Abstract
Background:
Most outbreaks of viral hepatitis in India are caused by hepatitis E. Recently in the year 2009, Modasa town of Sabarkantha district in Gujarat witnessed the outbreak of hepatitis B.
Purpose:
An attempt was made to study the outbreak clinically and serologically, to estimate the seropositivity of hepatitis B Virus among the cases and their contacts and to know the seroprevalence of hepatitis B envelope antigen (HBeAg) and IgM antibody against hepatitis B core antigen (IgM HBcAb) out of all the Hepatitis B surface Antigen (HBsAg) positive ones.
Materials and Methods:
Eight hundred and fifty-six (856) cases and 1145 contacts were evaluated for hepatitis B markers namely HBsAg, HBeAg and IgM HBcAb by enzyme-linked immuno Sorbent Assay (ELISA) test.
Results:
This outbreak of viral hepatitis B in Modasa, Gujarat was most likely due to unsafe injection practices. Evidence in support of this was collected by Government authorities. Most of the patients and approximately 40% of the surveyed population gave history of injections in last 1.5–6 months. Total 664/856 (77.57%) cases and 20/1145 (1.75%) contacts were found to be positive for HBsAg. 53.41% of the positive cases and 52.93% of the positive contacts were HBeAg-positive and thus in a highly infectious stage.
Conclusions:
Inadequately sterilized needles and syringes are an important cause of transmission of hepatitis B in India. Our data reflects the high positivity rate of a hepatitis B outbreak due to such unethical practices. There is a need to strengthen the routine surveillance system, and to organise a health education campaign targeting all health care workers including private practitioners, especially those working in rural areas, as well as the public at large, to take all possible measures to prevent this often fatal infection.
Keywords: Hepatitis B markers, Outbreak, Recycled syringes
INTRODUCTION
Viral hepatitis, an important world health problem, is responsible for acute infection and chronic sequel.[1] hepatitis B virus (HBV) infection is a common viral disease and the present data show that more than one-third of the world's population is infected with this virus.[2] HBV-infected patients show a variety of clinical symptoms ranging from an apparently healthy inactive carrier state to fulminant hepatitis or chronic liver disease, including cirrhosis and hepatocellular carcinoma.[3,4]
More than 2000 million people alive today have been infected with HBV at some time in their lives.[5] It is estimated that 350 million people worldwide are chronic HBV carriers, representing approximately 7% of the total population and approximately 1 million people die annually.[6–8] In India, hepatitis B surface antigen (HBsAg) prevalence in the general population ranges from 2% to 8% placing India in the intermediate HBV endemic zone and the number of HBV carriers is estimated to be 50 million forming a large global pool of chronic HBV infections second only to East Asia.[5,9,10]
In India, virtually all outbreaks of viral hepatitis are considered to be due to feco-orally transmitted hepatitis non-A non-B virus (hepatitis E).[11–13] But in the present record, we describe a major outbreak of HBV that was experienced in Modasa town in Sabarkantha district of Gujarat state in India in 2009. Sudden increased in the flow of the patients with clinical signs and symptoms of hepatitis with high serum alanine aminotransferase (ALT) and HBsAg positivity gave us the idea of hepatitis B outbreak. We suspected reuse of syringes in various hospitals and clinics in and around Modasa town in Gujarat to be the main risk factor for HBV infection in this outbreak.
Thus, the main purpose of this outbreak investigation was to estimate the HBV seroprevalence among cases and their household contacts along with biochemical markers for liver damage as well as investigate the route of HBV transmission in this outbreak.
MATERIALS AND METHODS
This study of the HBV outbreak in the Sabarkantha district was carried out in an observational cross-sectional setting at the referral teaching hospital in Ahmedabad, Gujarat, India. The study area, Sabarkantha district, is situated approximately 100 km away from Ahmedabad. It has a population of approximately 2082 531 according to 2001 census.
Rapid surveillance system was established in the affected Sabarkantha district and regular surveillance was carried out mainly in Modasa town and also in other affected areas in the district to find out suspected cases in the community.
