Abstract
Breaking the human-to-human transmission cycle remains the cornerstone of infection control during filoviral (Ebola and Marburg) hemorrhagic fever outbreaks. This requires effective identification and isolation of cases, timely contact tracing and monitoring, proper usage of barrier personal protection gear by health workers, and safely conducted burials. Solely implementing these measures is insufficient for infection control; control efforts must be culturally sensitive and conducted in a transparent manner to promote the necessary trust between the community and infection control team in order to succeed. This article provides a review of the literature on infection control during filoviral hemorrhagic fever outbreaks focusing on outbreaks in a developing setting and lessons learned from previous outbreaks. The primary search database used to review the literature was PUBMED, the National Library of Medicine website.
Keywords: Ebola hemorrhagic fever, Infection control, Marburg virus disease, Viral hemorrhagic fever
INTRODUCTION
Ebola and Marburg are single-stranded RNA viruses in the family filoviridae associated with viral hemorrhagic fever outbreaks, mainly in Africa. Although outbreaks are relatively rare, localized, and small, these diseases garner fear and media attention due to high fatality rates up to 90% and concern over their potential use as bioweapons.[1–3] Both Ebola and Marburg hemorrhagic fevers present with a sudden onset of symptoms following an incubation period of 2-21 days.[4] Initial symptoms are non-specific, including fever, malaise, anorexia, headache, sore throat, abdominal pain, vomiting, diarrhea, myalgias, arthralgias, and rash.[4,5] Hemorrhagic symptoms such as epistaxis, petechiae, bleeding from mucous membranes, and internal bleeding may later develop.[4,6] There are five known serotypes of Ebola, four of which are known to cause disease in humans (Bundibugyo, Cote d’Ivoire, Sudan, and Zaire), while the fifth (Reston) has so far been found to infect humans without making them ill; there is one serotype of Marburg virus.[5,7,8] Treatment of disease is supportive. No specific post-exposure therapy is currently available although recent studies using RNA interference and phosphorodiamidate morpholino oligonucleotides have demonstrated promising results among non-human primates.[9,10] Although there are no licensed vaccines for Ebola or Marburg at present, several candidates are currently in development, some of which provide post-exposure protection in animals.[11,12]
The exact mechanisms of filoviral transmission are unclear. Infection with Ebola or Marburg virus can occur as primary animal-to-human transmission, e.g. from handling and butchering infected animals such as bats, the putative reservoir hosts, or non-human primates and forest antelopes who are known to be accidental hosts, or as secondary human-to-human transmission, likely via fomites or close contact.[4,5,13–15] Viral excretion has been demonstrated in blood, breast milk, saliva, semen, stool, and tears.[15] Aerosol transmission has been demonstrated among laboratory primates, but never been documented among filoviral outbreaks in humans.[4,5] Nosocomial transmission historically played a key role in outbreak development due to reuse of contaminated needles and lack of basic infection control measures.[16–19]
This review addresses infection control during filoviral hemorrhagic fever outbreaks, primarily in a developing country setting, and lessons learned from previous outbreaks. A literature search was conducted on PubMed, the National Library of Medicine website. Search strategies included the MeSH categories “Hemorrhagic Fever, Viral,” “Hemorrhagic Fever, Ebola” and “Marburg Virus Disease.” Articles were restricted to the English language. Only articles specifically describing, providing guidance for, or critically analyzing infection control techniques during Ebola and/or Marburg hemorrhagic outbreaks were considered for review.
Infection control during filoviral hemorrhagic fever outbreaks is predominantly based on breaking the human-to-human transmission cycle, which is the principal transmission mode during most outbreaks.[6,16,20] Avoidance of contacts with animal species known to harbor filoviruses plays a role only in the minority of outbreaks for which there is evidence for repeated and widespread primary transmission.[21,22] Identifying new cases is based on a combination of clinical and epidemiological factors. High-risk persons within the community and in health care facilities, those who have within the incubation period come into direct contact with a case, should be followed for signs of illness but should not be isolated as long as they are healthy. Community exposure is minimized through management of cases in an isolation ward using barrier protective gear to protect health care workers providing care and visitors to the ward, as well as using protective materials to provide safe burials.
