Abstract
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) remains one of the most serious threats to global health. Today there are no HIV vaccines which can prevent HIV infection. All of the candidates being studied are in the experimental stage. Preventive vaccine candidates are being tested in HIV-negative people to see if they can prevent infection. With of the development of a safe and effective vaccine still likely to be years away, topical microbicide formulations that are applied vaginally and rectally are receiving greater interest as an effective alternative to slow down the global spread of HIV. Current microbicide trials that aim to prevent the sexual transmission of HIV are using gels, creams, rings, films and there is also work underway to explore other types of ‘delivery’ systems. There have been numerous reports on safety and lack of toxicity of the application of nanotechnology for targeted delivery and slow, sustained release of drugs, proteins, peptides or nucleic acids by any route to maximize effectiveness and minimize adverse effects. The application of nanotechnology for targeting drugs and macromolecules to specific tissues or cells is one of the most important areas in nanomedicine research. Thus far nanoparticles provide a strong platform to combine protein and DNA based vaccines/microbicides and will facilitate the production, preclinical evaluation and clinical testing in the future.
Keywords: HIV, Microbicides, Nanotechnology, Vaccines
INTRODUCTION
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) remains one of the most serious threats to global health. According to the 2007 AIDS epidemic update by the World Health Organization (WHO) and the Joint United Nations Program on HIV/AIDS (UNAIDS), approximately 33.2 million people were living with HIV in 2007. Out of this number, 2.5 million were due to new infections among adults and children.[1] Global spending for HIV vaccine research increased from $186 million in 1997 to $759 million in 2005, according to the Joint United Nations Program on HIV/AIDS. The NIH formed its own HIV vaccine trial network in 2000 to oversee clinical research sites in the US, Africa, Asia, the Caribbean, and South America. HIV isolates are grouped into two types, HIV-type 1 (HIV-1) and HIV-type-2 (HIV-2). The most predominant agent worldwide is HIV-1, while HIV-2 is found in some regions of Western and Central Africa. HIV is a member of the Retroviridae family, genus Lentivirus. The retrovirus genome is composed of two identical copies of single-stranded RNA molecules.[2] HIV-1 and HIV-2 have similar basic structural genes, gag, pol, and env, but present a complex combination of other regulatory or accessory genes. Similar to other retroviruses the gag gene encodes the core proteins (p24, p7, p6) and matrix (p17) and the env gene encodes the viral envelope glycoproteins, gp120, and gp41, which recognize cell surface receptors. The pol gene encodes the viral reverse transcriptase that converts viral RNA into DNA, the integrase that incorporates viral DNA into host chromosomal DNA and the protease that cleaves the Gag and Pol protein precursors into their active forms.
Recent advances in the understanding of HIV-1 pathogenesis and immunology have greatly contributed to current HIV-1 vaccine development strategies. Following mucosal exposure to HIV-1, a limited number of virions cross the mucosal barrier to establish primary infection.[3–5] Activated CD4+ T lymphocytes at mucosal surfaces serve as initial targets of infection.[6,7] Acute infection is characterized by early establishment of viral reservoirs and explosive viral replication resulting in massive destruction of CD4+ T lymphocytes in the gastrointestinal mucosa.[8] Progressive immunodeficiency results from chronic immune activation.[9]
FAILED VACCINE TRIALS: LESSONS LEARNED
Conventional vaccines work by stimulating the immune system to manufacture antibodies against an infectious organism, but such a vaccine has proved elusive for the rapidly mutating HIV. In the early 1980s, after identifying the HIV virus as the cause of AIDS, researchers were confident they could come up with a vaccine against it within a few years. The problems involved in developing a successful vaccine accumulated from the time of the first clinical trials of the Microgenesis vaccine to the popular VaxGen trial.[10–12] Early tests using an HIV envelope (gp120)-based vaccine that induced neutralizing antibodies looked promising. Unfortunately, the first trials failed as the vaccine only worked against strains of HIV that had adapted to conditions in the laboratory. In 2003, results finally came in from a phase III clinical trial of a gp120 vaccine manufactured by VaxGen; it failed to prevent infections or reduce the number of virus particles circulating in the blood. Some of the reasons for these failures include the genetic variability of the viral envelope proteins, allowing the virus to escape neutralizing antibodies, and the difficulty in recognizing immunogens and in developing immunization platforms that continuously produce antibodies for neutralizing several HIV clades.[13]
