Abstract
Background:
In recent years, trichomoniasis has emerged as the most common sexually transmitted disease and limited data are available on the effective screening technique for the diagnosis of Trichomonas vaginalis.
Aim
The aim was to compare and evaluate different diagnostic methods like wet mount microscopy, In Pouch TV culture, and Polymerase chain reaction (PCR) to establish which method or combination of methods was most effective for detection of Trichomonas vaginalis in vaginal swab specimens.
Settings and Design:
This is a cross-sectional study.
Materials and Methods:
A total of 200 patients complaining of vaginal discharge were included in the study. Three vaginal swabs were screened for trichomoniasis by wet mount microscopy, In Pouch TV culture system and PCR, using TVK3 and TVK7 specific primers.
Results
Of the 200 cases studied, 36 (18%) were positive by wet mount microscopy, 44 (22%) by In Pouch TV culture system and 60(30%) by PCR. Sensitivity and specificity of wet mount were 60% and 100%, respectively, whereas sensitivity and specificity of the In Pouch TV culture system were 73.33% and 100%, respectively when compared to PCR.
Conclusion
Comparison of different methods showed that at least two techniques, such as wet mount microscopy and culture have a better chance of detection of T. vaginalis infection. Diagnosis of trichomoniasis by PCR was found to be highly specific and sensitive, but its availability and cost effectiveness limit its use in routine diagnostic laboratories.
Keywords: In Pouch TV culture, PCR, Trichomonas vaginalis, Vaginal discharge, Wet mount microscopy
INTRODUCTION
Trichomonas vaginalis is the most common sexually transmitted pathogen accounting for 180 million infections annually[1,2] and is also known to increase the risk of acquisition of Human immunodeficiency virus (HIV) infection and other nonulcerative Sexually transmitted diseases (STDs).[3,4] The classical symptoms associated with the T. vaginalis infection include a yellowish-green frothy discharge, pruritis, dysuria, and the “strawberry” cervix which is characterized by punctuate hemorrhagic lesions.[5]
Diagnosis cannot be made solely on the basis of clinical presentation for several reasons like (i) the clinical symptom may be synonymous with those of other STDs, (ii) the classical “strawberry” cervix is seen in approximately 2% of patients, and (iii) frothy discharge is seen only in 12% of women with T. vaginalis infection. It has been demonstrated that if these classical features are used alone in the diagnosis of trichomoniasis, 88% of these cases will not be diagnosed and 29% uninfected will be falsely indicated as having infection.[6] This suggests that clinical manifestations are not reliable diagnostic parameters and hence laboratory diagnosis is necessary for early and accurate diagnosis.[7,8]
Various laboratory methods have been employed for the detection of T. vaginalis in vaginal discharge which varies in their sensitivity and specificity. Hence the present study has been undertaken to compare wet mount microscopy, In Pouch TV culture, and Polymerase chain reaction (PCR). Although wet mount microscopy is a simple, rapid, and inexpensive, this technique is less sensitive when compared to culture. Culture in a microaerophilic condition is more sensitive but it is time consuming and expensive. Diagnosis by either wet mount or culture depends on an experienced microscopist and also requires the presence and maintenance of viable trichomonads.[9] When compared to culture techniques, PCR offers an advantage of extreme sensitivity and ability to detect nonviable organisms.[10]
Several PCR-based diagnostic assays for trichomoniasis using vaginal specimens and urine have been described which vary in their sensitivity and specificity.[11] In the present study, PCR is carried out using TVK3-and TVK7-specific primers and amplified products were detected by digoxigenin-labeled ELISA.
MATERIALS AND METHODS
The study was conducted in the Department of Microbiology, Jawaharlal Nehru Medical College, Belgaum. Two hundred women of the reproductive age group (15- 45 years) attending the OBG Department of K. L. E. Prabhakar Kore Hospital and District Hospital, Belgaum with the complaint of vaginal discharge, were included in the study. Patients were selected using the convenient sampling method and we have included only symptomatic women in our study, since the objective of this study was to find out the prevalence of T. vaginalis infection only in the symptomatic women. Patient consent for voluntary participation in the study was obtained and the study was approved by the Institutional Review Board. A detailed history was taken with special emphasis on duration of complaints, nature of discharge, associated symptoms, and history of sexual exposure. Women presenting with white discharge due to noninfectious causes such as cervical ectropion, uterine prolapse, postcervical cauterization, etc. were excluded from the present study.
