Abstract
Background:
Although enterococci are relatively common nosocomial pathogens in surgical intensive care units (ICUs), their significance in blood cultures from patients in the medical ICU is unclear.
Materials and Methods:
In this retrospective study spanning 2 years, the clinical and microbiological characteristics of enterococcal bacteremia among medical ICU patients were evaluated.
Results:
Of 1325 admissions, 35 with enterococcal bacteremia accounted for 14.8% of positive blood cultures. They were significantly older (P=0.03) and had various co-morbidities. Most had vascular (96.9%) and urinary (85.3%) catheters, and 67.7% were mechanically ventilated. In addition to blood, enterococci were isolated from vascular catheters (8.6%) and other sites (20%), while no focus was identified in 77% of patients. Prior use of broad-spectrum antimicrobials was nearly universal. All isolates tested were sensitive to vancomycin and linezolid. Resistance to ampicillin and gentamicin were 44.7% and 52.6%, respectively. Compared with other medical ICU patients, patients with enterococcal bacteremia had a longer ICU stay (P<0.0001) and a trend toward higher ICU mortality (P=0.08).
Conclusions:
Enterococcal bacteremia is an important nosocomial infection in the medical ICU, with a predilection for older patients with multiple comorbidities. Its occurrence is associated with a significantly longer ICU stay and a trend to a higher mortality. The choice of antibiotics should be dictated by local susceptibility data.
Keywords: Bacteremia, Duration, Enterococcal, Medical intensive care unit, Mortality
INTRODUCTION
Enterococci are commensals in the human alimentary tract, traditionally considered to have low virulence. However, they are increasingly recognized as an important pathogen in the intensive care unit (ICU). It has been shown that the isolation of enterococci from blood–even of doubtful clinical significance–is associated with mortality, especially in elderly patients with underlying diseases like malignancy and diabetes.[1]
Enterococci rank third among nosocomial infections in the United States and are the third most common isolated bacteria in blood stream infections in developed nations.[2,3] The progressive development of resistance by enterococci to various antimicrobial agents in the past two decades has further heightened the importance of their isolation from critically ill patients.[4,5] Enterococcal infections have a predominantly nosocomial source in developing countries too,[6] where, like in developed nations, their treatment is frequently complicated by antimicrobial resistance.[7] The substantial costs associated with enterococcal infections were recently estimated by Butler and colleagues.[8]
The significance of enterococci in surgical ICUs is well established; it is the single most frequently reported pathogen from surgical site infections.[3] However, the importance of this isolate in patients admitted to the medical ICU is not well established. It is also unclear if enterococcal infections in a medical ICU patient impact outcome.[9] Our study seeks to describe the characteristics of patients with enterococcal bacteremia in the medical ICU in the setting of a developing country, the antibiotic susceptibility patterns of the organisms involved, and the risk factors for an adverse outcome.
MATERIALS AND METHODS
This retrospective descriptive study was performed in the 11-bed medical ICU of a tertiary care university teaching hospital in South India. The study was approved by the Institutional Review Board (IRB) and the Ethics Committee. Adult and adolescent patients, 15 years or older, with enterococcal bacteremia during ICU stay were included. The electronic medical records of patients admitted to the medical ICU over a 2-year period (January 2006 to December 2007), as well as the microbiology database, were screened for enterococci in blood cultures. A data abstraction form was developed after reviewing the available literature and scanning a sample of the patient records. Data were extracted from the charts and the electronic records of laboratory reports, radiologic studies and discharge summaries of patients. The demographic profile of patients, diagnosis at admission, baseline characteristics, risk factors for acquisition of enterococcal infections, antibiotic susceptibility, and outcomes were recorded. Severity of illness was determined with physiological variables from the ICU monitoring flow charts using APACHE II and SAPS scores. The primary end point was hospital mortality. Other outcomes included duration on ventilator and duration of ICU and hospital stay.