By that time, the field had turned its focus away from vaccines that elicit neutralizing antibodies toward those that control viral load after infection and thus reduce secondary infection.[14–17] One idea was to energize killer T cells by injecting DNA-encoding genes from circulating strains of the virus. Schmitz et al. demonstrated that the depletion of CD8+ cells in simian immunodeficiency virus (SIV)-infected macaques led to reappearance of viral load.[18] It was also shown that the control of viral load in HIV-infected individuals was associated with potent and broad cellular immune responses.[19,20] The so-called STEP trial, sponsored by Merck and Co. and the federally funded HIV Vaccine Trials Network (HVTN), was the first to test the idea of stimulating the immune system's killer T cells to hunt for the virus more aggressively; in this case using a weakened form of recombinant adenovirus serotype 5 (rAD5) to carry three genes from HIV.[21] It was not expected that the vaccine would prevent infection, but it was hoped that it might hinder the growth of the virus enough to delay the onset of full-blown AIDS and make it harder for an infected individual to transmit HIV to others, creating a stopgap while more effective therapy was looked for.
The STEP trial involved recruitment and immunization of 3000 non-infected healthy volunteers with three rAD5 vectors, each expressing HIV genes: Ad5-gag, Ad5-pol, and Ad5-Nef. Each individual in the trial received three injections of three rAd5 vectors, with the last two injections spaced six months apart. The same vaccine regimen was administered to another 3000 non-infected individuals in South Africa (Phambili trial) shortly after the initial results of the STEP trial became available.[22] The STEP trial included 12 Phase I trials with more than 1,300 volunteers which showed that the vaccine was safe and immunogenic as measured by a standardized interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay and experiments in primates also showed some protection aganst SHIV (SIV with an HIV envelope).[21] One aspect of the STEP trial that was completely unexpected was that previous adenovirus infection could enhance the susceptibility to HIV infection in vaccinated subjects.[22] The scientific community soon learned that not only had the STEP HIV vaccine trial failed to protect Ad5-seronegative individuals against infection, it may have enhanced infection in vaccines with prior immunity to adenoviruses.[22] In view of this, Harari et al. reported an alternative heterologous prime boost strategy in which the DNA priming improved the immunogenicity of a recombinant viral vector, in this case the vaccinia virus NYVAC.[23]
The current HIV vaccine clinical trial in Thailand is a continuation of more than ten years of effort to find an effective preventive measure to halt the HIV epidemic in the country. Subsequently, the vaccine manufacturer (Sanofi-Aventis) was approached to develop candidate vaccines that matched the HIV clades circulating in Thailand to be tested in phase I to III trials. In the late 1990s, an assessment of the ‘prime-boost’ vaccine concept was initiated with three candidate vaccine combinations in phase I/II testing.[24,25] One combination was chosen for further evaluation in a phase III trial, the ALVAC-HIV (vCP1521) ‘prime’ and AIDSVAX gp120 B/E ‘boost’.[26] The selection criteria were based on immunogenicity, post-injection reactogenicities, and manufacturing feasibility. ALVAC-HIV (vCP1521), produced by Aventis Pasteur, was a recombinant canarypox vector vaccine that had been genetically engineered to express antigens of subtypes B and E HIV-1: gp120 (subtype E) linked to the transmembrane anchoring portion of gp41 (subtype B), and HIV-1 gag and protease (subtype B). AIDSVAX gp120 B/E, produced by VaxGen Inc., was a bivalent HIV gp120 envelope glycoprotein vaccine containing a subtype E envelope from the HIV-1 strain A244 and a subtype B envelope from the HIV-1 strain MN. In a community-based, randomized, multicenter, double-blind, placebo-controlled efficacy trial, a test of four priming injections of ALVAC-HIV plus two booster injections of AIDSVAX gp120 B/E was evaluated. The trial was the world's largest HIV vaccine trial, and recruited 16,402 healthy men and women between the ages of 18 and 30 years at risk of heterosexual HIV infection in Rauong and Chon Buri provinces in Thailand. The outcome of this vaccine regimen trial was reduction in the risk of HIV infection in a community-based population. In the modified intention-to-treat analysis involving 16,395 HIV-infected subjects, however, the regimen failed to affect the viral load or CD4+ count.[26]
Taken together the failures of these vaccine trials should energize researchers to review and better understand the existing methods of vaccine design and efficacy, the immune mechanisms involved and the appropriateness of animal models. There is no question that the world needs an AIDS vaccine. Researchers, public health leaders, governments, private organizations and companies, and affected communities must work together closely to accelerate research and delivery of HIV vaccines. Table 1 lists some of the continuing HIV vaccine trials being conducted throughout the world.
