI), microbiological profile, bacterial resistance, length of stay (LOS), excess mortality and hand hygiene compliance in one intensive care unit (ICU) of a hospital member of the International Infection Control Consortium (INICC) in Beirut, Lebanon.
Materials and Methods:
An open label, prospective cohort, active DA-HAI surveillance study was conducted on adults admitted to a tertiary-care ICU in Lebanon from November 2007 to March 2010. The protocol and methodology implemented were developed by INICC. Data collection was performed in the participating ICUs. Data uploading and analyses were conducted at INICC headquarters on proprietary software. DA-HAI rates were recorded by applying the definitions of the National Healthcare Safety Network (NHSN) at the US Centers for Disease Control and Prevention (CDC). We analyzed the DA-HAI, mechanical ventilator-associated pneumonia (VAP), central line-associated bloodstream infection (CLA-BSI), and catheter-associated urinary tract infection (CAUTI) rates, microorganism profile, excess LOS, excess mortality, and hand hygiene compliance.
Results:
A total of 666 patients hospitalized for 5,506 days acquired 65 DA-HAIs, an overall rate of 9.8% [(95% confidence interval (CI) 7.6–12.3], and 11.8 (95% CI 9.1–15.0) DA-HAIs per 1000 ICU-days. The CLA-BSI rate was 5.2 (95% CI 2.8–8.7) per 1000 catheter-days; the VAP rate was 8.1 (95% CI 5.5–11.7) per 1000 ventilator-days; and the CAUTI rate was 4.1 (95% CI 2.6–6.2) per 1000 catheter-days. LOS of patients was 7.3 days for those without DA-HAI, 13.8 days for those with CLA-BSI, 18.8 days for those with VAP. Excess mortality was 40.9% [relative risk (RR) 3.14; P 0.004] for CLA-BSI. Mortality of VAP and CAUTI was not significantly different from patients without DA-HAI. Escherichia coli was the most common isolated microorganism. Overall hand hygiene compliance was 84.9% (95% CI 82.3–87.3).
Conclusions:
DA-HAI rates, bacterial resistance, LOS and mortality were moderately high, below INICC overall data and above CDC-NHSN data. Infection control programs including surveillance and antibiotic policies are essential and continue to be a priority in Lebanon.
Keywords: Catheter associated urinary tract infection, Central line associated bloodstream infection, Ventilator associated pneumonia, Intensive care unit, Lebanon, International Nosocomial Infection Control Consortium
INTRODUCTION
In the USA, as well as in several other high income countries, device-associated healthcare-associated infection (DA-HAI) surveillance in the intensive care unit (ICU) plays a substantial role in hospital infection control and quality assurance.[1] The Study of the Efficacy of Nosocomial Infection Control (SENIC) of the Centers for Disease Control (CDC) reported that surveillance was an efficacious tool to reduce DA-HAIs.[2]
In an increasingly large amount of scientific literature, DA-HAIs are considered the principal threat to patient safety in the ICU, and are among the main causes of patient morbidity and mortality.[3–5] The CDC's previous National Nosocomial Infection Surveillance System (NNIS) and current National Healthcare Safety Network (NHSN) have established standardized criteria for DA-HAI surveillance.[6,7] This standardized surveillance method allows for the determination of DA-HAI rates per 1000 device-days that can be used as benchmarks among healthcare centers, and provides infection control practitioners (ICP) with an in-depth look at the institutional problems they confront, so they can design an effective strategy to solve them.
In the context of an expanded framework for DA-HAI control, most of the relevant studies of ICU-acquired infections have been carried out in the industrialized countries.[8] In the developing countries, however, few published studies report on the data of DA-HAI rates by means of using standardized definitions.[9–17]
The International Infection Control Consortium (INICC) was founded in 1998 when selected hospitals from Latin America were invited to participate in the project in order to measure DA-HAI using standardized definitions and methodology.[18] Shortly afterwards, other hospitals located in different parts of the world joined the consortium. Nowadays, the INICC comprises a worldwide network of around 250 ICUs from 38 countries of Latin America, Asia, Africa and Europe.[9–17]
On a monthly basis, healthcare facilities send data to the INICC which are then entered into an international database. Hospitals members of INICC provide general medical and surgical inpatient services to adults and children hospitalized in the intensive care units.