Three vaginal fluid specimens were collected using sterile cotton swab from the posterior fornix. The first vaginal swab was placed in 10 ml screw-cap plastic tubes containing 0.5 ml of 0.9% saline to carry out the wet mount microscopy. Swab was vigorously rotated in the saline and pressed against the side of the tube to express as much fluid as possible. One drop of the expressed fluid was placed on glass slide with a cover slip and examined at magnification of 200× within 1 hour of collection of the sample.[12] Wet mount microscopy was carried out by the microbiologist and further confirmed by the senior microbiologist. The positive result is defined as the presence of one or more trichomonads with characteristic morphology and jerky motility.
The upper chamber of the In Pouch TV culture system (Biomed, Diagnostics, Santa Clara, California, USA) was inoculated with the second-vaginal swab. The contents of the upper chamber were immediately pushed into the lower chamber and the pouch was incubated at 37°C. Pouch cultures were examined microscopically on days 2, 3 and again on day 5 of inoculation. The presence of motile trichomonads indicated the positive result.[13]
The third swab was placed in a dry sterile plastic container and was frozen at or below –20°C until they were subjected for PCR. The PCR was carried out on pooled samples using oligonucleotide primers TVK3 and TVK7.[10]
Primer sequences
Forward primer–TVK3, 5′AT TGT CGA ACA TTG GTC TTA CCC TC3′, reverse primer–TVK7, 5′ TCT GTG CCG TCT TCA AGT ATG C3′ purified T. vaginalis DNA, and sterile water were used as positive and negative controls, respectively.
The PCR-amplified products were detected using an Amplicor CT detection kit based on digoxigenin labeled ELISA, wherein the microtitre plates were precoated with 40 ng/well TVK probe [5′ CCG AAG TTC ATG TCC TCT CCA AGG G-3′]. Test samples and both controls were tested in duplicate on each microtiter plate. In addition a biotinylated oligonucleotide corresponding to the reverse complement of TVK probe (40 ng/well) was used as positive control for ELISA in each plate. The sample was considered reactive if the absorbance was ≥0.2.[1]
RESULTS
Among 200 patients screened for T. vaginalis, majority of the patients belonged to the age group of 26-35 years, 116 (58%), and among these 44 (37.93%) were positive for T. vaginalis infection by either of the methods. The common symptom associated with vaginal discharge was abdominal pain, accounting for 18% of cases, followed by dysuria in 16% of cases and dyspareunia in 14% of cases.
It is very difficult to predict the T. vaginalis infection only on clinical findings, as in our study 100 (50%) of the patient had normal vaginal findings, 20 (20%) of them were positive for T. vaginalis infection, and 72 (36%) had cervicitis, 32 (44%) were positive for T. vaginalis infection.
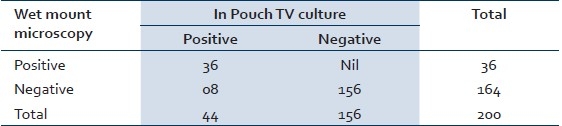
Of the 200 cases screened, 36 (18%) cases were positive by wet mount microscopy and 44 (22%) cases were positive by In Pouch TV culture and 60 (30%) were found positive by PCR. Eight cases which were missed by microscopy were picked by In Pouch TV culture and 16 cases were picked by PCR which were missed by In Pouch TV culture. Sensitivity and specificity of wet mount microscopy are 60% and 100% [Table 1], whereas sensitivity and specificity of In Pouch TV culture are 73.33% and 100% [Table 2], respectively when compared to PCR. The Mc Nemar test was used for paired observation between wet mount microscopy and In Pouch TV culture, which showed a significant difference between the two screening methods (Chi-square=7.03, P>0.008) with the In Pouch TV culture being more sensitive then wet mount microscopy [Table 3].
