Abstract
Background/Aim:
In contrast to diverticulosis of the colon, jejunal diverticulosis is a rare entity that often becomes clinically relevant only after exacerbations occur. The variety of symptoms and low incidence make this disease a difficult differential diagnosis.
Patients and Methods:
Data from all patients who were treated in our surgical department for complicated jejunal diverticulitis, that is, gastrointestinal hemorrhage or a diverticula perforation were collected prospectively over a 6-year period (January 2004 to January 2010) and analyzed retrospectively.
Results:
The median age among the 9 patients was 82 years (range: 54–87). Except for 2 cases (elective operation for a status postjejunal peridiverticulitis and a re-perforation of a diverticula in a patient s/p segment resection with free perforation), the diagnosis could only be confirmed with an exploratory laparotomy. Perforation was observed in 5 patients, one of which was a retroperitoneal perforation. The retroperitoneal perforation was associated with transanal hemorrhage. Hemodynamically relevant transanal hemorrhage requiring transfusion were the reason for an exploratory laparotomy in 2 further cases. In one patient, the hemorrhage was the result of a systemic vasculitis with resultant gastrointestinal involvement. A singular jejunal diverticulum caused an adhesive ileus in one patient. The extent of jejunal diverticulosis varied between a singular diverticulum to complete jejunal involvement. A tangential, transverse excision of the diverticulum was carried out in 3 patients. The indication for segment resection was made in the case of a perforation with associated peritonitis (n=4) as well as the presence of 5 or more diverticula (n=2). Histological analysis revealed chronic pandiverticulitis in all patients. Median operating time amounted to 142 minutes (range: 65–210) and the median in-hospital stay was 12 days (range: 5–45). Lethality was 0%. Major complications included secondary wound closure after s/p repeated lavage and bilateral pleural effusions in one case. Signs of malabsorption as the result of a short bowel syndrome were not observed. Minor complications included protracted intestinal atony in 2 cases and pneumonia in one case. Median follow-up was 6 months (range: 1–18).
Conclusion:
Complicated jejunal diverticulitis often remains elusive preoperatively due to its unspecific clinical presentation. A definitive diagnosis can often only be made intraoperatively. The resection of all diverticula and/or the complete diverticula-laden segment is the goal in chronic cases. The operative approach chosen (tangential, transverse excision vs segment resection) should be based on the extent of the jejunal diverticulosis as well as the intraoperative findings.
Keywords: Diverticulosis, jejunum, surgery
Diverticulosis of the jejunum is a rare disease. Reported frequencies vary between 0.06% and 5%.[1,2] Patient age at diagnosis is usually well beyond the 40-year mark. In addition to chronic disease courses characterized by unspecific symptoms, such as intermittent abdominal pain, nausea, diarrhea, or malabsorption, which often lead to wrong diagnoses, the typical complications of an acute diverticulitis are also observed.[3] It usually becomes clinically relevant when complications occur, such as perforation, ileus, and gastrointestinal hemorrhage.[4] The frequency of complicated disease courses is reported at 10%.[5] The high mortality rate, which has been reported in up to 40%, is often the result of a delayed diagnosis.[6] We report a series of 9 patients treated for a complicated case of jejunal diverticulitis in our clinic.
PATIENTS AND METHODS
The data of all patients who were treated for complicated jejunal diverticulitis, that is, with gastrointestinal hemorrhage or a diverticulum perforation, in our clinic over a 6-year period (January 2004 to January 2010) were documented in a prospective data bank and analyzed retrospectively. Characteristics, such as age, gender, and clinical findings, diagnostic modalities, operative strategies, and resection methods, localization of the perforation/hemorrhage, intra- and postoperative complications, as well as the postoperative course were analyzed. Major complications were defined as all complications that required invasive treatment (operative revision, drainage).
RESULTS
Over the observation period given, a total of 9 patients were treated at our clinic for complicated jejunal diverticulitis.
Relevant biographical and patient history data as well as clinical characteristics are shown in Table 1. The median age was 82 years (range: 54–87).
Table 1.