In Lebanon, published data on DA-HAI rates are scarce (Azzam, Tohme). The findings of the present study on Lebanon form an integral part of INICC and reflect the outcome and process surveillance data that were systematically collected.
MATERIALS AND METHODS
Setting
The study was carried out in an ICU at the American University of Beirut Medical Center (AUBMC), a tertiary care teaching hospital in Lebanon from November 2007 to March 2010. The hospital has an infection control team composed of two physicians (a director and a hospital epidemiologist), two ICPs with more than fifteen years of experience in infection control, and an infection control officer with a nursing background. The clinical microbiology laboratory, which is accredited by the College of American Pathologists (CAP) provides in vitro antibiotic susceptibility testing of clinical isolates using standardized methods. The Institutional Review Board of the hospital approved the study protocol. Patient confidentiality was protected by codifying the recorded information, making it only identifiable to the infection control team.
Surveillance
On a daily basis, data were collected prospectively from all the patients admitted to the ICUs by means of specifically designed forms. The data were gathered according to the DA-HAI definitions provided by the CDC-NNIS and CDC-NHSN,[6,7] and methodology of INICC.[18]
Collection of specimens
Central line associated bloodstream infection (CLA-BSI): Central lines were removed aseptically and the distal 5 cm of the catheter was amputated and cultured using a standardized semi-quantitative method.[19] Concomitant blood cultures were drawn from the lines prior to removal and percutaneously in nearly all cases.
Ventilator associated pneumonia (VAP): In most cases, a deep tracheal aspirate from the endotracheal tube was cultured aerobically and Gram-stained.
Catheter-associated urinary tract infection (CAUTI): A urine sample was aseptically aspirated from the sampling port of urinary catheter and cultured quantitatively.
In all cases, standard laboratory methods were used to identify microorganisms, and a standardized susceptibility test was performed.[20]
Device-associated infections rates calculation
Outcomes measured during the surveillance period included the incidence density rate of CLA-BSI (number of cases per 1000 central venous catheter days), of CAUTI (number of cases per 1000 urinary catheter days), and of VAP (number of cases per 1000 mechanical ventilator days).
In order to calculate DA-HAI rates of VAP, CLA-BSI, and CAUTI per 1000 device-days were calculated by dividing the total number of DA-HAI by the total number of specific device-days and multiplying the result by 1000.[21]
Device utilization (DU) ratios were calculated by dividing the total number of device-days by the total number of patient days. Device-days are the total number of days of exposure to the device (central line, ventilator, or urinary catheter) by all of the patients in the selected population during the selected time period. Patient-days are the total number of days that patients are in the ICU during the selected time period.[21]
Length of stay and mortality calculation
Length of stay (LOS) and mortality was collected prospectively when filling out INICC forms daily.
The excess LOS is the difference between the LOS of patients with a DA-HAI and the LOS of patients hospitalized in the ICU during that period who did not acquire a DA-HAI.[18]
The crude excess mortality was calculated as the difference between the crude overall case-fatality of patients with a DA-HAI and the crude case-fatality of patients hospitalized in the ICU during that period who did not acquire a DA-HAI.[18]
Statistical analysis
EpiInfo® version 6.04b (CDC, Atlanta, GA) and SPSS 16.0 (SPSS Inc. an IBM company, Chicago, Illinois) were used to conduct data analysis.
Chi square analyses for dichotomous variables and t-test for continuous variables were used to analyze baseline differences among rates. Relative risk (RR) ratios, 95% confidence intervals (CIs) and P values were determined for all primary and secondary outcomes.
RESULTS
Features of population studied
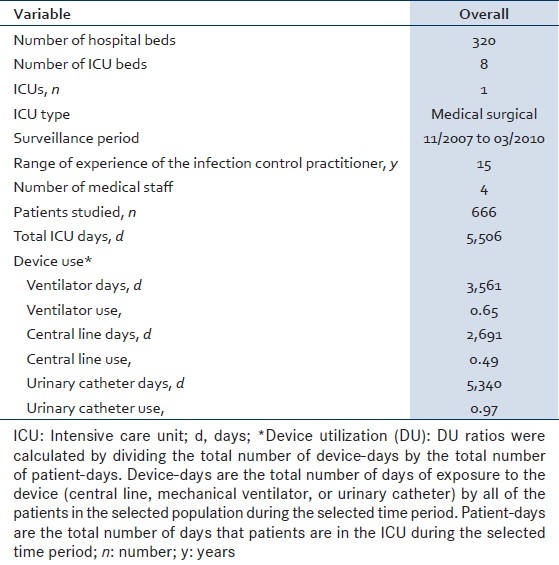
During the two years and four months of study, surveillance data were prospectively collected on 666 patients hospitalized in the ICUs for 5,506 ICU-days. The characteristics of the ICU, the number of patients enrolled in the study, the number of ICU days and device use days are shown in Table 1. Device utilization was 0.65 for mechanical ventilation, 0.49 for CL, and 0.97 for urinary catheters. The total number of hand hygiene (HH) opportunities observed was 843. Overall hand hygiene compliance rate was 84.9% (95% CI 82.3-87.3).
