Abstract
Apart from their association in familial and hereditary cancer syndromes, sporadic double malignancies of the gastrointestinal tract involving the ampulla of Vater and colon are extremely rare. Although synchronous resection of the two by adding a colectomy to a pancreaticoduodenctomy can be accomplished with minimal increase in the surgical morbidity, a few patients, however, are best managed by a staged resection. We report a case of sporadic double malignancy of the ampulla of Vater and right colon who despite the best attempts continued to bleed and remained malnourished and was successfully managed by staged right hemicolectomy followed by a pancreaticoduodenectomy.
Keywords: Ampulla of Vater, colon, neoplasms
Synchronous double malignancies of the gastrointestinal tract are rare. Although ampullary tumors are reported to occur in the presence of familial cancer syndromes such as familial adenomatous polyposis (FAP), hereditary non-polyposis colon cancer (HNPCC), and Gardner's syndrome, sporadic double malignancies involving the ampulla of Vater and colon are extremely uncommon.[1] Older age, environmental factors (such as diet and smoking), and adenoma-carcinoma sequence involving genetic mutations of oncogenes and tumor suppressor genes have been implicated as possible risk factors.[2] We report a patient of synchronous adenocarcinoma of the ampulla of Vater and ascending colon who was successfully managed by staged operations.
CASE REPORT
A 72-year-old man presented to our department with complaints of painless jaundice and passage of maroon-colored stools for the past 2 months. He also complained of pruritus, decreased appetite, and significant weight loss in the same period. There was no significant past or family history of malignancy of the bowel or the biliary tract. On examination, the patient was pale, icteric, and had bilateral pitting pedal edema. Abdominal examination was unremarkable except for mild hepatomegaly. His hematological and biochemical investigations showed a hemoglobin of 6.2 g/dL, bilirubin 6.8 mg/dL, alkaline phosphatase 1589 IU/L, and serum albumin 2.2 g/dL. On imaging with ultrasound followed by contrast-enhanced CT scan of the abdomen, bilateral intrahepatic biliary radical dilatation, dilated common bile duct up to the lower end, and a non-dilated main pancreatic duct were found [Figure 1a]. There was thickening of the right colon with multiple mesocolic lymph nodes and no evidence of liver metastases [Figure 1b]. A side-viewing duodenoscope demonstrated an ulcerated and bulky ampulla of Vater, the biopsy from which revealed a well-differentiated adenocarcinoma. On colonoscopy [Figure 2], a large constricting proliferative growth was detected in the ascending colon with a small pedunculated polyp about 2 cm proximal to it. The remaining colon was normal. Biopsy of the colonic growth revealed a moderately differentiated adenocarcinoma. His CEA and CA 19-9 levels were 5 (0–5.5 ng/mL) and 77.7 (0–39 IU/mL), respectively. A diagnosis of synchronous carcinoma of the colon and ampulla of Vater was made. In view of his suboptimal nutritional status, the patient was stented (with a plastic biliary stent) and started on high-protein diet supplemented with parenteral nutrition. However, despite 2 weeks of nutritional support, the serum albumin remained low and the patient continued to require blood transfusions due to bleeding from the colonic lesion. Hence, a decision was taken to perform a staged resection. A right hemicolectomy was done as the first operation via a midline incision. Histopathology revealed a multicentric moderately differentiated adenocarcinoma of the cecum (T3) and ascending colon (T2) with a tubular adenoma close to the ascending colonic lesion. The resection margins were free and two lymph nodes showed tumor metastases. Following discharge the patient had significant improvement in his nutritional status (a weight gain of 10 kg, improved serum albumin to 4.2 g/dL, and stabilization of hemoglobin) and was readmitted 6 weeks later for the second-stage surgery. A pancreaticoduodenectomy was done via the prior midline incision and the patient had an uneventful postoperative recovery. Histopathology of the resected specimen revealed a moderately differentiated adenocarcinoma of the ampulla (pT2). All the resection margins were free from tumor with no nodal metastases. Patient was started on 5-flurouracil and oxaliplatin-based chemotherapy. At the last follow-up of 8 months, patient is asymptomatic and disease-free.
Figure 1a.
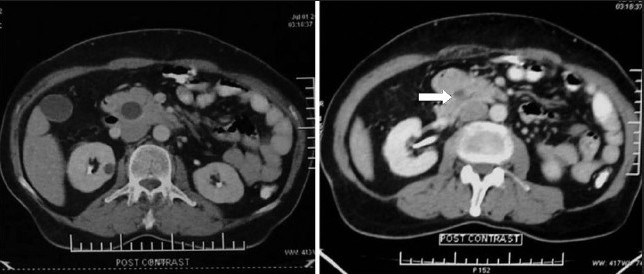
Contrast-enhanced CT abdomen showing distended gallbladder with dilated intrapancreatic common bile duct and an ampullary lesion (arrow)
Figure 1b.