The following protocol was used for enterococcal isolates from blood cultures. Growth-positive blood cultures with Gram-positive oval cocci in tetrads and short chains were subcultured onto blood and MacConkey agar plate and incubated overnight at 37°C. Magenta pink colored fine colonies were further speciated and subjected to antimicrobial susceptibility testing using standard recommended biochemical tests as per the Clinical and Laboratory Standards Institute guidelines.[10] Enterococcal bacteremia was defined as enterococci isolated from one or more blood cultures obtained by separate venipuncture drawn with strict aseptic precautions.[1] Nosocomially acquired bacteremia was defined as a blood culture drawn 48 hours after admission to the hospital yielding enterococci. Community acquired bacteremia was defined as a blood culture drawn within 48 hours of hospital admission being positive for enterococci.[11] Where two isolates from the same patient showed different sensitivities, the one with the higher resistance was used for statistical analyses in the study.
Data on age, ICU mortality and duration of stay in the ICU for the entire medical ICU population during the study period 2006–2007 were available from audit records and used for comparing patients with and without enterococcal bacteremia. Statistical analysis was performed using R statistical software version 2.11.[12] Continuous variables were assessed using the Mann–Whitney test for non-parametric data. Dichotomous variables were analyzed using Fisher's exact test.
RESULTS
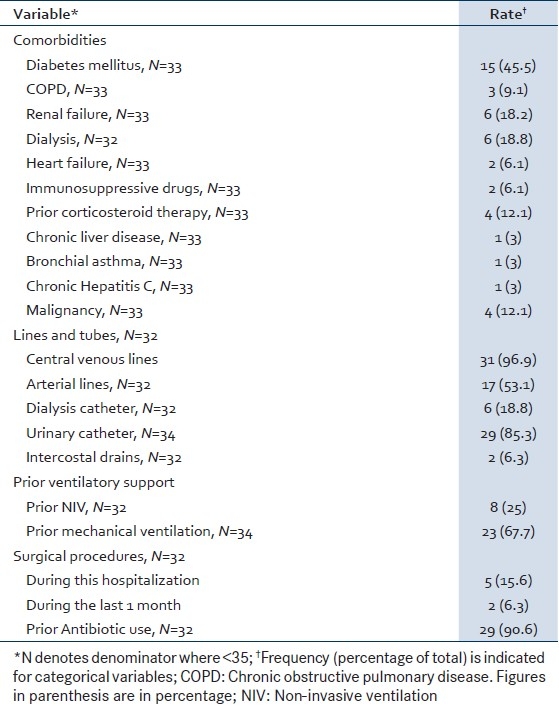
Of 1327 patients admitted to the medical ICU over 2 years, 237 (17.89%) had positive blood cultures, among whom 35 (14.8%) patients had enterococcal bacteremia, yielding 41 enterococcal blood culture isolates. Patients with enterococcal bacteremia were older than those without enterococcal bacteremia (P=0.03; 95% CI=1–14) and the ratio of males to females was 0.88 [Table 1].
Table 1.
Baseline characteristics of patients with enterococcal bacteremia
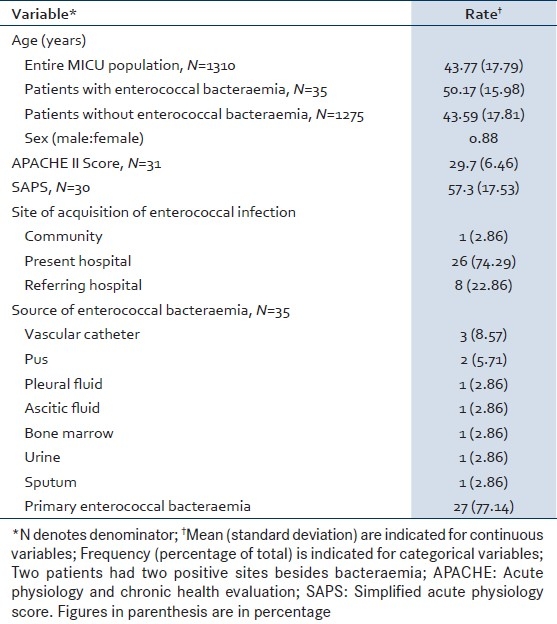
The commonest comorbidity was diabetes mellitus (45.5%). The rates of comorbidities and interventions previously identified as risk factors for acquisition of enterococcal infection[6,13,14] are listed in Table 2. Non-invasive (25%) and invasive ventilation (67.7%) were prominent among these.[13] Six (21.9%) patients underwent a surgical procedure during the month before the appearance of enterococcal bacteremia, including five (15.6%) patients who had surgery during the current hospitalization. The majority of patients (90.6%) had received antibiotics prior to isolation of enterococci from blood [Table 2].