Table 1.
Characteristics of the intensive care unit at the American University of Beirut Medical Center, member of the International Nosocomial Infection Control Consortium
DA-HAI rates, mortality and LOS
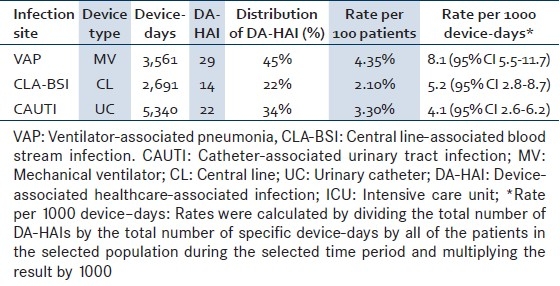
The patients acquired 65 DA-HAIs, giving an overall rate of 9.8% (95% CI 7.6–12.3) or 11.8 DA-HAIs per 1000 ICU-days (95% CI 9.1–15.0). VAP were the most commonly encountered type of infection, accounting for 45% of all DA-HAIs, followed by CAUTI at 34% and CLA-BSI at 22% [Table 2].
Table 2.
Device associated infections rates (VAP, CLA-BSI, and CAUTI) from 11/2007 to 03/2010
CLA-BSI
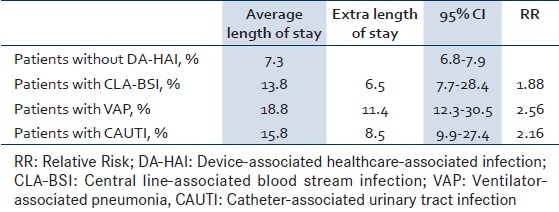
The CLA-BSI rate was 5.2 per 1000 CL-days (95% CI 2.8–8.7) [Table 2]. The crude mortality of patients with CLA-BSI was 60% compared to 19% in patients without DA-HAI, amounting to an excess mortality rate of 41% (RR 3.14, 95% CI 1.4-7.1, P=0.004) [Table 3]. Similarly, LOS was longer in patients with CLA-BSI compared to those without DA-HAI (13.8 days; 95% CI 7.7–28.4 vs. 7.3 days; 95% CI 6.8–7.9), yielding an extra LOS of 6.5 days (RR 1.88) [Table 4].
Table 3.
Excess mortality of patients with deviceassociated infections from 11/2007 to 03/2010
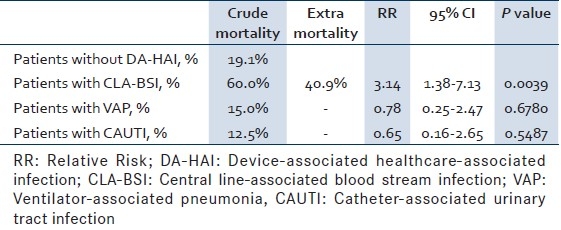
Table 4.
Excess length of stay of patients with device-associated infections from 11/2007 to 03/2010
VAP
As for VAP, the rate was 8.1 per 1000 MV-days (95% CI 5.5-11.7) [Table 2]. The crude mortality of patients with VAP was 15% comparable to that of patients without DA-HAI (RR 0.78; 95% CI 0.3-2.5; P 0.678) [Table 3]. The LOS of patients with VAP was 18.8 days (95% CI 12.3-30.5), yielding an extra LOS of 11.4 days (RR 2.56) [Table 4].
CAUTI
The CAUTI rate was 4.1 per 1000 UC-days (95% CI 2.6-6.2) [Table 2]. The LOS of patients with CAUTI was 15.8 days (95% CI 9.9-27.4), yielding an extra LOS of 8.5 days (RR 2.16) [Table 4].