Detailed history containing name, age, sex, occupation, date of onset of illness, date of hospitalization, signs, and symptoms and former injections/vaccinations was taken. If the person was a contact then the name of the concerned patient was also noted along with the other details of the contact person. Clinical signs and symptoms were icterus, anorexia, nausea, vomiting, malaise, dark colour urine and right-sided pain in the abdomen.
Government authorities interviewed the patients, their family members, and their doctors to identify the mode of transmission.
We evaluated 856 consecutive jaundiced cases presenting with signs and symptoms of acute hepatitis. We also evaluated 1145 household contacts of the confirmed cases. Samples from household contacts were collected by house to house survey in affected areas. Three to five millilitres of blood sample was collected aseptically in plain vacutainers from each individual. Sample was centrifuged at 3000 rpm, serum separated, and screened on the same day of receiving for all the serological tests. Samples were evaluated for the liver function tests namely serum ALT/serum glutamic pyruvate transaminase (SGPT), serum bilirubin, and serum alkaline phosphatase. The HBsAg test was done by ELISA (advanced ELISA, Morepen, India–a sandwich immunoassay that uses immobilised specific monoclonal antibodies to HBsAg with sensitivity 0.5 ng/mL and specificity 99.9%) IgM HBcAb (Hepatitis B core IgM Antibody), and HBeAg (Hepatitis B e Antigen) markers ELISA (ImmunoLISA, Orgenics, Israel–capture ELISA with sensitivity and specificity >98%) were also evaluated in the HBsAg positive cases. The upper limit of normal ALT was 40 IU/L and of alkaline phosphatase was 290 U/L. Case definition of acute hepatitis B according to CDC (2000) is (a) discrete onset of symptoms, (b) jaundice or elevated serum aminotransferase levels with laboratory criteria of (a) IgM HBcAb positive or (b) HBsAg positive.
We sent randomly selected 12 samples to a private laboratory for genotyping assay. Six samples were also sent to NIV (National Institute of Virology), Pune for further investigation.
Statistical analysis was done using the software Epi Info 3.5.1. Association was studied using the chi-square test. The association was considered to be statistically significant if P<0.05.
RESULTS
The epidemic was long lasting; from mid-February 2009 to August 2009. A total of 856 consecutive cases with signs and symptoms of acute hepatitis were evaluated. The first case was admitted to Sarvajanic hospital, Modasa on 26th January 2009. Of the 856 hospitalized cases 664 (77.57%) were from Sabarkantha district and were included in this study.
Liver function tests showed that all the cases had marked high ALT with mean of 1630.14±1601.12 IU/L. Serum bilirubin was elevated in 595 (92.11%) cases with mean of 5.54±4.78 mg/dL and alkaline phosphatase was elevated in 350 (54.18%) cases with mean of 531.25±474.96 U/L. Out of the 664 cases, 164 (24.70%) had an ALT value more than 2000 IU/L, 229 cases (34.49%) had ALT in the range of 1000–2000 IU/L, 95 (14.31%) cases had value in the range of 500–1000 IU/L and 176 (26.50%) had a value less than 500 IU/L.
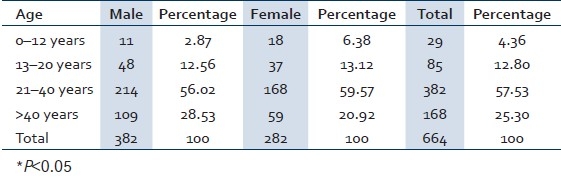
Table 1 shows the age and sex wise distribution of HBV confirmed cases. Males were affected more than females with the overall male to female ratio 1.36:1 and highest prevalence found in the age group of 21 to 40 years that was statistically significant.
Table 1.
Age and sex wise distribution of the HBs Ag positive cases in Sabarkantha district, Gujarat province, India, January 2009 to August 2009
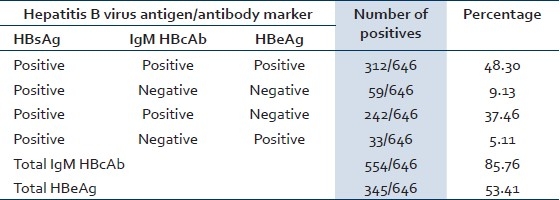
A total of 664 hepatitis B cases were confirmed by ELISA test at our institute. Out of 664 HBsAg positive cases, HBeAg and IgM HBcAb marker tests were done by ELISA on 646 cases. Rest of the HBsAg positive samples were not tested for the following reasons: either the quantity was insufficient or the sample was not suitable for testing, for example, haemolysed sample. The seroprevalence of HBeAg was 53.41% (345/646) and IgM HBcAb was 85.76% (554/646). Seropositivity of hepatitis B markers is shown in Table 2.