CASE IDENTIFICATION
Interrupting transmission of Ebola and Marburg hemorrhagic fever requires timely identification of cases and close monitoring of persons at high risk. Early recognition of outbreaks is difficult, due to the non-specificity of symptoms, a low index of suspicion for filoviral disease, and a weak public health system in many countries where filovirus outbreaks occur. Delay in recognition of filoviral disease results in further exposure and transmission prior to the onset of infection control efforts. Timely confirmation of infection with Ebola or Marburg can be made by positive results from IgM or antigen detection ELISA assays or PCR.[4,18] Laboratory facilities capable of diagnosing infection with these viruses are few and far between. Once an outbreak has been identified, real-time confirmation of infection is possible and usually requires that temporary laboratory facilities are set up in close proximity to the outbreak zone. Operational definitions of suspected and probable cases, based on clinical and epidemiological factors, are a necessity. Although similar across outbreaks, these definitions should be tailored to the unique epidemiological patterns of each outbreak.
All persons presenting to health care facilities during filoviral outbreaks with illness consistent with possible filoviral hemorrhagic fever must be triaged and evaluated for possible admission to the isolation unit.[23] Tracing the contacts of each infected patient helps identify additional persons at high-risk who require close follow up for signs of illness. This includes all persons with direct physical contact with the patient, the patient's body fluids, or their clothing or linens within the previous 21 days (the maximum incubation period).[18] All case contacts, including all health care personnel who enter the isolation ward, should be followed for signs of illness, and it has been suggested to take the temperature at least once a day if feasible. Monitoring continues for 21 days after the last known contact with the case.[23] Contacts who develop signs of illness should be transported by medical personal wearing basic personal protective gear to the isolation ward for further evaluation and possible admission to the isolation unit.
ISOLATION UNIT
Patient isolation reduces the potential for transmission of filoviruses by minimizing community exposure. Isolation units capable of caring for filoviral hemorrhagic fever patients are lacking in most developing country health facilities and must be established in existing buildings or temporary constructions at the beginning of outbreak control efforts. The isolation unit is housed in a single or multiple buildings physically separated from other health care facilities by transparent fencing. It contains clean areas for donning protective gear, taking breaks, maintaining a pharmacy and temporary laboratory facilities, as well as patient care wards, a morgue or area for burial preparation, and outdoor space for incineration of contaminated items.[24] The isolation wards should be spacious enough to house confirmed and probable cases separately. Crowding should be avoided to minimize cross-contamination and provide a safe, pleasant environment. A regular power and water supply are vital, as well as adequate access to washroom facilities. The fence surrounding the isolation unit should be constructed using mesh or a combination of mesh and opaque fencing materials; mesh fencing promotes the transparency of control activities, which may enhance compliance with control efforts, while opaque fencing provides a better sense of security to the surrounding community.[23,25,26] During development of the isolation unit, it is vital that care for patients continues using barrier protection measures, as a lack of care may severely hamper relations between the community and infection control team.[23]
Any item in the isolation ward, including human excreta, must be disinfected prior to removal. Effective disinfectants include bleach solutions in concentrations of 1:10 for heavily contaminated objects such as human excreta, body bags, and large spills, and 1:100 for disinfection of everyday objects. Calcium hypochlorite solution ranging from 0.02% to 2% concentration is an acceptable alternative disinfectant.[27] Difficult to disinfect items, such as mattresses, should be covered in plastic to facilitate decontamination.[28] Solid sharps containers must be easily accessible on the isolation ward and recapping of needles discouraged. Contaminated disposable objects should be disinfected and incinerated following removal from the isolation ward.[29]