Overall microorganism profile and bacterial resistance
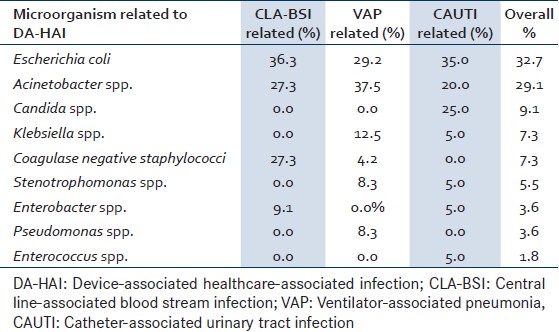
DA-HAI were caused by E. coli (32.7%), Acinetobacter spp. (29.1%), Candida spp (9.1%), Klebsiella spp. (7.3%), coagulase negative staphylococci (CNS) (7.3%), Stenotrophomonas spp. (5.5%), by Pseudomonas spp. (3.6%), Enterobacter spp. (3.6%), and Enterococcus spp (1.8%). Staphylococcus aureus was not isolated [Table 5]. Antibiotic susceptibilities showed that all E. coli isolates were resistant to amikacin, ciprofloxacine, and to ampicillin. Resistance to cefalotin, ceftazidime, gentamicin, and ceftriaxone was observed in 66%, 50%, 25%, and 33% of the isolates, respectively. However, all strains were susceptible to amikacin, imipenem, piperaciline-tazobactam, cefoperazone. For Acinetobacter spp. Isolates, we showed that all were resistant to amikacin, ceftazidime, ciprofloxacine, gentamicin, imipenem, and to piperacillin-tazobactam. All Klebsiella spp. isolates were resistant to ampicillin, cefalotin, and none was resistant to amikacin or imipenem, 33% were resistant to ceftazidime and to ciprofloxacine, 50% to gentamicin and to piperaciline-tazobactam. CNS isolates were all resistant to methicilin, clindamycin, and penicillin, however none was resistant to gentamicin or to vancomycine. Stenotrophomonas spp.-0% of which were resistant to levofloxacin. Resistance to ceftazidime, to ciprofloxacine, to gentamicin, and to piperaciline-tazobactam was not detected any Pseudomonas spp. isolate. Enterobacter spp. isolates were resistant to levofloxacin and susceptible to all other antibiotics tested. Finally, all Enterococcus spp. were resistant to ampicilin, to erythromycin, and to vancomycin.
Table 5.
Distribution of pathogens involved in device-associated infections from 11/2007 to 03/2010
DISCUSSION
Although DA-HAIs have been a primary and serious cause of patient morbidity and attributable mortality in the developing countries,[9–17] this is the first prospective study to examine DA-HAI rates in an ICU of a university hospital in Lebanon. Additionally, DA-HAIs have been shown to be a critical factor, predisposing hospitals to increased healthcare costs.[9,10,22,23] However, several research studies conducted in the US have indicated that the incidence of DA-HAI can be reduced by as much as 30%, which would result in correlative reduced healthcare costs. It is noteworthy that US hospitals that were able to reduce their DA-HAI rates relied on strategies developed by their infection control programs, which included targeted device-associated surveillance.[2] In addition, compliance with hand hygiene has also been found to be central to any infection control intervention. In this study, hand hygiene compliance rate was higher than in the overall INICC ICUs: 84.9% (95% CI 82.3–87.3) vs. 54.1% (95% CI 53.6–54.4).[12] This is largely due to the efforts made by the infection control team at AUBMC by launching several campaigns over the past 4 years to increase awareness of the importance of hand hygiene among healthcare workers, and by making alcohol based hand rubs widely available in all patient care areas. However, although hand hygiene compliance rate was high if compared to INICC overall HH rates, the fact that DA-HAI rates shown in this study continue to be high, when benchmarked against NHSN rates, may anticipate that further prevention efforts need to be implemented.