Table 2.
Results of common serological profile of HBsAg positive cases in Sabarkantha district, Gujarat province, India, January 2009 to August 2009
NIV, Pune, and the private laboratory reported that all the sent samples were HBV genotype D (the most prevalent genotype in India) and tests for total antibody to hepatitis D virus were negative.
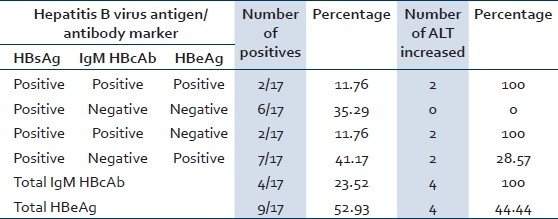
We also investigated 1145 healthy contacts. Out of all the contacts, 588 were males and 557 were females. Twenty (1.75%) contacts out of 1145 were positive for HBsAg ELISA. Males were more affected and the age group involved maximally was >40 years (40%). Out of these 20 contacts, three could not be tested for HBeAg and IgM HBcAb as the quantity of the samples was insufficient. Eleven out of 17 contacts tested were chronic carriers and six were acutely infected with HBV. Results of serological profile of HBV positive 17 contacts are shown in Table 3.
Table 3.
Results of common serological profile of contacts of HBsAg positive cases in Sabarkantha district, Gujarat province, India, January 2009 to August 2009
It was observed that this outbreak was due to use of unsterile needles and syringes by local practitioners. Evidence in support of this was collected by Government authorities. Most of the patients and approximately 40% of the surveyed population gave history of injections in the last 1.5–6 months. Some of the private practitioners were not using disposal syringes and standards of sterilization/universal precautions were at unacceptable levels. Many patients had taken treatment/injections from these doctors.
DISCUSSION
Based on the available clinical, epidemiological, and laboratory investigation reports, it was confirmed that the outbreak was due to acute hepatitis B viral infection leading to acute fulminant viral hepatitis in some patients, and that it was centered mainly in Modasa town, but also involving some other surrounding regions of Sabarkantha district.
Looking at the gravity of the situation in the community, a rapid operation was launched to control the epidemic. Expert teams from NICD (National Institute of Communicable Diseases), Delhi and NIV, Pune visited the place and the State Health Authorities took control measures in consultation with them as and when required. To guide district health authorities, a protocol for diagnosis and treatment was developed.
Government authorities confirmed that the mode of transmission was from unsafe injection practices by private practitioners in the Modasa town and surrounding areas by interviewing the patients, their family members and their doctors.
As shown in Table 1, adults in the age group of 21–40 years were maximally affected (57.53%), which is statistically significant (*P<0.05). This could be due to the reason that people in this age group have to earn livelyhood for their families and hence they prefer injectables for faster recovery whereas other age groups that are children and old people avoid injectables as they are afraid of it.
Table 2 shows seroprevalence of hepatitis B markers in the cases. As HBeAg is a qualitative marker of HBV replication and relative infectivity,[9] 53.41% (HBeAg positive) confirmed cases were in the stage of high infectivity in our investigation (study). Also as shown in literatures, HBsAg carrier mothers who are HbeAg positive almost invariably (>90%) transmit hepatitis B infection to their offsprings, whereas HBsAg-positive, HBeAg negative mothers rarely (10–15%) infect their offsprings.[9] Amongst all the females in the reproductive age group (15–44 years) in our study, 26.7% females were positive for both markers (HBsAg and HBeAg) and special attention should be given to such groups to prevent mother to child (vertical) transmission.