Patient data and clinical characteristics

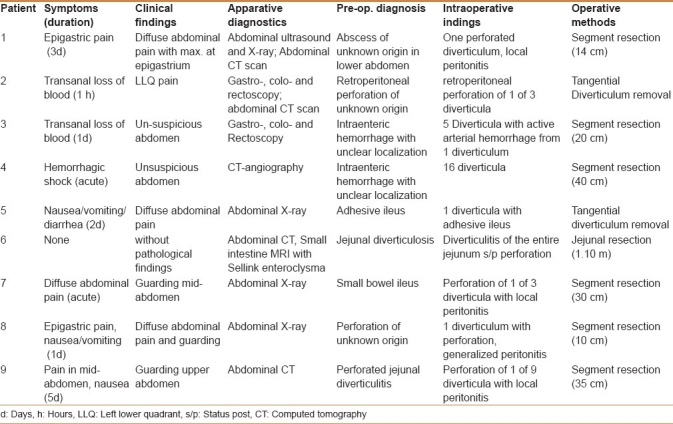
Table 2 shows clinical symptoms, duration of complaints, clinical and apparative examination results, suspected diagnoses, and intraoperative findings. The patients complained of nausea, transanal hemorrhage, and even abdominal pain in the sense of an acute abdomen. Symptoms presented acutely or had been present for several days. The same variation was apparent in the results of clinical examination. These varied between isolated pain on palpation to abdominal rigidity. There was no correlation between clinical signs and the extent of intraoperative findings.
Table 2.
Preoperative findings and diagnostics
Diagnostics
The apparative diagnostics ranged from abdominal ultrasound/X-ray to endoscopy and computed tomography (CT)- or magnetic resonance imaging (MRI) scans in different combinations [Table 2]. Preoperative diagnosis and intraoperative findings were only congruent in 2 patients (patient No. 9 and patient No. 6).
Patient No. 9 had already undergone segment resection due to diverticulum perforation at an outside clinic. Evidence of massive diverticula affliction of the entire jejunum, accentuated in the proximal third, without evidence of stenosis but with peridiverticulitis was obtained in patient No. 6 at an outside clinic via abdominal CT scan and an MRI with Sellink enteroclysis. In this case, the peridiverticulitis had first been treated conservatively with antibiotics. The patient then presented to our clinic for elective operative therapy.
Indication for surgical therapy, intraoperative findings, and performed surgical procedure
With the suspicion of intra-abdominal adhesions the indication for diagnostic laparotomy was stated for patient No. 5.
Abdominal Ultrasound and X-ray revealed distention of the small bowel (3.8 cm) with pendulum-like peristalsis, suggesting a proximal small bowel ileus. Intraoperatively, an adhesion originating from a jejunal diverticulum caused twisting of the small bowel and venous stasis. After the adhesion was dissected, closed small bowel compression was performed, followed by transverse stapler-assisted removal of the singular 3.5 cm long jejunal diverticulum.
Three patients (No. 2–4) presented with transanal hemorrhage.
Patient No. 2 had no complaints but showed an elevation in inflammatory markers (white blood cells 19,000/μL, C-reactive protein 166 mg/L). The localization of the hemorrhage source could not be accomplished by gastroscopy, colonoscopy, or rectoscopy. The subsequent abdominal CT scan showed free air ventrally to the aorta and pneumotosis intestinalis. The indication for operative therapy was given with the suspicion of retroperitoneal perforation. A diagnostic laparotomy was performed. This revealed 3 diverticula within the first jejunal loop distal to the ligament of Treitz with perforation of the first diverticulum. The diverticula were removed tangentially transversely using an Endo-GIA and covering sutures were performed.
In patient No. 3 rectoscopy and colonoscopy exposed pandiverticulosis, but was unable to identify the source of hemorrhage. Gastroscopy only showed antrum gastritis. Throughout the following clinical course, hemodynamically relevant peranal hemorrhage occurred and the indication for laparotomy was stated. Intraoperatively, the small bowel was completely filled with blood beginning 50 cm distally to the Treitz ligament. Five diverticula were found in the mid-section of the jejunum. This section was enterotomized. One diverticulum neck was found to have an active arterial hemorrhage. Under full heparinization, discontinuous enteroscopy of the entire small bowel was performed to exclude further sources of hemorrhage. After additional hemorrhages could be ruled out, small bowel segment resection (20 cm) was performed.