In a point prevalence study from Lebanon, the HAI prevalence per 100 patients in the ward was 6.8% (95% CI 5.1–8.4), which is similar to our DA-HAI rate of 9.8% (95% CI 7.6–12.3). Also, as in our present study, VAP was the most common infection.[24] In a retrospective study from another university hospital from Lebanon, CAUTI was the most common infection, followed by VAP.[25]
The CLA-BSI rate was 5.2 (95% CI 2.8–8.7) per 1,000 CL days in this study, which is lower than the INICC report rate (7.4 per 1,000 CL days),[12] but higher than the 1.5 NHSN rate (95% CI 1.4–1.6).[1] VAP rate was also lower in this study (8.1 per 1,000 MV days, [95% CI 5.5–11.7]) than the one in the INICC report (14.7 per 1,000 MV days [95% CI 14.2–15.2])[12] but higher than the NHSN rate (1.9 per 1,000 MV days [95% CI 1.8–2.1]).[1] The CAUTI rate was 4.1 (95% CI 2.6–6.2) per 1,000 catheter days in this study, which is comparable with the 6.1 rate (95% CI 5.9–6.4) of overall INICC ICUs,(12) and with the 3.1 NHSN rate (95% CI 3.0–3.3).[1]
The mortality of patients without DA-HAI was higher in this study than in the overall INICC ICUs: 19.1% (95% CI 16.1–22.5) vs. 14.4% (95% CI 14.1–14.7)[12] which may be explained due to the increased severity of the patients’ underlying conditions in the ICU. Such conditions were measured according to INICC methodology,[18] and CDC NNIS/NHSN criteria,[21] which include the calculation and recording of Severity of illness scores—namely, APACHE II, and Average Severity Illness Score (ASIS)—for each patient at ICU admission. CLA-BSI mortality was not significantly higher in this study in comparison to the overall INICC ICUs: 60.0% (95% CI 26.2–88.0) vs. 38.1% (95% CI 35.7–40.4).[12] The average LOS of patients without DA-HAI, with CLA-BSI and with VAP was similar in this study to the overall INICC ICUs.[12]
Several factors have probably contributed to the observed DA-HAI rates in this study, many of which are particular to the country and to the hospital setup itself. First, in Lebanon, guidelines on specific infection control practices are in place, but national infection control surveillance is not conducted. Recently, AUBMC was granted accreditation by the Joint Commission International, which attests to the rigorous infection control program currently in place. Practice bundles for the prevention of DA-HAI have now become central to the care of patients in the ICU. Other hospitals in the country are also working towards accreditation. In addition, national accreditation by the Ministry of Health has become mandatory. Second, in Lebanon, as in most developing countries, administrative and financial support is limited, which almost inevitably results in limited funds and resource availability to deal with infection control.[26] Third, there are insufficient supplies and wards are over-crowded. Fourth, there is a lower nurse-to-patient ratio compared to US hospitals, which has also been associated with increased risk of DA-HAI.[27] Finally, and unlike in US hospitals, our DA-HAI rates might be higher than the NHSN rates because the ICU at our center admits many patients who are terminally ill with advanced chronic illnesses, and who receive multiple courses of antibiotics and are colonized and/or infected with multi-drug resistant pathogens.
The first step that would contribute to a reduction in DA-HAI risk in hospitalized patients is the institution of surveillance of DA-HAI.[2] Next, basic, but effective, infection control practices need to be adopted for improving the prevention of DA-HAIs.[28–31] Needless to say, shared knowledge and accurate information on this serious problem in hospital ICUs can be highly motivating for developing effective high-quality infection control strategies. In this regard, there is evidence from several centers in INICC suggesting positive modifications in hospital practices: Substantial increase in hand hygiene compliance, institution of performance feedback programs for hand hygiene, and subsequent significant reduction in CLA-BSI, CAUTIs, and VAP rates.[14,32–37]
However, this study presents many limitations, the first one being the fact that these data may not be generalized to primary or other tertiary medical centers in Lebanon. During two years and four months, we have prospectively collected data as an integral part of the implementation of a comprehensive surveillance system in 1 ICU from a Lebanese hospital. There is a likelihood that the efficacy of surveillance could have affected the observed rates, which constitutes a possible bias. In addition, variations in DA-HAI rates among the INICC member hospitals and between countries might be accounted for by heterogeneity in the patient populations, the severity of illness, and the efficacy of infection control interventions. Thirdly, processing and interpretation of culture specimens is currently being performed at individual member hospitals’ laboratories rather than at a central laboratory. However, most laboratories follow the CLSI's criteria (updated to M100-S20-U) and definitions so that variability is kept at a minimum.