Recent and remote HBV infections can be distinguished by determination of the immunoglobulin class of HBcAb. As HBcAb of IgM class predominates during the first 6 months after acute infection, patients with current or recent acute hepatitis B, including those in the window period have IgM HBcAb in their serum.[9] In this study, 85.76% (554/646) cases were diagnosed as acute hepatitis B cases and all of them match with the criteria of CDC case definition for acute hepatitis B.
Out of all the 53.41% cases that were in the stage of high infectivity, 48.30% (312/646) cases had acute infection and 5.11% (33/646) were HBsAg and HBeAg positive and IgM HBcAb negative, which suggests that they were either having late/chronic HBV infection or were in the carrier state. There were seven children among these but we were not able to confirm whether any of these children had acquired infection perinatally. Fifty nine (9.13%) out of 646 cases had low infectivity and had late or chronic hepatitis B infection or were in carrier state. These 92 (33 + 59) cases that probably had acquired infection earlier and are not a part of this epidemic had elevated ALT levels. This can be attributed to (a) sero conversion from replicative phase to the nonreplicative phase or (b) other infection leading to elevated ALT.[14] Two hundred and forty two (37.46%) out of 646 had HBsAg positive, IgM HBcAb positive, and HBeAg negative. These include patients in incubation period, patients with acute hepatitis B, or those in the persistent carrier state.[15,16] This category of patients were in the stage of low infectivity. One limitation of our study is that we did not test the cases of acute hepatitis after 6 months to confirm chronic carriage. Thus, a high percentage of acute cases and a high infectivity were identified in this outbreak. If these cases become super carriers in future there will be a danger of perpetuation of the virus in the community that is serious and needs to be dealt with. Super carrier is defined as one with high titre of HBsAg, along with HBeAg, DNA polymerase and HBV in circulation, and generally elevated transaminases.[16]
Following acute HBV infection, the risk of developing chronic infection varies with age. Chronic HBV infection occurs among about 90% of infants infected at birth, 25–50% of children infected at 1–5 years of age, and about 1–5% of persons infected as older children or adults.[5] In our study there were no infants infected, 1.05% was in the age group of 1–5 years, 98.95% had age of more than 5 years out of which adults were 65.23%. Most adult patients recover completely from HBV infections but about 5–10% will not clear the virus and will progress to become asymptomatic carriers or develop chronic hepatitis possibly resulting in cirrhosis and/or liver cancer. Rarely, others may develop fulminant hepatitis and die.[5]
Evidences suggest that HBV genotypes/subgenotypes can significantly influence HBeAg seroconversion rates, viremia levels, mutational patterns that could significantly influence the heterogeneity in clinical manifestations and even response to antiviral therapy.[17–19] It was found from NIV, Pune and private laboratory reports that all the 18 (6 + 12) samples were HBV genotype D (the most prevalent genotype in India) and tests for total antibody to hepatitis D virus were negative. Genotype D and genotype A appear to be the most predominant among Indian patients with HBV-induced acute and chronic liver diseases.[20] In this outbreak, we lost many people. We have records of 72 patients who died up to 6/4/09, but further data are not available to us. We were not able to determine the genotype of the rest, and this is a limitation of our study.
The contacts were surveyed to find out if they too had acquired the virus from the same practitioners as the cases or there had been horizontal transmission. Only 1.75% (20/1145) contacts were found positive for hepatitis B virus infection and since this percentage matches with that in the general population, it reflects that an external risk factor (contaminated syringes) caused the outbreak. As shown in Table 3, 9/17 HBsAg positive contacts were highly infectious, out of which four had acute infection and five with late/chronic infection or carrier state. Out of the remaining eight contacts, six were with late/chronic HBV infections or carrier stage with low infectivity and two were with simple infectious carrier stage. The cases pertaining to the chronically infected household contacts might have acquired infection via horizontal transmission or via reuse of needles and syringes. The HBsAg positive contacts could contribute further to the spread of the virus in the community.
Due to awareness, mass public health actions, and administrative and legislative actions with scientific approach, considerable success was achieved in controlling this epidemic.
Our findings emphasize the following. There were 664 total HBsAg positive confirmed cases, males were more affected than females and persons in the age group of 21–40 years were maximally affected. HBV infection was also detected in 1.75% (20) of the contacts. This seropositivity in the contacts may also reflect the normal prevalence of the HBV in that community. 53.41% of positive cases and 52.93% of the positive contacts were in the highly infectious stage. Use of inadequately sterilised needles and syringes was identified as the main culprit behind this outbreak.