Patients refuse isolation for many reasons: stress, fears of abandonment by family members, stigma associated with infection, and belief in the certainty of death on the ward due to neglect or intentional killing.[30,31] These concerns may be minimized by efforts to make the isolation ward a pleasant environment, promoting the transparency of activities within the ward, and focusing on providing quality care to each patient. Patients can be discharged if they no longer exhibit signs and symptoms of an active filoviral hemorrhagic fever for at least 3 days and if they are able to feed, wash, and walk independently. If laboratory support is within reach (so that results are available within 48 hours), PCR results that turn negative after being positive previously can help with deciding on discharge (Paul Roddy and Benjamin Jeffs, personal communication). If a patient adamantly refuses hospitalization, risk reduction and care in the home may be attempted.[25] Coordination and supervision of home risk reduction may be difficult, particularly during large outbreaks; thus patients should continue to be encouraged to accept care on the isolation ward. Management in the home includes encouraging the patient to stay in a single, private room, family-based education, having a single caregiver equipped with personal protective gear, and daily visits by medical staff.[24,32] Home risk reduction for all patients may include disinfection of patients’ personal possessions and households using 1:100 bleach solution to decrease the risk of transmission to other members of the household. Compensation for any damages this causes to items improves the acceptability of this practice. Family education is crucial prior to attempting home disinfection, as spraying of disinfectant may be misinterpreted as an attempt to poison the family.[32]
PERSONAL PROTECTIVE GEAR
During filoviral hemorrhagic fever outbreaks, protective equipment demands are high. Implementation of barrier nursing techniques is crucial to minimizing the risk of infection among health workers. Standard personal protective gear for filoviral hemorrhagic fever management includes a scrub suit, gown, apron, rubber boots, head covering, mask, eyewear, and two pairs of gloves [Figure 1].[29] Standard medical gloves are suitable for patient care and should be disinfected between contacts with different patients. Thick neoprene or rubber gloves should be used as the outer glove layer when dealing with spills, disinfecting excreta, laundering linens, and conducting burials.[29] Masks containing HEPA-filters should be utilized if available. Protective gear needs to be removed in a specific order to prevent contamination during undressing, instructions for which are detailed in guidelines published by the WHO and CDC.[29] Personal protective gear and education on their proper use must be available for all personnel working within the isolation area, laundering potentially infected linens, disinfecting items or houses, transporting patients, or providing safe burials. Basic protective gear, including gloves, masks, and disinfectant, should be supplied to all persons manning triage stations.
Figure 1.
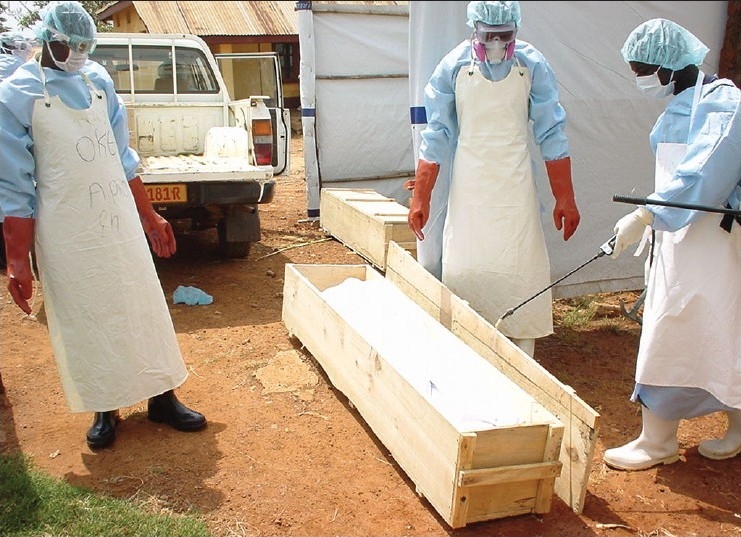
Health workers in protective gear disinfecting a body bag in a coffin. The appearance of control team members is often perceived as frightening. The figure on the right lacks eyewear and heavy-duty gloves in violation of protective gear guidelines
Shortages of protective gear are common in filoviral hemorrhagic outbreak settings, particularly in the early stages, often requiring that gear be flown in by international medical organizations.[17,33] Many hospitals have a limited supply of infection control gear, such as gloves and face masks, which hinders implementation of basic infection control practices.[20,34] The lack of basic infection control gear contributes to the spread of disease, particularly among health care workers prior to disease recognition and in the early stages of outbreak management.[27,35] Reuse of needles without proper sterilization has also been implicated in disease spread.[19] Temporary closure of medical facilities may be required to contain nosocomial spread of disease, but is problematic when there is no alternative where patients can receive emergency care.[27,33,36] Concerns regarding potential transmission within health care facilities may deter patients from seeking necessary medical care for other health problems.[16,18] In addition to equipping workers involved directly in outbreak management, basic protection supplies and training should be provided to other local medical facilities. Implementation of basic infection control precautions in all health facilities reduces the risk of disease transmission from unidentified cases and helps keep health care facilities open, thereby reducing the potential for increased mortality due to other conditions from avoidance of medical care.