Patient No. 4 experienced hemorrhagic shock following diagnostic laparoscopy and transferred to the medical clinic for the initiation of cortisone therapy for systemic vasculitis. The urgently performed laparotomy revealed 16 diverticula in the region of the jejunum as the suspected source for an upper Gastro-Intestinal bleeding. After segment resection (40 cm) within this region, no further bleeding was observed.
Perforated jejunal diverticulitis was observed in 4 patients (No. 1,7,8,9).
In 2 patients (patients No. 8 and 9), perforation was suspected preoperatively. Only in one case (patient No. 9) the suspicion of diverticula re-perforation within the jejunum had arisen on abdominal CT scan, as this patient had a history of previous jejunal diverticulum perforation. The diagnosis of perforation with local peritonitis was confirmed intraoperatively [Figure 1]. The remaining diverticula affected portion of the jejunum (30 cm) was then resected. In another patient (patient No. 8) the indication for exploratory laparotomy was stated as the consequence of free air found on the abdominal X-ray. The localization of the perforation was unclear preoperatively. Intraoperatively, generalized peritonitis was present after a singular perforated diverticulum of the jejunum. A 10 cm segment was resected and an abdominal lavage was performed. After repeated lavage, on the third postoperative day, definitive closure of the abdomen was performed with the aid of a Safil®-net.
Figure 1.
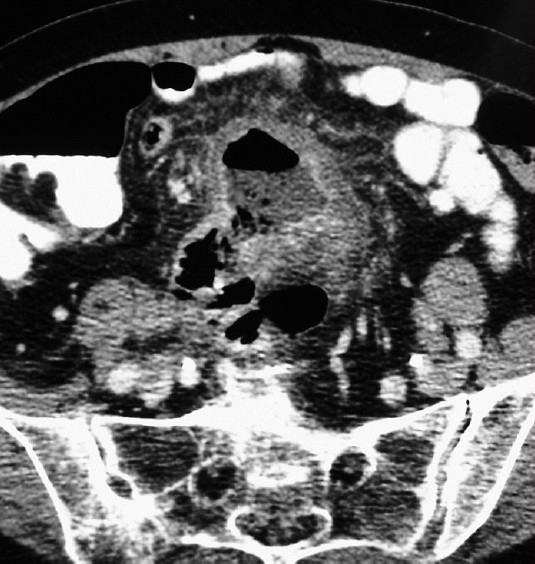
Abdominal CT in perforated jejunal diverticulitis
With the diagnosis of an acute abdomen and the radiologic evidence of an ileus, the indication for surgery was stated for patient No. 7. Exploratory laparotomy revealed 3 jejunal diverticula with a perforation of the middle diverticulum and local peritonitis. The jejunal segment affected measuring 30 cm in length was then resected.
In patient No. 1, when ultrasound and abdominal X-ray remained inconclusive, an abdominal CT scan was performed. The imaging revealed a 7 cm long process with contrast-enhancing borders suspicious of an abscess, with air-fluid levels within the entrance to the pelvis but without evidence of free air or fluid. Exploratory laparotomy showed an inflammatory process (6 cm) with perforation of a jejunal diverticulum and local peritonitis. A 14 cm segment resection was then performed.
The indication for elective operative therapy was stated for one patient (No. 6).
The referral for elective surgical therapy was the result of the evidence of massive diverticulosis after peridiverticulitis had resolved. Intraoperatively, there was evidence of florid diverticulitis status postperforation of several giant diverticula. A laparoscopically assisted jejunum resection was performed. The resection length was 110 cm.
As demonstrated in Table 2, the operative method chosen is based on the extent of the jejunal diverticulosis as well as intraoperative findings and diagnosis. Hemorrhage from singular diverticula and perforations without peritonitis were treated via tangential removal. Multiple diverticula (>3) or free perforation with peritonitis represented an indication for segment resection.