CONCLUSION
In conclusion, we recommend the following actions to prevent such fatal epidemics that are due to use of inadequately sterilised needles and syringes.
Strengthening of the routine surveillance system.
Organization of a health education campaign targeting all health care workers, including private practitioners, especially those in rural areas and the public at large, to take possible measures to prevent spread of infection.
Safe injection practices and Screening of blood and blood products to ensure blood safety.
Consistent management of the waste from the point of generation (cradle) to the point of final disposal (grave).
Hepatitis B vaccination.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Preston H, Wright TL. Interferon therapy for hepatitis C. Lancet. 1996;384:973–4. doi: 10.1016/S0140-6736(05)64920-3. [DOI] [PubMed] [Google Scholar]
- 2.van Damme P, Kane M, Meheus A. Integration of hepatitis b vaccination into national immunization programmes. BMJ. 1997;314:1033. doi: 10.1136/bmj.314.7086.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Pisani P, Munoz N, Ferlay J. The global health burden of incidence of infection associated cancers. Cancer Surv. 1999;33:25–33. [Google Scholar]
- 5.WHO. Hepatitis B. WHO/CDS/CSR/LYO/2002. 2:Hepatitis B. [Last accessed on 2010 Aug 08]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo/20022/en/print.html .
- 6.Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40:4068–71. doi: 10.1128/JCM.40.11.4068-4071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki H, Darmstadt GL, Baten A, Ahsan CR, Saha SK. Seroepidemiology of hepatitis B and dalta virus infection in Bangladesh. J Trop Pediatr. 2003;49:371–4. doi: 10.1093/tropej/49.6.371. [DOI] [PubMed] [Google Scholar]
- 8.WHO: Hepatitis B (fact sheet) 2000. [Last accessed on 2010 Aug 10]. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
- 9.Datta S. An overview of molecular epidemiology of hepatitis B virus (HBV) in India. J Virol. 2008;5:156. doi: 10.1186/1743-422X-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Gupta R, Joshi YK, Singh S. Role of horizontal transmission in hepatitis B virus spread among household contacts in north India. Intervirology. 2008;51:7–13. doi: 10.1159/000118790. [DOI] [PubMed] [Google Scholar]
- 11.Tandon BN, Gandhi BM, Joshi YK. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B infection in India. Bull World Health Organ. 1984;62:67–73. [PMC free article] [PubMed] [Google Scholar]
- 12.Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 13.Singh J, Agarwal NR, Bhattacharjee J, Prakash C, Bora D, Jain DC, et al. An outbreak of viral hepatitis E: Role of community practices. J Commun Dis. 1995;27:92–6. [PubMed] [Google Scholar]
- 14.Dienstag JL, Isselbacher KJ. Acute viral hepatitis, Chap 285. In: Kasper DL, Braunwald E, Fanci AS, Hanser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. Vol. 2. U.S: Mc Graw-Hill companies; 2005. pp. 1822–38. [Google Scholar]
- 15.Singhal V, Bora D, Singh S. Hepatitis B in health care workers: Indian Scenario. J Lab Physicians. 2009;1:41–8. doi: 10.4103/0974-2727.59697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anantnarayan R, Paniker CK. In: Anantnarayan and Panicar's Text book of Microbiology. 8th ed. India: University press (India) private limited; 2009. Hepatitis viruses; pp. 537–48. [Google Scholar]
- 17.Osiowy C. Detection of HBsAg Mutants. J Med Virol. 2006;78(Suppl 1):S48–51. doi: 10.1002/jmv.20607. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer S. Hepatitis B virus: Significance of genotypes. J Viral Hepat. 2005;12:111–24. doi: 10.1111/j.1365-2893.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 19.Echevarria JM, Avellon A. Hepatitis B virus genetic diversity. J Med Virol. 2006;78:S38–42. doi: 10.1002/jmv.20605. [DOI] [PubMed] [Google Scholar]
- 20.Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006;19:203–17. [PubMed] [Google Scholar]