Table 1.
Continuing preventive and therapeutic HIV vaccine trials

STATE OF THE ART: MICROBICIDES
With the development of a safe and effective vaccine still likely to be years away, topical microbicide formulations that are applied vaginally and rectally are receiving greater interest as an effective alternative to slow down the global spread of HIV. Nearly half of the HIV-infected individuals world-wide are now women, who acquire virus mostly by heterosexual sex. Although methods of prevention already exist, these are insufficient to address the problems of the epidemic especially in developing countries. The “ABC” approach (Abstinence, Be faithful, and Use Condoms) has been used with some success in African countries. One of the most important rationales for developing a microbicide is that high risk women need protection in a form that they themselves can control. Many women, because of economic situations and gender inequality cannot negotiate sexual encounters, and therefore are more vulnerable to sexually transmitted infections including HIV. In fact, researchers developed a mathematical model that shows that if even a small proportion of women in lower income countries used a 60% efficacious microbicide in half the sexual encounters where condoms are not used, 2.5 million HIV infections could be averted over the space of three years. Microbicides offer both primary protection in the absence of condoms and back-up protection if a condom breaks or slips off during intercourse.
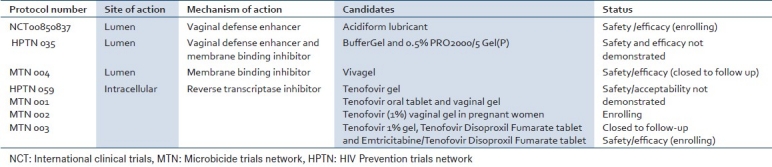
A microbicide could be produced in many forms, including gels, creams, suppositories, films, or as a related device (sponge or ring) that when inserted vaginally or rectally releases the active ingredient over time and acts to prevent infection of a woman or a man by HIV during sexual intercourse. A microbicide should be effective for several hours after use but must not induce inflammation because any increase in the frequency or activation status of immune system cells near the site of virus deposition might facilitate HIV transmission. The type of formulation of the microbicide is also important as any product for use during sexual intercourse must be acceptable to both partners. A practical microbicide must not only be effective, safe and user friendly but also economically affordable in the developing world. Although protection against HIV is the primary goal, it is hoped that microbicides that could protect against other STIs as well as HIV might also become available in the future. To date, the performance of microbicide candidates in efficacy trials has been disappointing, but scientists are pursuing more than fifty product leads, including about a dozen that have proven safe and effective in animals and are now being tested in people [Table 2].
Table 2.
Selected microbicide candidates in clinical phases of development
No one strategy or technology will help solve the AIDS pandemic. All existing prevention strategies such as behavior change, voluntary counseling and testing, STI diagnosis and treatment, broad access to male and female condoms, and antiretroviral interventions must be employed along with continuing to expand existing tools and technologies.
HOW CAN NANOTECHNOLOGY PLAY A ROLE IN HIV/AIDS VACCINE OR MICROBICIDES?