In addition to supply difficulties, problems with protective gear arise due to fear and difficulty in prolonged use. Patients and community members may find personal protection gear frightening.[32] Health care workers dressed in protective gear have been likened to looking like cartoons or monkeys.[26] This fear may be partially mitigated by arriving in the village in normal dress and dressing in protective gear on-site and by using protective gear that minimizes facial distortion, such as face shields instead of goggles.[26,37] Difficulties may arise from health care workers being unfamiliar with the proper use of protective gear, thus training and education is essential.[16] Some health care workers may choose not to use it as a gesture of solidarity with sick colleagues.[38] Protective gear becomes unbearably hot after a few hours due to the hot, humid conditions encountered during outbreaks.[28] Perspiration may contribute to fogging of goggles and the accompanying visual impairment poses a safety risk to health care workers. This may be avoided by using alternative gear, such as face shields, provided adequate protection is maintained.[26,37]
BURIALS
Burial teams usually conduct all burials during Ebola and Marburg outbreaks to minimize the potential for exposure from handling the bodies of known infected patients and unrecognized cases.[19] Standard burial practice involves decontamination of the body using 1:10 bleach solution and placement in a body bag.[29] Family members should be offered the opportunity to identify the body from a safe distance at this time. The body bag is then closed and the outside of the bag is similarly decontaminated.[29] Further opening of the body bag is avoided to reduce the possibility of disease transmission. A coffin should be used in addition to a body bag if available and culturally appropriate. The burial is conducted as soon as possible.
Conducting safe burials in a culturally sensitive manner has been a challenge during previous Ebola and Marburg outbreaks.[32] Difficulties arise from families being unable to view the body of the deceased to confirm their identity and being unable conduct traditional rites, such as washing or touching the body.[26,32,39] This leads to distrust between the infection control team and contributes rumors of malicious activities, such as stealing organs or intentional killings, which have resulted in violence towards control team members and interrupted outbreak control practices.[25,26,31,32] Allowing family members to watch the body being placed inside the body bag has been used with moderate success to facilitate body identification, although family members may decline due to fear of infection.[32] The use of body bags with a viewing window to facilitate identification without requiring the body bag to be opened has been suggested, although not yet implemented.[26]
Traditional burial rites that do not increase the infection risk, such as song and dance, should be incorporated into burials according to local practices and aid in establishing a respectful relationship between the burial team and community.[25,32] Some practices may be easily adapted to reduce the risk of infection, such as using gloves to carry a coffin, while others pose more risk. One frequently encountered tradition has particularly been incriminated in facilitating the spread of infection: washing of and giving enemas to the body by family members prior to burial.[31,35,36,40] A modified washing of the body conducted by burial team members in protective gear may be considered acceptable alternatives depending on the family's wishes and comfort level of the burial team.[32]
COMMUNITY SENSITIZATION