Postoperative course and histological findings
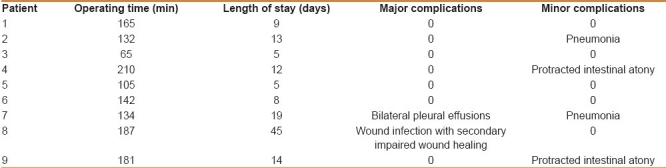
Table 3 offers an overview of peri- and postoperative course data. The median operating time was 142 min (65–210). The median length of stay was 12 days (5–45). Lethality was 0%. Morbidity was 55%. Two major complications and 3 minor complications were observed. One major complication (patient No. 8) was related to the immediate operating site. In this case, wound infection occurred after repeated lavage for peritonitis and the placement of a Safil®-net and thus secondary wound healing was necessary. There was no evidence for malabsorption.
Table 3.
Peri- and postoperative course
Throughout the median follow-up interval of 6 months (range: 1–18), no further complications were observed.
Histologic examination revealed high-grade chronic active ulcerous–phlegmonous partially perforated diverticulitis with peritonitis in 7 patients (No. 1–3, 6–9). Systemic vasculitis was additionally present in patient No. 4. Patient No. 5 had the anomaly of a nonexistent muscularis propria layer, although the submucosa and mucosa were well developed, thus the pathology must be referred to as pseudodiverticula of the jejunum.
DISCUSSION
As early as 1932, Andreas Christ reported a rare and unusual disease, namely, diverticulosis of the upper jejunum.[7] Fifteen percent to forty percent of the population in industrial nations over 40 years of age have evidence of diverticulosis of the large intestine. In 35% of cases, they are associated with the occurrence of small intestine diverticula.[8] In congruence with the published literature, all our patients had colonic diverticulosis in addition to diverticulosis of the small intestine. The incidence for small intestinal diverticulosis is reported at 2–3% and for jejunal diverticula alone mostly at 0.02–1%.[9] However, the reported frequencies for jejunal diverticulosis vary between 0.06% and 5%.[1,2]
As a remnant of the omphalomesenteric duct, the Meckel's diverticulum is the only congenital true diverticulum of the small intestine. The origin of the acquired small intestinal diverticulum has not been definitively clarified.[6] Hypotonic small intestinal motility dysfunction with consequent bacterial overgrowth is often deemed the cause. The occurrence at an advanced age, as in the median age of 82 years shown in our patient collective, suggests a correlation with degenerative processes such as myo- and neuropathy of the myenteric plexus as well as scleroderma. As in diverticulosis of the large intestine, the mucosa herniates through the vessel gap at the “locus minoris resistentae.”[10,11] Acquired diverticula of the duodenum and of the proximal jejunum are the most common type.[12] In our series, the diverticula were also observed within the proximal jejunum, especially 10-20 cm distal to the ligament of Treitz. Three patients had a solitary diverticulum and 4 patients had more than 3 diverticula. In one case, the entire jejunum was afflicted. Further variations were present in the size of the diverticula, which ranged between 1 and 10 cm in length.
With regard to the present results of the histologic examination, the assertion that the disease often remains asymptomatic appears questionable.[13] Except for one case, all patients had histologic evidence of chronic recurrent inflammation. The chronic recurrent course is difficult to diagnose.[14] Unspecific symptoms, such as dyspepsia, anorexia, and upper abdominal pain, lead to misdiagnosis such as irritable bowel syndrome.[15] It can thus be assumed that there are a large number of undetected cases of chronic jejunal diverticulitis that are never diagnosed as such. The intermittency of symptoms, typical for diverticulitis of the large intestine, is often missing in these cases. In the cases analyzed, it was only present in 1 patient, who had diverticulitis of the entire jejunum. In most cases, disease exacerbations with serious complications often prompt extensive diagnostics.[16] The factors that contribute to the exacerbation of diverticulitis remain unclear.
As presented in this study, such cases often remain undetected preoperatively, even in the stage of exacerbation and/or when typical complications arise. Only in one patient with known jejunal diverticulosis and status post–segment resection after perforation, the preoperative diagnosis matched the intraoperative findings. In all other cases, the definite diagnosis could only be made by exploratory laparotomy. The failure to confirm the diagnosis preoperatively is the result of the rarity of the disease, its location, and the great variation in symptoms of exacerbation.