Table 2.
Comorbidities and co-interventions among patients with enterococccal bacteraemia
Thirty-nine of 41 isolates underwent antibiotic susceptibility testing. In vitro resistance to ampicillin was observed in 17 (44.7%) isolates and to gentamicin [high level gentamicin resistance (HLGR)][15] in 20 (52.6%). None of the isolates demonstrated resistance to vancomycin or linezolid [Figure 1]. Fourteen (40%) patients were treated for enterococcal bacteremia with either vancomycin or teicoplanin. The rest received combinations of ampicillin and gentamicin.
Figure 1.
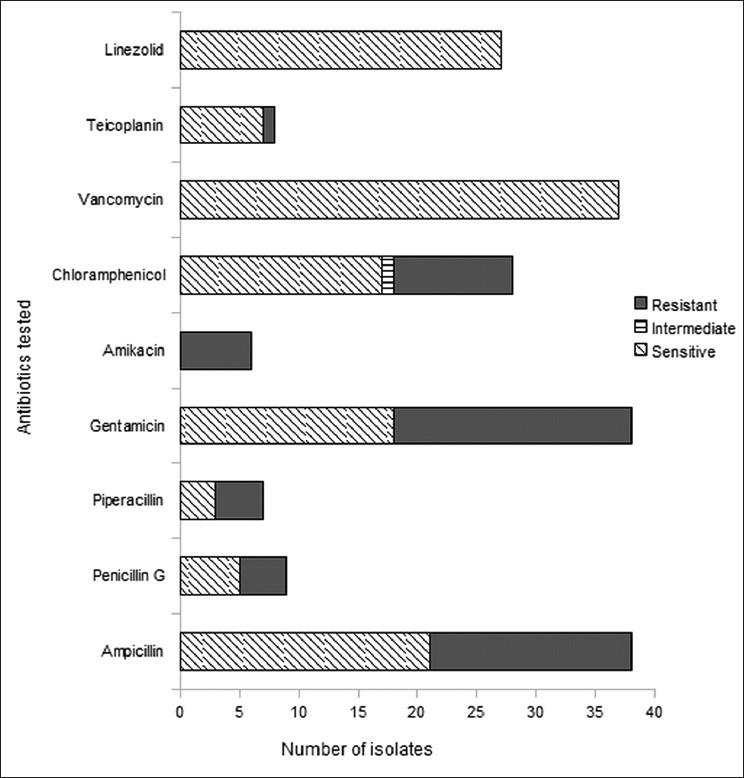
Antibiotic resistance profile of enterococci isolated in blood cultures from the medical ICU during 2006–2007. The vertical axis shows the antibiotics for which sensitivity testing was done. The horizontal axis shows the number of isolates tested for each antibiotic, classified as resistant, sensitive and (for chloramphenicol) intermediate
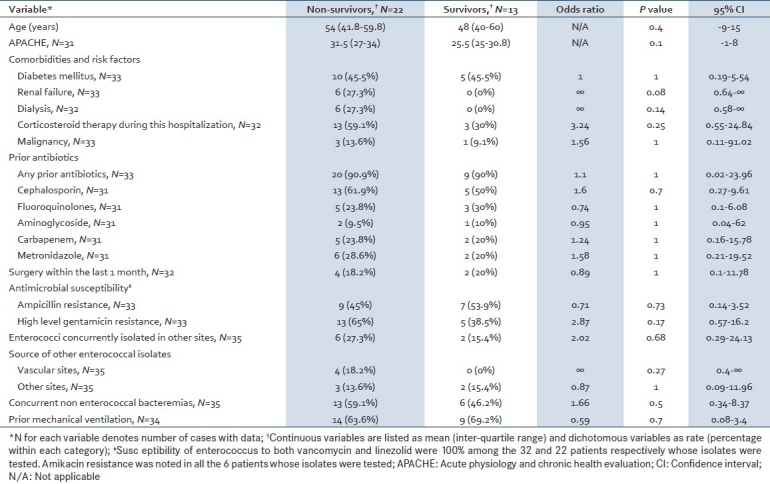
There was a trend toward a higher ICU mortality amongst patients who developed enterococcal bacteremia compared with those who did not develop enterococcal bacteremia in the medical ICU (P=0.08). The mean stay in the ICU was significantly longer in those who developed enterococcal bacteremia compared with patients who did not develop enterococcal bacteremia (15.9 vs. 5.7 days; P<0.0001) [Table 3]. In-hospital mortality among patients with enterococcal bacteremia was 62.9% (n=22); their mean duration of stay in hospital was 33.2±25.6 days (that of survivors was 37±26.7 days). In univariate analysis, a trend toward increased mortality was noted among patients with renal failure (P=0.08; 95% CI=0.64–∞). There were no statistically relevant differences between survivors and non-survivors in other parameters [Table 4].
Table 3.
Comparison of patients with and without enterococcal bacteremia
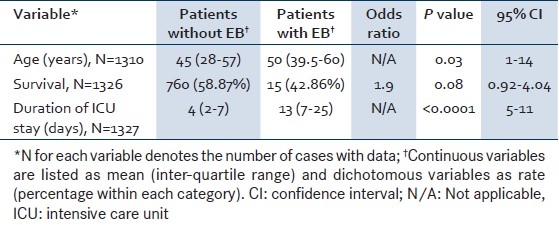
Table 4.
Comparison of survivors and non-survivors
DISCUSSION
Enterococcal bacteremia is nosocomially acquired, with rare exceptions. This condition, often associated with ICU patients having surgical problems particularly intra-abdominal pathology, is not uncommon amongst patients with medical problems admitted to the ICU, as demonstrated in this study. Our study found that nearly 15% of positive blood cultures of patients admitted to the medical ICU were due to enterococci. In the medical ICU, enterococcal bacteremia occurred in older patients who were very ill (as reflected by the severity scores) with multiple comorbidities, and was associated with a prolonged stay in ICU and high mortality.