One important aspect of treatment for HIV/AIDS is the method of administration of the drugs to patients. Several studies have shown that inflammation in the vaginal and rectal epithelium plays a greater role in successful HIV infection than was previously thought and the clinical significance of the vaginal-rectal route of infection makes this area an important therapeutic target. Improvements in the delivery of drugs via the oral route or by systemically administered nanoparticle preparations allow a reduction in the drug dose required for symptomatic relief thereby lessening the risk of side-effects. In addition, poorly water-soluble antiretroviral therapy (ART) drugs may have additional bioavailability problems due to poor plasma solubility.
A matrix consisting of internal hydrophobic moieties surrounded by an external sheath of hydrophilic groups provides a pseudomicelle structure capable of being loaded with a hydrophobic drug and transporting it through the blood to the desired target where it can be released within the cytoplasm after uptake of the nanoparticles.[27,28] The application of nanotechnology for targeting drugs and macromolecules to specific tissues or cells is one of the most important areas in nanomedicine research.[29] The advantages of targeted delivery include minimizing systemic exposure with its risk of harmful side-effects, reduction in the amount of drug needed to achieve a therapeutic dose and the potential for producing a nanoparticle system capable of sensing conditions in the target organ and responding with a therapeutic agent.
Development of chitosan-based nanoparticles for drug delivery
Chitosan has been studied extensively as a matrix for nanoparticle-mediated drug transport. Evidence shows that chitosan, a natural biocompatible cationic polysaccharide extracted from crustacean shells, has the potential to be a safe and effective carrier for genes and drugs since it has strong immunostimulatory properties combined with very low immunogenicity.[30–35] Chitosan has also been found to have anticoagulant activity,[33] wound-healing properties,[36] and anti-microbial properties.[37] Additionally, chitosan is non-toxic, non-hemolytic, slowly biodegradable and has been widely used in controlled drug delivery.[34,38] Chitosan also increases transcellular and paracellular transport across the mucosal epithelium[39–41] and, thus, may facilitate mucosal drug delivery and modulate immunity of the mucosal and bronchus-associated lymphoid tissue. It has been demonstrated before that plasmid DNAs can be encapsulated in chitosan nanoparticles to effectively transfer and express genes in lung cells. For example, particles containing plasmids encoding nine different antigens of RSV effectively protected mice from viral infection and chitosan-plasmid nanoparticles expressing IFN-γ were capable of reversing ongoing asthma in a mouse model.[27,28] Chitosan, therefore, possesses all the attributes required for an ideal in vivo drug carrier.
The toxicity of mucosally administered chitosan has been studied in rodents, and no significant difference in behavior, external appearance, body weight or food consumption between control and treated rats was seen. No change was observed upon urinalysis, hematology, and blood chemistry analysis. Relative organ weights and histopathology were similar in control rats compared to chitosan-treated rats. The results suggested that the subacute toxicity of chitosan oligosaccharides is low and that the limit of toxicity must be over 2 g/kg in rats.[42,43] After years of use in food and nutritional supplements, there have been no reports of chitosan toxicity in the database of 2,700 complaints and the EPA has ruled chitosan exempt from its tolerance guidelines.
Given these numerous reports on safety and lack of toxicity, the application of chitosan nanoparticles provides a great opportunity for delivery of proteins, peptides, drugs and nucleic acids. Furthermore, a number of investigators have taken advantage of the cationic property of chitosan to utilize it for targeted delivery and slow-sustained release of drugs and peptides by the mucosal route to maximize effectiveness and minimize adverse effects.