CONCLUSION
DA-HAIs pose a huge and largely under-recognized threat to patient safety in the developing countries. In Lebanon, rates of most DA-HAIs are lower than INICC rates but higher than NHSN rates, emphasizing that there is still room for improvement to lower infection rates and provide safer care to patients. Through continued and systematic surveillance, healthcare personnel at INICC member hospitals are provided with simple, but effective and inexpensive preventive strategies.[14,32–38] We expect that this results in wider acceptance of infection control programs in all hospitals members of the consortium, thereby leading to significant reductions in DA-HAI rates, particularly in the ICU setting. For that reason, as in the case of this Lebanese hospital, any hospital may participate in the INICC network, which was created in an understanding of the paramount need of developing countries to significantly prevent, control and reduce DA-HAI and their adverse consequences. In INICC, not only are investigators freely provided with training and methodological tools to conduct outcome and process surveillance, but through the publication of these confidentially collected data, relevant scientific evidence-based literature is fostered as well.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Haley RW, Quade D, Freeman HE, Bennett JV. The SENIC Project.Study on the efficacy of nosocomial infection control (SENIC Project). Summary of study design. Am J Epidemiol. 1980;111:472–85. doi: 10.1093/oxfordjournals.aje.a112928. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: Morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552–7. doi: 10.1086/647371. [DOI] [PubMed] [Google Scholar]
- 4.Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gibert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275:866–9. [PubMed] [Google Scholar]
- 5.Laupland KB, Zygun DA, Doig CJ, Bagshaw SM, Svenson LW, Fick GH. One-year mortality of bloodstream infection-associated sepsis and septic shock among patients presenting to a regional critical care system. Intensive Care Med. 2005;31:213–9. doi: 10.1007/s00134-004-2544-6. [DOI] [PubMed] [Google Scholar]
- 6.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 7.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Safdar N, Crnich CJ, Maki DG. Nosocomial Infections in the Intensive Care Unit Associated with Invasive Medical Devices. Curr Infect Dis Rep. 2001;3:487–95. doi: 10.1007/s11908-001-0085-5. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal VD, Guzman S, Migone O, Crnich CJ. The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina: A prospective, matched analysis. Am J Infect Control. 2003 Dec;31:475–80. doi: 10.1016/j.ajic.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal VD, Guzman S, Migone O, Safdar N. The attributable cost and length of hospital stay because of nosocomial pneumonia in intensive care units in 3 hospitals in Argentina: A prospective, matched analysis. Am J Infect Control. 2005;33:157–61. doi: 10.1016/j.ajic.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal VD, Guzman S, Orellano PW. Nosocomial infections in medical-surgical intensive care units in Argentina: Attributable mortality and length of stay. Am J Infect Control. 2003;31:291–5. doi: 10.1067/mic.2003.1. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal VD, Maki DG, Jamulitrat S, Medeiros EA, Todi SK, Gomez DY, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95–104. doi: 10.1016/j.ajic.2009.12.004. e2. [DOI] [PubMed] [Google Scholar]
- 13.Lynch P, Rosenthal VD, Borg MA, Eremin SR. Infection control in developing countries. In: Jarvis WR, editor. Bennett and Brachman's Hospital Infections. Philadelphia: Lipppincott Williams and Wilkins; 2007. p. 255. [Google Scholar]
- 14.Higuera F, Rosenthal VD, Duarte P, Ruiz J, Franco G, Safdar N. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med. 2005;33:2022–7. doi: 10.1097/01.ccm.0000178190.89663.e5. [DOI] [PubMed] [Google Scholar]
- 15.Moreno CA, Rosenthal VD, Olarte N, Gomez WV, Sussmann O, Agudelo JG, et al. Device-associated infection rate and mortality in intensive care units of 9 Colombian hospitals: Findings of the International Nosocomial Infection Control Consortium. Infect Control Hosp Epidemiol. 2006;27:349–56. doi: 10.1086/503341. [DOI] [PubMed] [Google Scholar]