As in diverticulitis of the large intestine, perforation is the most common complication in diverticulitis of the small intestine.[17] Perforation is associated with peritonitis in most cases. The severity of the peritonitis determines the clinical picture, which ranges from mild pain upon palpation to a full-scale acute abdomen.[18] As a result of the varying location of small intestinal diverticula, there is no “typical” localization. Thus, within our collective, cases of perforated diverticulitis were associated with mid-abdominal pain as well as pain throughout the entire abdomen.
Preoperative diagnostics often reveal indirect signs. Perforation with only retroperitoneal or mesenterial free air escape detection on abdominal X-rays (in both upright and lateral views). Evidence of subphrenic free air, indicating free perforation, which is rare in cases of small intestine diverticulitis, was only found in one case in our collective. Abdominal ultrasound can only be complementary. The meteorism that inevitably accompanies peritonitis with reflectory ileus as well as the continually increasing average patient body mass index hinder the success of ultrasound studies.[19] The typical inhomogeneous process located close to the bowel wall with unspecific irregular formation in relation to the small bowel can often not be visualized. Thus, ultrasound alone is not a suitable diagnostic method.[20] Double-contrasted CT scans with enteric and i.v. contrast is the standard method. As shown in our patient collective, these studies often only reveal secondary changes. Thus, indirect signs such as increased wall thickness, entrapped air or fat tissue inhibition must aid the diagnosis of diverticulitis.[21] Typical signs of free perforation such as contrast leak or abscess formation are rare, but should serve as sufficient indication for operative clarification when coupled with surrounding small intestinal loops. Intestinal hemorrhage related to small intestinal diverticulitis is an extremely rare complication and very difficult to localize. It can occur separately from or in combination with systemic diseases. For example, one patient suffering from acute systemic vasculitis had a hemorrhage from a diverticula affected portion of the jejunum despite ongoing corticosteroid therapy. Diverticula hemorrhage was also observed in a patient under phenprocumon therapy as well as in another patient with normal coagulation parameters. The localization of the small intestinal hemorrhage represents a diagnostic challenge. Typical diagnostics such as colonoscopy or gastroscopy do not provide the localization. Push enteroscopy and capsule endoscopy can be valuable tools in cases of hemorrhages of unknown localization. The push enteroscopy can detect bleedings of the proximal jejunum with good sensitivity and even simultaneous therapy might be performed. The capsule endoscopy is able to detect the localization of the hemorrhage in up to 70% cases. However, both diagnostics are mainly used in patients with occult bleeding and strongly depend on the experience of the examiner. Furthermore, these techniques are still limited to a few clinics, and therefore the widespread use is restricted.[22] In hemodynamically stable patients, one can consider performing a CT-angiography, which can demonstrate hemorrhages in cross-sections. Evidence of intestinal hemorrhage via angiography is only successful in cases where the bleeding exceeds 1 mL/min.[23] Thus, such studies should be performed under full heparin therapy and in OR-standby. A further option is the erythrocyte scintigraphy. In contrast to CT-angiography studies, however, this method has a much lower sensitivity.[22] In hemodynamically unstable patients and those requiring blood transfusions, immediate operative therapy is indicated. Our study shows that discontinuous enteroscopy is a safe intraoperative method to rule out or confirm a source of small intestinal hemorrhage. However, there are no studies on the matter available.