Filovirus outbreaks produce an enormous amount of fear and many communities are unfamiliar with the techniques used to manage them.[33,41] Efforts to sensitize the community to control efforts and establish a trusting, respectful relationship between the community and outbreak control team are of paramount importance.[26,41,42] Misunderstanding, stigma, and distrust during previous outbreaks resulted in patients being hidden from outbreak personnel and verbal and physical harassment towards health workers from the isolation unit in the form of death threats, throwing stones, and burning down houses.[26,27,31–34,41]
To establish a trusting relationship with the community and facilitate the acceptance of control efforts, measures must be transparent and culturally sensitive.[23,26,32,37] In addition to using transparent fencing and burial measures described previously, allowing family members and community leaders wearing protective gear to visit patients on the ward increases the acceptance of isolation.[23] There is significant benefit to providing psychosocial support to patients, family members, and health workers.[23,30,32] Collaboration with anthropologists has aided in adapting control efforts to reflect local traditions, including the integration of traditional explanatory models of illness, and identify traditions which may contribute to disease spread.[25,31] Involvement of community leaders can help mobilize persons to adopt measures to protect themselves, including acceptance of the isolation ward.[32] Regular reports through media outlets are another possible route of social mobilization; however this reporting needs to be conducted in an organized, ethical manner, so that the media may aid rather than hinder outbreak management.[17,35] It is always important to convey a message of hope rather than fear; filoviral disease is survivable and treatment likely improves the chance of survival.[32]
SUMMARY
Successful infection control during filoviral hemorrhagic fever outbreaks requires breaking the human-to-human transmission cycle. Control efforts are labor and equipment intensive. Core infection control activities include effective identification and isolation of cases, timely contact tracing and monitoring, adequate barrier personal protection gear for health care workers, safe burials, and community sensitization. Established guidelines for management of filoviral hemorrhagic fever outbreaks exist, however, do not sufficiently address the community distrust and insensitivity to local culture that has complicated control efforts during previous outbreaks. The main challenge for infection control during filoviral hemorrhagic fever outbreaks remains the cultural acceptability and the ability to adapt efforts to reflect local customs while maintaining bio-safety.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Centers for Disease Control and Prevention. History of Ebola outbreaks. [Last cited on 2010, Nov 27]. Available from: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/ebola/ebolatable.htm .
- 2.Centers for Disease Control and Prevention. History of Marburg outbreaks. [Last cited on 2010, Nov 27]. Available from: http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/marburg/marburgtable.htm .
- 3.Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, et al. Hemorrhagic fever viruses as biological weapons: Medical and public health management. JAMA. 2002;287:2391–405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 4.Hartman AL, Towner JS, Nichol ST. Ebola and Marburg hemorrhagic fever. Clinical Laboratory Medicine. 2007;30:161–77. doi: 10.1016/j.cll.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Schou S, Hansen AK. Marburg and Ebola virus infections in laboratory non-human primates: A literature review. Comp Med. 2000;50:108–23. [PubMed] [Google Scholar]
- 6.Ndambi R, Akamituna P, Bonnet M, Tukadila AM, Muyemba-Tamfun J, Colebunders R. Epidemiologic and clinical aspects of the Ebola virus epidemic in Mosango, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S8–10. doi: 10.1086/514297. [DOI] [PubMed] [Google Scholar]