The principle of targeted nanoparticle-mediated drug delivery
The ‘drug’ (small molecule, peptide, gene, oligonucleotide, siRNA) is complexed with thiolated cross-linked chitosan (TCC) and nanoparticles are administered intranasally as an aerosol to the lung. Thiolation targets the TCC particles to lung cells after which the drug is slowly released over a period of days. Even longer exposures can be achieved by encapsulating plasmid DNAs in TCCs which express therapeutic peptides or oligonucleotides. Recently, a thiol-modified chitosan matrix for nanoparticles showed enhanced targeting of a green-fluorescent protein (GFP)-expressing plasmid to mucosal epithelial cells in a mouse model of allergic asthma.[44,45] Nanocomplexes were generated by mixing different ratios of plasmid to thiolated chitosan at a pH of about 5.2, allowing them to coacervate with agitation, then adjusting the pH to about 7. The complexes can be lyophilized and stored indefinitely at room temperature for later use after reconstitution with water. This thermostability and storability of the nanocomplexes provides a great advantage in remote areas of countries where there is no means for refrigeration. Safely encapsulated in nanoparticles, the drugs and immunomodulatory agents for treatment of respiratory diseases, viral infections, asthma or rhinitis can be easily transported, stored and used as a nasal spray. There have been recent suggestions that thiol-modified N,O-SOCC/CS nanoparticles might be useful as novel materials for specific delivery of basic fibroblast growth factor with mitogenic activity.[46]
Given that HIV's port of entry is mucosal, Le Buanec et al, took advantage of the mucotropic properties of polymeric nanoparticle carriers such as chitosan and poly lactic-glycolic acid (PLGA) and tested them in mice in a series of anti-Tat immunization experiments, utilizing different immunization protocols, routes and vectors, with a view to preferentially triggering the production of specific IgA antibodies in addition to IgG antibodies.[47] Since HIV Tat is a true viral toxin in its extracellular configuration, can exercise a wide array of deleterious effects, and retains its immunogenicity in toxoid form, it was used to develop a vaccine for HIV. Both carriers promoted the development of an effective mucosal vaccine equally.
In an effort to develop a more effective genetic immunization strategy for HIV, Locher et al. developed an HIV-2 env DNA vaccine and evaluated adjuvant formulations including cationic liposomes and chitosan. The results showed that liposome formulations given by the intradermal route was superior to chitosan.[48]
Towards developing a biocompatible vaccine delivery system, Drogoz et al. studied the interaction of HIV capsid protein p24 with colloids obtained from polyelectrolyte complexes (PECs) involving two polysaccharides: chitosan and dextran sulfate (DS).[49] The authors investigated p24 sorption kinetics, isotherms, and loading capacities for positively and negatively charged particles of chitosan and dextran sulfate differing in degrees of polymerization (DP) or acetylation (DA). Compared with positive particles, negatively charged colloids had higher binding capacities, faster kinetics, and greater stability of the adsorbed p24. Also, the immunogenicity of the p24-covered particles was assessed for vaccine purposes in mice. The antibody titers obtained with immobilized p24 was dose dependent and in the same range as for Freund's adjuvant, the gold standard for humoral responses.
Further work by Weber et al showed that dendritic cells were capable of uptake of the PECs and subcutaneous delivery of the vaccine generated significant humoral and cellular immune response.[50]
Targeted delivery using chitosan nanoparticles