In our study, patients who developed enterococcal bacteremia were significantly older than patients without enterococcal bacteremia (P=0.03). Our observations are in keeping with a recent study that demonstrated age to be an independent risk factor (OR 1.2; P=0.009) for the acquisition of enterococcal infections including bacteremia.[14] However, age per se was not associated with mortality in our cohort with enterococcal bacteremia or in other studies.[5,14,16] The increased predilection for enterococcal bacteremia among the elderly may be related to the need for more intensive monitoring with invasive vascular devices, indwelling urinary catheters and the greater risk of skin breakdown at pressure sites.
The absence of vancomycin and linezolid resistance and the high rate of ampicillin and aminoglycoside resistance in our cohort is similar to the findings by Indian[6,7] and other[16] investigators reporting on general hospital populations. However, vancomycin-resistant enterococci (VRE) have been detected in fecal and urine samples in Indian hospitals as early as 2007.[17] Experience from South Korea suggests that an outbreak of VRE would require multifaceted interventions for effective control, including cohorting of infected patients, active rectal and environmental surveillance cultures, daily extensive cleaning of environmental surfaces, antibiotic restriction, and education of hospital staff.[18]
We would echo Agrawal's caution against the empiric use of amikacin for synergy with cell wall-active agents, given the much higher prevalence of resistance to amikacin/kanamycin than to gentamicin/tobramycin/netilmicin among enterococci (48.8% vs. 8.3% in their study).[19,20] The variation in aminoglycoside susceptibility profiles among Indian studies probably arises from varying antimicrobial usage practices and emphasizes the importance of referring to data on local antimicrobial susceptibility patterns when deciding empiric therapy. The high rate of resistance to first-line drugs (ampicillin and aminoglycosides) observed by us may warrant the inclusion of vancomycin or teicoplanin in the initial treatment of life-threatening enterococcal infections while awaiting sensitivity reports, in centers with antimicrobial susceptibility profiles similar to ours.