- 7.Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki S, Nichol ST, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–6. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 8.Wamala JF, Lukwago L, Malimbo M, Nguku P, Yoti Z, Musenero M, et al. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007-2008. Emerg Infect Dis. 2010;16:1087–92. doi: 10.3201/eid1607.091525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Lee ACH, Robbins M, Geisbert JB, Honko A, Sood V, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: A proof-of-concept study. Lancet. 2010;375:1896–905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, van Tongeren SA, et al. Advanced antisense therapies for postexposure protection against lethal filovirus infection. Nat Med. 2010;16:991–4. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 11.Geisbert TW, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and Ebola viruses. Rev Med Virol. 2010;20:344–57. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Ströher U, Grolla A, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathogens. 2007;3:54–61. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leroy E, Epelboin A, Mondonge V, Pourret X, Gonzalez J, Muyemba-Tamfum J, et al. Human Ebola outbreak resulting from direct exposure to fruit bats in Leubo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 2009;9:723–8. doi: 10.1089/vbz.2008.0167. [DOI] [PubMed] [Google Scholar]
- 14.Leroy EM, Rouquet P, Formenty P, Souquière S, Kilbourne A, Froment J, et al. Multiple Ebola virus transmission events and rapid decline in central African wildlife. Science. 2004;303:387–90. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 15.Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–7. doi: 10.1086/520545. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Hoch SP. Lessons from nosocomial viral haemorrhagic fever outbreaks. Brit Med Bull. 2005;73-4:123–37. doi: 10.1093/bmb/ldh054. [DOI] [PubMed] [Google Scholar]
- 17.Heymann DL, Barakamfitiye D, Szczenioski M, Muyemba-Tamfum J, Bele O, Rodier G. Ebola hemorrhagic fever: Lessons from Kikwit, Democratic Republic of the Congo. J Infect Dis. 1999;179(Suppl 1):S283–6. doi: 10.1086/514287. [DOI] [PubMed] [Google Scholar]
- 18.Jeffs B. A clinical guide to viral haemorrhagic fevers: Ebola, Marburg, and Lassa. Trop Doct. 2006;36:1–4. doi: 10.1258/004947506775598914. [DOI] [PubMed] [Google Scholar]
- 19.Peters CJ. Marburg and Ebola-arming ourselves against the deadly filoviruses. N Engl J Med. 2005;352:2571–3. doi: 10.1056/NEJMp058109. [DOI] [PubMed] [Google Scholar]
- 20.Okware S, Omaswa M, Zaramba S, Opio A, Lutwama JJ, Kamugisha J, et al. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002;7:1068–75. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 21.Bausch DG, Nichol ST, Muyemba-Tamfum JJ, Borchert M, Rollin P, Sleurs H, et al. Marburg haemorrhagic fever associated with multiple genetic lineages of virus. N Eng J Med. 2006;355:909–19. doi: 10.1056/NEJMoa051465. [DOI] [PubMed] [Google Scholar]
- 22.Bausch DG, Borchert M, Grein T, Roth C, Swanepoel R, Libande ML, et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg Infect Dis. 2003;9:1531–7. doi: 10.3201/eid0912.030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffs B, Roddy P, Weatherill D, de la Rosa O, Dorion C, Iscla M, et al. The Medecins Sans Frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. I. Lessons learned in the hospital. J Infect Dis. 2007;196(Suppl 2):S154–61. doi: 10.1086/520548. [DOI] [PubMed] [Google Scholar]
- 24.Colebunders R, Sleurs H, Pirard P, Borchert M, Libande M, Mustin JP, et al. Organisation of health care during an outbreak of Marburg haemorrhagic fever in the Democratic Republic of Congo, 1999. J Infection. 2004;48:347–53. doi: 10.1016/S0163-4453(03)00122-1. [DOI] [PubMed] [Google Scholar]
- 25.Hewlett BS, Epelboin A, Hewlett BL, Formenty P. Medical anthropology and Ebola in Congo: Cultural models and humanistic care. Bull Soc Pathol Exot. 2005;98:230–6. [PubMed] [Google Scholar]