Table 1.
Comparison of wet mount microscopy and PCR
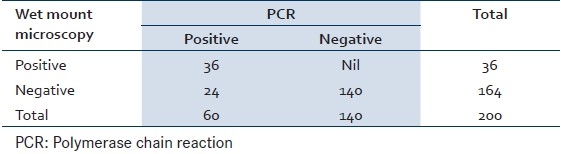
Table 2.
Comparison of In Pouch TV culture and PCR
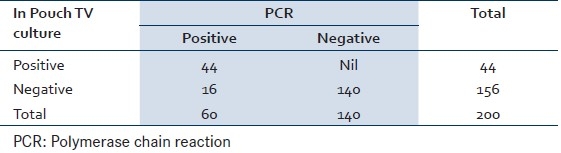
Table 3.
Comparison of wet mount microscopy and In Pouch TV culture
DISCUSSION
Till today, little emphasis has been laid on the importance of decreasing the rates of T. vaginalis infection even though it has been associated with human immunodeficiency virus acquisition. Vaginal discharge being the nonspecific symptom, laboratory diagnosis is must for detecting the infection.
One strategy for increasing the diagnosis and treatment of trichomoniasis is the use of a screening test with the increased sensitivity compared to the traditional wet mount preparation. In the present study 36 (18%) cases were detected by wet mount microscopy, with the sensitivity and specificity of 60% and 100%, when compared to PCR. This finding is consistent with that of other studies.[14] Although wet preparation examination of vaginal fluid is inexpensive, rapid, requiring only a microscope and trained personnel, the sensitivity of this method is low, compared to culture. It is highly dependent on the expertise of microscopist, prompt transport, and laboratory processing of the sample before the organisms lose their motility or become nonviable.[15,16] In the present study, the In Pouch TV culture system has been able to detect (44) 22% of cases with the sensitivity of 73.33%. It was found better than wet mount microscopy since it has picked up additional 4% cases which were missed by wet mount microscopy. A similar study conducted by Madico et al. showed the sensitivity of 70% by the In Pouch TV culture system; the findings are consistent with our study.[4]
The In Pouch TV culture system offers many advantages when compared to other culture media. It has a life of 6 months and is stable; moreover it has the versatility of being used both for specimen transport and culture.[17] The additional advantage of the In Pouch TV system is that, microscopy can be performed on the pouch itself, which alliates the need to enter the broth culture. This decreases the contamination and speeds up the examination time. Yet another advantage of the In Pouch TV system is that positive cultures with counts less than 10 organisms/ml can be detected. Some of the limitations of using this method are that 2-7 days are required for identification of positive culture and the medium is expensive.[15]
In this study PCR has shown 30% positivity. Additional 16 cases which were negative by in pouch TV culture were detected by PCR. The oligonucleotide primers TVK3 and TVK7 used for PCR amplification help in specifically amplifying a 312 bp sequences from repetitive DNA in T. vaginalis genome. Hence the amplification is highly specific and does not amplify human DNA, other organisms found in the human genitourinary tract, and other Trichomonas species.[1] In addition, PCR has the ability to detect nonviable or defective T. vaginalis. Moreover detection of PCR products by ELISA increases the sensitivity of PCR compared to the detection by agarose gel electrophoresis.[14]
Several groups of investigators have reported their findings on the development of a PCR technique for trichomonads. In 2003, a study conducted on comparison of different PCR assays for detection of T. vaginalis in vaginal swab specimens, showed sensitivity and specificity of PCR using the TVK3/TVK7 primer set was 92.8% and 94.6% respectively whereas sensitivity and specificity of PCR with primer set TVA5/TVA6 were 63.9% and 94.7% respectively.[18]
Limitation of this study is that we need to include asymptomatic patients for detection of T. vaginalis infection as we have considered only the symptomatic women, as one-third of the infected cases are asymptomatic.