As a result of the chronicity we observed, all diverticula should be resected, regardless of whether perforation or hemorrhage has occurred, in order to avoid further complications, such as re-perforation after segment resection, continuing pandiverticulitis or adhesive ileus. The choice of operative method should be based on the basic principles of septic surgery implying immediate decontamination and healthy wound edges. If only one diverticulum can be observed, tangential/transverse diverticulum resection can be performed safely as shown in one of our cases according to the surgical therapy of a Meckel's diverticulum. When patients suffered perforation with accompanying peritonitis and/or more than 3 diverticula, the indication for segment resection with primary anastomosis was made. Usually only a short segment is afflicted even when several diverticula are present.[1] The jejunal segments we resected were 5–40 cm long. Extensive small bowel resection (110 cm) was only necessary in one case. As with most other acute operations and/or diseases, the postoperative course is determined by previous medical conditions, the timing of diagnosis and/or indication for operative therapy, as well as the extent of intraoperative findings.[24] The occurrence at an advanced age is associated with an increased number of previous medical conditions, as shown in our patient collective, which cause intraoperative complications such as diverticulum hemorrhage under anti-coagulatory therapy as well as postoperative complications such as postoperative pneumonia with bilateral pleural effusions in a patient with decompensated Chronic obstructive pulmonary disease. The most common unspecific complication associated with acute processes is pneumonia, which is often the result of volume shifts intra- and postoperatively.[25] This caused delays in postoperative recovery in 3 patients in our series. In one case, the rare complication of fungal pneumonia occurred after aspiration. Except for one case of generalized peritonitis, only the occurrence of localized peritonitis was observed. The patient with generalized peritonitis was first treated with repeated lavage; wound infection followed, but secondary wound closure could eventually be achieved, although the length of stay was extended in this case. For this particular aspect, conclusions are not possible due to the small number of cases.
Complicated jejunal diverticulitis is an entity that is difficult to diagnose. When therapy is delayed, further complications can have serious consequences. Thus, one should always include this disease in the list of differential diagnoses for unclear and acute abdomens, especially in elderly patients.
CONCLUSION
As a result of its rarity and localization as well as the large variation of symptoms, jejunal diverticulitis is often first diagnosed intraoperatively despite extensive preoperative diagnostics. The operative method chosen (tangential/transverse diverticulum resection versus segment resection) should be based on the extent of jejunal diverticulosis and general intraoperative findings as well as the diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tsiotos GG, Farnell MB, Ilstrup DM. Nonmeckelian jejunal or ileal diverticulosis: An analysis of 112 cases. Surgery. 1994;116:726–31. discussion 731-2. [PubMed] [Google Scholar]
- 2.Chugay P, Choi J, Dong XD. Jejunal diverticular disease complicated by enteroliths: Report of two different presentations. World J Gastrointest Surg. 2010;2:26–9. doi: 10.4240/wjgs.v2.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanrykel F, Pattyn P, Vuylsteke P, Smet B. Perforated jejunal diverticulitis: a rare presentation of acute abdomen. Acta Chir Belg. 2010;110:367–9. doi: 10.1080/00015458.2010.11680637. [DOI] [PubMed] [Google Scholar]
- 4.Fang M, Agha S, Lee R, Culpepper-Morgan J, D’Souza A. Perforation of jejunal diverticulum: Case report and review of literature. Conn Med. 2000;64:7–10. [PubMed] [Google Scholar]
- 5.Akhrass R, Yaffe MB, Fischer C, Ponsky J, Shuck JM. Small-bowel diverticulosis: Perceptions and reality. J Am Coll Surg. 1997;184:383–8. [PubMed] [Google Scholar]