- 16.Madani N, Rosenthal VD, Dendane T, Abidi K, Zeggwagh AA, Abouqal R. Health-care associated infections rates, length of stay, and bacterial resistance in an intensive care unit of Morocco: Findings of the International Nosocomial Infection Control Consortium (INICC) Int Arch Med. 2009;2:29. doi: 10.1186/1755-7682-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta A, Rosenthal VD, Mehta Y, Chakravarthy M, Todi SK, Sen N, et al. Device-associated nosocomial infection rates in intensive care units of seven Indian cities.Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67:168–74. doi: 10.1016/j.jhin.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal VD, Maki DG, Graves N. The International Nosocomial Infection Control Consortium (INICC): Goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control. 2008;36:e1–e12. doi: 10.1016/j.ajic.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296:1305–9. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 20.Villanova P. Minimum Inhibitory Concentration Interpretive Standards M7-A4: USA: National Committee for Clinical Laboratory Standards (NCCLS) 1997 [Google Scholar]
- 21.Emori TG, Culver DH, Horan TC, Jarvis WR, White JW, Olson DR, et al. National nosocomial infections surveillance system (NNIS): Description of surveillance methods. Am J Infect Control. 1991;19:19–35. doi: 10.1016/0196-6553(91)90157-8. [DOI] [PubMed] [Google Scholar]
- 22.Tarricone R, Torbica A, Franzetti F, Rosenthal VD. Hospital costs of central line-associated bloodstream infections and cost-effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Eff Resour Alloc. 2010;8:8. doi: 10.1186/1478-7547-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higuera F, Rangel-Frausto MS, Rosenthal VD, Soto JM, Castanon J, Franco G, et al. Attributable cost and length of stay for patients with central venous catheter-associated bloodstream infection in Mexico City intensive care units: A prospective, matched analysis. Infect Control Hosp Epidemiol. 2007;28:31–5. doi: 10.1086/510812. [DOI] [PubMed] [Google Scholar]
- 24.Azzam R, Dramaix M. A one-day prevalence survey of hospital-acquired infections in Lebanon. J Hosp Infect. 2001;49:74–8. doi: 10.1053/jhin.2001.1043. [DOI] [PubMed] [Google Scholar]
- 25.Tohme A, Karam-Sarkis D, El-Rassi R, Chelala D, Ghayad E. Agents and consequences of nosocomial infections in a Lebanese university hospital.Retrospective study over a two-year period. Ann Med Interne (Paris) 2001;152:77–83. [PubMed] [Google Scholar]
- 26.Chandra PN, Milind K. Lapses in measures recommended for preventing hospital-acquired infection. J Hosp Infect. 2001;47:218–22. doi: 10.1053/jhin.2000.0904. [DOI] [PubMed] [Google Scholar]
- 27.Hugonnet S, Harbarth S, Sax H, Duncan RA, Pittet D. Nursing resources: A major determinant of nosocomial infection? Curr Opin Infect Dis. 2004;17:329–33. doi: 10.1097/01.qco.0000136931.83167.d2. [DOI] [PubMed] [Google Scholar]
- 28.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319–26. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 29.Lo E, Nicolle L, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S41–50. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 30.Coffin SE, Klompas M, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–40. doi: 10.1086/591062. [DOI] [PubMed] [Google Scholar]
- 31.Marschall J, Mermel LA, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S22–30. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 32.Rosenthal VD, Guzman S, Safdar N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am J Infect Control. 2005;33:392–7. doi: 10.1016/j.ajic.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal VD, Maki DG. Prospective study of the impact of open and closed infusion systems on rates of central venous catheter-associated bacteremia. Am J Infect Control. 2004;32:135–41. doi: 10.1016/j.ajic.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal VD, Guzman S, Safdar N. Effect of education and performance feedback on rates of catheter-associated urinary tract infection in intensive care units in Argentina. Infect Control Hosp Epidemiol. 2004;25:47–50. doi: 10.1086/502291. [DOI] [PubMed] [Google Scholar]
- 35.Rosenthal VD, McCormick RD, Guzman S, Villamayor C, Orellano PW. Effect of education and performance feedback on handwashing: The benefit of administrative support in Argentinean hospitals. Am J Infect Control. 2003;31:85–92. doi: 10.1067/mic.2003.63. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31:405–9. doi: 10.1067/mic.2003.52. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal VD, Guzman S, Crnich C. Impact of an infection control program on rates of ventilator-associated pneumonia in intensive care units in 2 Argentinean hospitals. Am J Infect Control. 2006;34:58–63. doi: 10.1016/j.ajic.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal V, Maki D, Rodrigues C, Alvarez-Moreno C, Leblebicioglu H, Sobreyra-Oropeza M, et al. Impact of international nosocomial infection control consortium (INICC) strategy on central line-associated bloodstream infection rates in the icus of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31:1264–72. doi: 10.1086/657140. [DOI] [PubMed] [Google Scholar]