Ninety percent of the patients in our study received antibiotics for various indications prior to the enterococcal bacteremia. A number of studies link prior antibiotic use to the acquisition of enterococcal infections and the selection of antibiotic resistance among enterococci. The use of carbapenems and cefepime in the first 48 hours in ICU has been independently associated with acquisition of enterococcal infections in the ICU.[14] Third-generation cephalosporins, quinolones, and carbapenems have been associated with HLGR enterococcal bacteremia in a hospital setting.[5,20] In spite of these concerns, prior antibiotic use did not appear to directly correlate with mortality in our cohort, as well as in other ICU and hospital environments.[5,14,20] Nevertheless, care should be taken in the initiation and choice of antibiotics.
Patients with enterococcal bacteremia had a longer ICU stay (P<0.0001) and a trend toward higher mortality (P=0.08), when compared with other patients in the medical ICU. A review of the literature identified several risk factors for death among patients with enterococcal bacteremia, including surgery, presence of a nasogastric tube, arterial lines, higher APACHE score, renal replacement therapy, cirrhosis, malignancy, and immunosuppression.[14,16,21,22] Many of these appear to be markers of severity of the primary illness.[14] Our findings on the impact of enterococcal bacteremia on outcome support the observation of Hoge and colleagues that early and appropriate treatment of enterococcal bacteremia significantly reduces mortality (relative risk 0.46; 95% CI=0.27–0.77).[1]
The study has a few limitations. As it was retrospective, antimicrobial susceptibility data were incomplete, being governed by clinical requirements, and species were not routinely characterized. Nevertheless, relevant analyses and inferences were possible in this area. Only limited information from audit records could be obtained for the “control” population (medical ICU patients without enterococcal bacteremia). The subgroup of medical ICU patients with positive blood cultures other than enterococci would have been a better group against which to compare our study cohort, but these data were unavailable. The paucity of significant associations precluded multivariate analysis of risk factors for mortality in the study cohort.
CONCLUSION
Enterococci cause 15% of the bacteremias in the medical ICU, with a predilection for older patients with multiple comorbidities. Enterococcal bacteremia is associated with a longer ICU stay and a trend toward increased mortality. The wide variation in their resistance profiles in various centers in India calls for close attention to local antimicrobial susceptibility patterns when initiating treatment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hoge CW, Adams J, Buchanan B, Sears SD. Enterococcal bacteremia: To treat or not to treat, a reappraisal. Rev Infect Dis. 1991;13:600–5. doi: 10.1093/clinids/13.4.600. [DOI] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 4.Reik R, Tenover FC, Klein E, McDonald LC. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn Microbiol Infect Dis. 2008;62:81–5. doi: 10.1016/j.diagmicrobio.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Jang H, Lee S, Song K, Jeon JH, Park WB, Park S, et al. Clinical features, risk factors and outcomes of bacteremia due to enterococci with high-level gentamicin resistance: Comparison with bacteremia due to enterococci without high-level gentamicin resistance. J Korean Med Sci. 2010;25:3–8. doi: 10.3346/jkms.2010.25.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanty S, Kapil A, Das BK. Enterococcal bacteraemia in a tertiary care hospital of North India. J Indian Med Assoc. 2005;103:31–7. [PubMed] [Google Scholar]
- 7.Mendiratta DK, Kaur H, Deotale V, Thamke DC, Narang R, Narang P. Status of high level aminoglycoside resistant Enterococcus faecium and Enterococcus faecalis in a rural hospital of central India. Indian J Med Microbiol. 2008;26:369–71. doi: 10.4103/0255-0857.43582. [DOI] [PubMed] [Google Scholar]
- 8.Butler AM, Olsen MA, Merz LR, Guth RM, Woeltje KF, Camins BC, et al. Attributable costs of enterococcal bloodstream infections in a nonsurgical hospital cohort. Infect Control Hosp Epidemiol. 2010;31:28–35. doi: 10.1086/649020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimailho A, Lampl E, Riou B, Richard C, Rottman E, Auzepy P. Enterococcal bacteremia in a medical intensive care unit. Crit Care Med. 1988;16:126–9. doi: 10.1097/00003246-198802000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility testing: Eighteenth Informational Supplement. CLSI document M100-S18. Wayne, Pennsylvania: 2008. [Google Scholar]
- 11.Steinberg JP, Clark CC, Hackman BO. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: Impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996;23:255–9. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 12.R Development Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Last cited on 2010 Oct 14]. ISBN 3-900051-07-0-Available from: http://www.R-project.org .