CONCLUSION
In conclusion, wet mount microscopy for detection of T. vaginalis is a rapid, inexpensive screening technique, but has low sensitivity. The In Pouch TV culture system has higher sensitivity when compared to wet mount microscopy and has unique advantages when compared to other culture media, but it is expensive and not easily available. In resourceful settings the PCR method offers advantage of extreme sensitivity in potentially shorter time, whereas in places with limited resources, combination of wet mount microscopy and culture methods could be adapted.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kaydos SC, Swygard H, Wisc SL, Sena AC, Leone PA, Miller WC, et al. Development and validation of a PCR-based enzyme-linked immunosorbent assay with urine for use in clinical research settings to detect Trichomonas vaginalis in women. J Clin Microbiol. 2002;40:89–95. doi: 10.1128/JCM.40.1.89-95.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ken HL. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165:1168–76. doi: 10.1016/s0002-9378(12)90722-x. [DOI] [PubMed] [Google Scholar]
- 3.Madico G, Quinn CT, Rampalo A, Kelly T, McKee JT, Jr, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36:3205–10. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coth MF, Pastorek JG, Nugent RP, Hiller SH, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Transm Dis. 1997;24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;1:300–17. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fouts AC, Kraus SJ. Trichomonas vaginalis: Reevalaution of its clinical presentation and laboratory diagnosis. J Infect Dis. 1980;141:137–43. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Wolner-Hanssen P, Krieger JN, Steven CE, Kiviat NB, Roven JD, Hiller S, et al. Clinical manifestation of vaginal trichomoniasis. JAMA. 1989;264:571–6. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]
- 8.McLellan R, Spence MR, Brockman M, Raffel L, Smith JL. The clinical diagnosis of trichomoniasis. Obstet Gynecol. 1982;60:30–4. [PubMed] [Google Scholar]
- 9.Heine RP, McGregor JA. Trichomonas vaginalis: A reemerging pathogen. Clin Obstet Gynecol. 1993;36:137–44. doi: 10.1097/00003081-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Riley DE, Roberts MC, Takayama T, Krieger JN. Development of polymerase chain reaction-based diagnosis of Trichomonas vaginalis. J Clin Microbiol. 1992;30:465–72. doi: 10.1128/jcm.30.2.465-472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaydos-Daniels SC, Miller WC, Hoffman I, Banda T, Dzinyemba W, Martinson F, et al. Validation of a urine-based PCR enzyme linked immunosorbent assay for use in clinical research settings to detect Trichomonas vaginalis in men. J Clin Microbiol. 2003;41:318–23. doi: 10.1128/JCM.41.1.318-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCann JS. Comparison of direct microscopy and culture in the diagnosis of trichomoniasis. Br J Vener Dis. 1974;50:450–2. doi: 10.1136/sti.50.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borchardt KA, Smith RF. An evaluation of an In Pouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med. 1991;67:149–52. doi: 10.1136/sti.67.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawing LF, Hedges SR, Schwebbe JR. Detection of trichomoniasis in vaginal and urine specimens from women by culture and PCR. J Clin Microbiol. 2000;38:3585–8. doi: 10.1128/jcm.38.10.3585-3588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draper D, Parker R, Patterson E, Jones W, Beulz M, French J, et al. Detection of Trichomonas vaginalis in pregnant women with the In Pouch TV culture system. J Clin Microbiol. 1993;31:1016–8. doi: 10.1128/jcm.31.4.1016-1018.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barenfanger J, Drake C, Hanson C. Timing of inoculation of the pouch makes no difference in increased detection of Trichomonas vaginalis by the In Pouch TV method. J Clin Microbiol. 2002;40:1387–9. doi: 10.1128/JCM.40.4.1387-1389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwebke SR, Venglarik MF, Morgan SC. Delayed versus immediate bedside inoculation of culture media for diagnosis of vaginal trichomoniasis. J Clin Microbiol. 1999;37:2369–70. doi: 10.1128/jcm.37.7.2369-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crucitti T, van Dyck E, Tehe A, Abdellati S, Vuylsteke B, Buve A, et al. Comparison of culture and different PCR assays for detection of Trichomonas vaginalis in self collected vaginal swab specimens. Sex Transm Infect. 2003;79:393–8. doi: 10.1136/sti.79.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