A useful feature of chitosan is its ability to transport therapeutic genes and target them to specific cell types. For in vivo therapy, it is necessary to target gene delivery vehicles to specific cell types in order to avoid unwanted effects on non-target cells. Active targeting can be achieved by incorporating structures which facilitate the exclusive uptake of the vector by certain tissues or cell types. In some cases, targeting can be achieved passively by taking advantage of a particular physiological condition in the target tissue, such as irregular endothelial fenestration in tumors. Effective cell-specific targeting in vivo requires that unspecific interactions be reduced by using low nitrogen-to-phosphate ratios to shield net positive surface charges on the complexes and that the targeting moiety, which enables uptake into a specific cell type, is correctly incorporated. Various target-specific ligands, such as folate, transferrin, lactose, galactose, mannose, low density lipids, fibroblast growth factors, epidermal growth factor and several antibodies are being tested. Leong and associates[38] have developed methods to conjugate targeting ligands to the surface of DNA-chitosan nanospheres through a polyethylene glycol ‘spacer’. The spacer facilitates binding of the ligand to its cognate receptor on the target cell and minimizes aggregation of nanospheres in solution allowing them to be lyophilized. These strategies have been reviewed in detail.[38,39]
CONCLUSION
Despites decades of research, development of an effective HIV vaccine or microbicide has not been successful. There are many reasons including the immunepathologic mechanisms underlying the HIV infection which leads to AIDS. The very immune cells that are needed for vaccine effctiveness are destroyed by the virus. However, several new approaches are being used including those of nanoparticle technology. Chitosan polymeric nanoparticles provide a strong platform to combine protein and DNA based vaccines and will facilitate the future production, pre-clinical evaluation, and clinical testing of these vaccines.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2007 AIDS epidemic update. [PubMed] [Google Scholar]
- 2.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 3.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J Virol. 2008;82:5548–61. doi: 10.1128/JVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–67. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 7.McDougal JS, Mawle A, Cort SP, Nicholson JK, Cross GD, Scheppler-Campbell JA, et al. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T antigen. J Immunol. 1985;135:3151–62. [PubMed] [Google Scholar]
- 8.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 10.Billich A. AIDSVAX VaxGen. Curr Opin Investig Drugs. 2004;5:214–21. [PubMed] [Google Scholar]
- 11.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006;6:930–9. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 12.Robinson HL. HIV/AIDS vaccines: 2007. Clin Pharmacol Ther. 2007;82:686–93. doi: 10.1038/sj.clpt.6100408. [DOI] [PubMed] [Google Scholar]
- 13.Phogat S, Wyatt RT, Karlsson Hedestam GB. Inhibition of HIV-1 entry by antibodies: Potential viral and cellular targets. J Intern Med. 2007;262:26–43. doi: 10.1111/j.1365-2796.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–55. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 15.Emini EA, Koff WC. AIDS/HIV. Developing an AIDS vaccine: Need, uncertainty, hope. Science. 2004;304:1913–4. doi: 10.1126/science.1100368. [DOI] [PubMed] [Google Scholar]
- 16.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–3. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorner AR, Barouch DH. HIV-1 vaccine development: Progress and prospects. Curr Infect Dis Rep. 2007;9:71–5. doi: 10.1007/s11908-007-0025-0. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 19.Bernard NF, Pederson K, Chung F, Ouellet L, Wainberg MA, Tsoukas CM. HIV-specific cytotoxic T-lymphocyte activity in immunologically normal HIV-infected persons. AIDS. 1998;12:2125–39. doi: 10.1097/00002030-199816000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Pontesilli O, Klein MR, Kerkhof-Garde SR, Pakker NG, de Wolf F, Schuitemaker H, et al. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: A predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–18. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 21.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 22.Sekaly RP. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harari A, Bart PA, Stöhr W, Tapia G, Garcia M, Medjitna-Rais E, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 27.Kumar M, Behera AK, Lockey RF, Zhang J, Bhullar G, De La Cruz CP, et al. Intranasal gene transfer by Chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum Gene Ther. 2002;13:1415–25. doi: 10.1089/10430340260185058. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Yang H, Kong X, Mohapatra S, San Juan-Vergara H, Hellermann G, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S, Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine. 2010;6:64–9. doi: 10.1016/j.nano.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moingeon P, de Taisne C, Almond J. Delivery technologies for human vaccines. Br Med Bull. 2002;62:29–44. doi: 10.1093/bmb/62.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002;14:666–72. doi: 10.1016/s0952-7915(02)00384-9. [DOI] [PubMed] [Google Scholar]