- 6.Peters R, Grust A, Gerharz CD, Dumon C, Furst G. Perforated jejunal diverticulitis as a rare cause of acute abdomen. Eur Radiol. 1999;9:1426–8. doi: 10.1007/s003300050862. [DOI] [PubMed] [Google Scholar]
- 7.Christ A. Multiple Diverticula of the Jejunum. Langenbecks Arch Surg. 1932;236:560–70. [Google Scholar]
- 8.Baskin RH, Jr, Mayo CW. Jejunal diverticulosis: A clinical study of 87 cases. Surg Clin North Am. 1952:1185–96. doi: 10.1016/s0039-6109(16)33700-8. [DOI] [PubMed] [Google Scholar]
- 9.Garg N, Khullar R, Sharma A, Soni V, Baijal M, Chowbey P. Total laparoscopic management of large complicated jejunal diverticulum. J Minim Access Surg. 2009;5:115–7. doi: 10.4103/0972-9941.59311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huguenin A, Tirveilliot F, Dell’Erba U, Fabre S, Triboulet JP, Durand F. Acquired jejunal and ileal diverticula (Meckel's excluded) Ann Chir. 1999;53:522–6. [PubMed] [Google Scholar]
- 11.Chow DC, Babaian M, Taubin HL. Jejunoileal diverticula. Gastroenterologist. 1997;5:78–84. [PubMed] [Google Scholar]
- 12.Hamada N, Ishizaki N, Shirahama K, Nakamura N, Murata R, Kadono J, et al. Multiple duodeno-jejunal diverticula causing massive intestinal bleeding. J Gastroenterol. 2000;35:159–62. doi: 10.1007/s005350050030. [DOI] [PubMed] [Google Scholar]
- 13.Franke C, Grust A, Frieling T, Simon D. Jejunum diverticulosis-a rare cause of gastrointestinal hemorrhage. Zentralbl Chir. 2001;126:707–9. doi: 10.1055/s-2001-18237. [DOI] [PubMed] [Google Scholar]
- 14.Tankova L, Berberova M, Purvanov P, Tsankov Ts, Gegova A. Complicated small bowel diverticulosis-a case report and literature review. Chirurgia (Bucur) 2007;102:603–6. [PubMed] [Google Scholar]
- 15.Franzen D, Gurtler T, Metzger U. Solitary duodenal diverticulum with enterolith as a rare cause of acute abdomen. Swiss Surg. 2002;8:277–9. doi: 10.1024/1023-9332.8.6.277. [DOI] [PubMed] [Google Scholar]
- 16.Nightingale S, Nikfarjam M, Iles L, Djeric M. Small bowel diverticular disease complicated by perforation. ANZ J Surg. 2003;73:867–9. doi: 10.1046/j.1445-2197.2003.02792.x. [DOI] [PubMed] [Google Scholar]
- 17.Makris K, Tsiotos GG, Stafyla V, Sakorafas GH. Small intestinal non-Meckelian diverticulosis. J Clin Gastroenterol. 2009;43:201–7. doi: 10.1097/MCG.0b013e3181919261. [DOI] [PubMed] [Google Scholar]
- 18.Staszewicz W, Christodoulou M, Proietti S, Demartines N. Acute ulcerative jejunal diverticulitis: Case report of an uncommon entity. World J Gastroenterol. 2008;14:6265–7. doi: 10.3748/wjg.14.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelekis AD, Poletti PA. Jejunal diverticulitis with localized perforation diagnosed by ultrasound: A case report. Eur Radiol. 2002;12(Suppl 3):S78–81. doi: 10.1007/s00330-002-1414-2. [DOI] [PubMed] [Google Scholar]
- 20.Kleemann M, Kujath P, Stellmacher F, Gellissen J, Bruch HP, Eckmann Ch. Perforated jejunal divertikula - a rare differential diagnosis of acute abdominal pain. Zentralbl Chir. 2006;131:521–4. doi: 10.1055/s-2006-955454. [DOI] [PubMed] [Google Scholar]
- 21.Macari M, Faust M, Liang H, Pachter HL. CT of jejunal diverticulitis: Imaging findings, differential diagnosis, and clinical management. Clin Radiol. 2007;62:73–7. doi: 10.1016/j.crad.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Klar E, Stowhas M, Foitzik T. A surgical approach to acute intestinal bleeding. Chirurg. 2006;77:133–8. doi: 10.1007/s00104-005-1143-6. [DOI] [PubMed] [Google Scholar]
- 23.Ortner MA, Dorta G. Endoskopische diagnostik und therapie der gastrointestinalen blutung. Chirurg. 2006;77:111–6. doi: 10.1007/s00104-006-1151-1. [DOI] [PubMed] [Google Scholar]
- 24.Mossner J. Acute abdomen. Internist (Berl) 2005;46:974–81. doi: 10.1007/s00108-005-1455-0. [DOI] [PubMed] [Google Scholar]
- 25.Pereira ED, Fernandes AL, da Silva Ancao M, de Arauja Pereres C, Atallah AN, Faresin SM. Prospective assessment of the risk of postoperative pulmonary complications in patients submitted to upper abdominal surgery. Sao Paulo Med J. 1999;117:151–60. doi: 10.1590/s1516-31801999000400003. [DOI] [PubMed] [Google Scholar]