- 13.Chou Y, Lin T, Lin J, Wang N, Peng M, Chang F. Vancomycin-resistant enterococcal bacteremia: Comparison of clinical features and outcome between Enterococcus faecium and Enterococcus faecalis. J Microbiol Immunol Infect. 2008;41:124–9. [PubMed] [Google Scholar]
- 14.Chatterjee I, Dulhunty JM, Iredell J, Gallagher JE, Sud A, Woods M, et al. Predictors and outcome associated with an Enterococcus positive isolate during intensive care unit admission. Anaesth Intensive Care. 2009;37:976–82. doi: 10.1177/0310057X0903700610. [DOI] [PubMed] [Google Scholar]
- 15.Weinbren MJ, Johnson AP, Woodford N. Defining high-level gentamicin resistance in enterococci. J Antimicrob Chemother. 2000;45:404–5. doi: 10.1093/jac/45.3.404. [DOI] [PubMed] [Google Scholar]
- 16.McBride SJ, Upton A, Roberts SA. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia-a five-year retrospective review. Eur J Clin Microbiol Infect Dis. 2010;29:107–14. doi: 10.1007/s10096-009-0830-5. [DOI] [PubMed] [Google Scholar]
- 17.Khudaier BY, Tewari R, Shafiani S, Sharma M, Emmanuel R, Sharma M, et al. Epidemiology and molecular characterization of vancomycin resistant Enterococci isolates in India. Scand J Infect Dis. 2007;39:662–70. doi: 10.1080/00365540701203501. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YK, Sim HS, Kim JY, Park DW, Sohn JW, Roh KH, et al. Epidemiology and control of an outbreak of vancomycin-resistant enterococci in the intensive care units. Yonsei Med J. 2009;50:637–43. doi: 10.3349/ymj.2009.50.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal J, Kalyan R, Singh M. High-level aminoglycoside resistance and Beta-lactamase production in enterococci at a tertiary care hospital in India. Jpn J Infect Dis. 2009;62:158–9. [PubMed] [Google Scholar]
- 20.Vigani AG, Oliveira AM, Bratfich OJ, Stucchi RS, Moretti ML. Clinical, epidemiological, and microbiological characteristics of bacteremia caused by high-level gentamicin-resistant Enterococcus faecalis. Braz J Med Biol Res. 2008;41:890–5. doi: 10.1590/s0100-879x2008001000010. [DOI] [PubMed] [Google Scholar]
- 21.Al-Otaibi FE, Kambal AM, Baabbad RA. Enterococcal bacteremia in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25:21–5. [PubMed] [Google Scholar]
- 22.Olivier CN, Blake RK, Steed LL, Salgado CD. Risk of vancomycin-resistant Enterococcus (VRE) bloodstream infection among patients colonized with VRE. Infect Control Hosp Epidemiol. 2008;29:404–9. doi: 10.1086/587647. [DOI] [PubMed] [Google Scholar]