- 32.Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52:145–50. doi: 10.1016/s0169-409x(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 33.Erbacher P, Zou S, Bettinger T, Steffan AM, Remy JS. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm Res. 1998;15:1332–9. doi: 10.1023/a:1011981000671. [DOI] [PubMed] [Google Scholar]
- 34.Sugitachi A, Kashiwaba M, Shimada Y, Terashima M, Asahi H, Saitoh K, et al. Novel biodegradable materials for drug delivery systems (DDS) Gan To Kagaku Ryoho. 2001;28:1530–3. [PubMed] [Google Scholar]
- 35.Bowman K, Leong KW. Chitosan nanoparticles for oral drug and gene delivery. Int J Nanomedicine. 2006;1:117–28. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonov SF, Filippov YI, Shinkarev SM, Frolova MA. Study of wound-healing properties of chitosan. Russian Agric Sci. 2008;34:426–7. [Google Scholar]
- 37.Roller S, Covill N. The Antimicrobial properties of chitosan in mayonnaise and mayonnaise-based shrimp salads. J Food Prot. 2000;63:202–9. doi: 10.4315/0362-028x-63.2.202. [DOI] [PubMed] [Google Scholar]
- 38.Leong KW, Mao HQ, Truong-Le VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release. 1998;53:183–93. doi: 10.1016/s0168-3659(97)00252-6. [DOI] [PubMed] [Google Scholar]
- 39.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 40.Borchard G, Junginger HE. Modern drug delivery applications of chitosan. Adv Drug Deliv Rev. 2001;52:103. doi: 10.1016/s0169-409x(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 41.Schipper NG, Olsson S, Hoogstraate JA, deBoer AG, Vårum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs 2: Mechanism of absorption enhancement. Pharm Res. 1997;14:923–9. doi: 10.1023/a:1012160102740. [DOI] [PubMed] [Google Scholar]
- 42.Kim SK, Park PJ, Yang HP, Han SS. Subacute toxicity of chitosan oligosaccharide in Sprague-Dawley rats. Arzneimittelforschung. 2001;51:769–74. doi: 10.1055/s-0031-1300113. [DOI] [PubMed] [Google Scholar]
- 43.Anzai N, Taniyama T, Nakandakari N, Sugiyama C, Negishi T, Hayatsu H, et al. Inhibition of DNA adduct formation and mutagenic action of 3-amino-1-methyl-5h-pyrido[4,3-b]indole by chlorophyllin-chitosan in rpsL transgenic mice. Jpn J Cancer Res. 2001;92:848–53. doi: 10.1111/j.1349-7006.2001.tb01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC. Thiolation of chitosan. Attachment of proteins via thioether formation. Biomacromolecules. 2005;6:880–4. doi: 10.1021/bm049352e. [DOI] [PubMed] [Google Scholar]
- 45.Lee D, Zhang W, Shirley SA, Kong X, Hellermann GR, Lockey RF. Thiolated Chitosan/DNA nanocomplexes exhibit enhanced and sustained gene delivery. Pharm Res. 2006;24:157–67. doi: 10.1007/s11095-006-9136-9. [DOI] [PubMed] [Google Scholar]
- 46.Ho YC, Wu SJ, Mi FL, Chiu YL, Yu SH, Panda N, et al. Thiol-Modified chitosan sulfate nanoparticles for protection and release of basic fibroblast growth factor. Bioconjug Chem. 2010;21:28–38. doi: 10.1021/bc900208t. [DOI] [PubMed] [Google Scholar]
- 47.Le Buanec H, Vetu C, Lachgar A, Benoit MA, Gillard J, Paturance S, et al. Induction in mice of anti-Tat mucosal immunity by the intranasal and oral routes. Biomed Pharmacother. 2001;55:316–20. doi: 10.1016/s0753-3322(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 48.Locher CP, Witt SA, Ashlock BM, Levy JA. Evaluation of genetic immunization adjuvants to improve the effectiveness of a human immunodeficiency virus type 2 (HIV-2) envelope DNA vaccine. DNA Cell Biol. 2004;23:107–10. doi: 10.1089/104454904322759911. [DOI] [PubMed] [Google Scholar]
- 49.Drogoz A, Munier S, Verrier B, David L, Domard A, Delair T. Towards biocompatible vaccine delivery systems: Interactions of colloidal PECs based on polysaccharides with HIV-1 p24 antigen. Biomacromolecules. 2008;9:583–91. doi: 10.1021/bm701154h. [DOI] [PubMed] [Google Scholar]
- 50.Weber C, Drogoz A, David L, Domard A, Charles MH, Verrier B, et al. Polysaccharide-based vaccine delivery systems: Macromolecular assembly, interactions with antigen presenting cells, and in vivo immunomonitoring. J Biomed Mater Res A. 2010;93:1322–34. doi: 10.1002/jbm.a.32605. [DOI] [PubMed] [Google Scholar]