- 26.Raabe VN, Mutyaba I, Roddy P, Lutwama JJ, Geissler W, Borchert M. Infection control during filoviral hemorrhagic fever outbreaks: Preferences of community members and health workers in Masindi, Uganda. Trans R Soc Trop Med Hyg. 2010;104:48–50. doi: 10.1016/j.trstmh.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Kerstiëns B, Matthys F. Interventions to control virus transmission during an outbreak of Ebola hemorrhagic fever: Experience from Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S263–7. doi: 10.1086/514320. [DOI] [PubMed] [Google Scholar]
- 28.Guimard Y, Bwaka MA, Colebunders R, Calain P, Massamba M, De Roo A, et al. Organization of patient care during the Ebola hemorrhagic fever epidemic in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S268–73. doi: 10.1086/514315. [DOI] [PubMed] [Google Scholar]
- 29.Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting. Atlanta: Centers for Disease Control and Prevention; 1998. Centers for Disease Control and Prevention and World Health Organization. [Google Scholar]
- 30.De Roo A, Ado B, Rose B, Guimard Y, Fonck K, Colebunders R. Survey among survivors of the 1995 Ebola epidemic in Kikwit, Democratic Republic of Congo: Their feelings and experiences. Trop Med Int Health. 1998;3:883–5. doi: 10.1046/j.1365-3156.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 31.Hewlett BS, Amola RP. Cultural contexts of Ebola in northern Uganda. Emerg Infect Dis. 2003;9:1242–8. doi: 10.3201/eid0910.020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roddy P, Weatherill D, Jeffs B, Abaakouk Z, Dorion C, Rodriguez-Martinez J, et al. The Medecins Sans Frontieres intervention in the Marburg hemorrhagic fever epidemic, Uige, Angola, 2005. II. Lessons learned in the community. J Infect Dis. 2007;196(Suppl 2):S162–7. doi: 10.1086/520544. [DOI] [PubMed] [Google Scholar]
- 33.Hall RC, Hall RC, Chapman MJ. The 1995 Kikwit Ebola outbreak: Lessons hospitals and physicians can apply to future viral epidemics. Gen Hosp Psychiatry. 2008;30:446–52. doi: 10.1016/j.genhosppsych.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewlett BL, Hewlett BS. Providing care and facing death: Nursing during Ebola outbreaks in central africa. J Transcult Nurs. 2005;16:289–97. doi: 10.1177/1043659605278935. [DOI] [PubMed] [Google Scholar]
- 35.Lamunu M, Lutwama JJ, Kamugisha J, Opio A, Nambooze J, Ndayimirije N, et al. Containing a haemorrhagic fever epidemic: The Ebola experience in Uganda (October 2000-January 2001) Int J Infect Dis. 2004;8:27–37. doi: 10.1016/j.ijid.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Muyemba-Tamfum J, Kipasa M, Kiyunga C, Colebunders R. Ebola outbreak in Kikwit, Democratic Republic of the Congo: Discovery and control measures. J Infect Dis. 1999;179(Suppl 1):S259–62. doi: 10.1086/514302. [DOI] [PubMed] [Google Scholar]
- 37.Bausch DG, Feldmann H, Geisbert TW, Bray M, Sprecher AG, Boumandouski P, et al. Outbreaks of filovirus hemorrhagic fever: Time to refocus on the patient. J Infect Dis. 2007;196:S136–41. doi: 10.1086/520542. [DOI] [PubMed] [Google Scholar]
- 38.Borchert M, Mulangu S, Lefevre P, Tshomba A, Libande ML, Kulidiri A, et al. Use of protective gear and the occurrence of occupational Marburg hemorrhagic fever in health workers from Watsa health zone, democratic republic of the Congo. J Infect Dis. 2007;196:S168–75. doi: 10.1086/520540. [DOI] [PubMed] [Google Scholar]
- 39.Enserink M. Crisis of confidence hampers Marburg control in Angola. Science. 2005;308:489. doi: 10.1126/science.308.5721.489. [DOI] [PubMed] [Google Scholar]
- 40.LeGrand J, Grais RF, Boelle PY, Valleron AJ, Flahault A. Understanding the dynamics of Ebola epidemics. Epidemiol Infect. 2007;135:610–21. doi: 10.1017/S0950268806007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ndayimirije N, Kindhauser MK. Marburg hemorrhagic fever in Angola-fighting fear and a lethal pathogen. N Engl J Med. 2005;352:2155–7. doi: 10.1056/NEJMp058115. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Outbreak(s) of Ebola haemorrhagic fever in the Republic of the Congo, January-April 2003. Weekly epidemiological record. 2003;78:285–96. [PubMed] [Google Scholar]