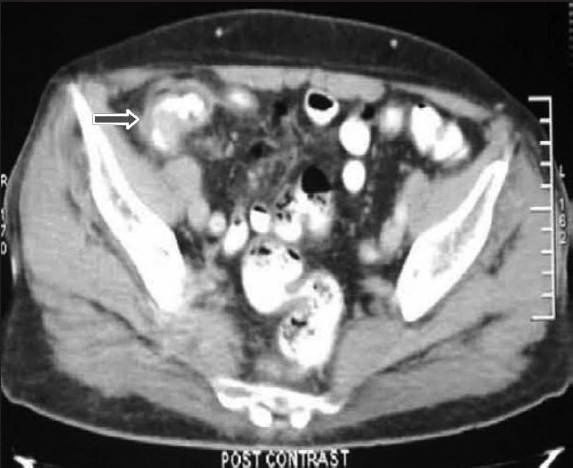
Contrast-enhanced CT scane of the abdomen showing mural thickening in the ascending colon (arrow)
Figure 2.
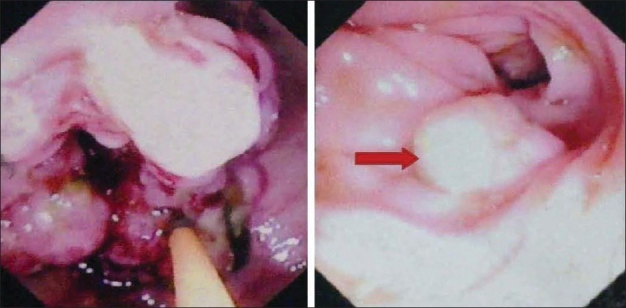
Colonoscopy showing ulceroproliferative lesion in the ascending colon with a polyp 2cm proximal to it (arrow)
DISCUSSION
Double malignancies of the gastrointestinal tract are very rare. In a review of more than 2000 patients, Minni et al. found the incidence of a second primary tumor in gastrointestinal tract to be 4.3%.[3] This association most often occurs in patients with familial cancer syndromes. Patients with hereditary colon cancer syndromes such as FAP, its attenuated variant, and HNPCC have an increased life time risk of developing cancer of the ampulla in addition to various other sites.[4]
Sporadic variants of both colonic and ampullary adenocarcinomas occur in a step-wise fashion involving activation of oncogenes (K-ras, Her-2/neu) and suppression of tumor suppressor genes (APC, DCC, p53, DPC4, BRCA2).[5,6] This results in a sequential transformation of the normal colonic and ampullary epithelial cells to an adenoma and finally carcinoma. The synchronous occurrence of the two malignancies in this patient may be explained by activation of a common oncogene or inhibition of a common tumor suppressor gene involved in this pathway. A recent study has also shown a higher risk of colorectal adenomas and malignancy in patients with sporadic duodenal adenomas.[7] In another analysis of the surveillance epidemiology and end results database, Das et al.[8] reported a two-fold increase in the risk for the development of colorectal cancer in a patient with ampullary malignancy and vice versa. Although a detailed genetic evaluation of this patient for MMR mutations would have been worthwhile, this facility was not available to us and hence it was not done.
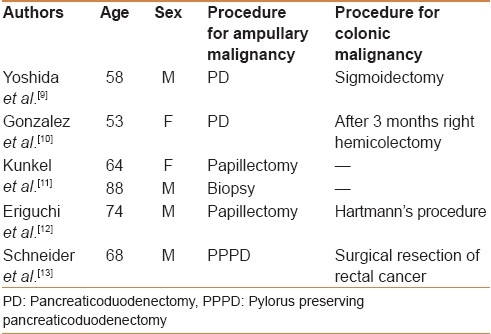
To the best of our knowledge, only six cases of sporadic synchronous ampullary and colorectal malignancies have been reported till date [Table 1]. The mean age of these patients was 67.5±12.4 years with predominance of males. The first case was reported in 1990 where sigmoid colon malignancy was incidentally detected during the surgical resection of an ampullary tumor.[9] Interestingly, this patient had two adenomas near the colonic lesion similar to our patient. This might favor a common adenoma-carcinoma hypothesis. In the majority of these case reports as tabulated, the two tumors were managed by simultaneous resections (pancreaticoduodenectomy/ampullectomy with colonic resection) which can be accomplished via the same incision. Similarly in our patient the initial plan was to improve the nutritional status and perform a simultaneous resection. However, despite 2 weeks of aggressive attempts to improve the nutritional and performance status, the serum albumin continued to remain low, and he had persistent bleeding from the colonic tumor with a drop in hemoglobin necessating blood transfusions. Hence a decision was taken to do a right hemicolectomy as the first part of the staged resection as this was thought to be of lesser magnitude than a pancreaticoduodenectomy, would prevent further blood loss (from the colonic tumor), and may allow the patient to be nutritionally optimized for a subsequent pancreaticoduodenectomy. As expected, the patient had remarkable improvement in his nutritional status and gained 10 kg weight over a period of 6 weeks. He could thus be taken up for a pancreaticoduodenectomy which could have been hazardous in the first stage. Although a staged resection (pancreaticoduodenectomy followed by a right hemicolectomy 3 weeks later) was performed by Gonzalez et al. in a similar situation, we believe that a patient who can undergo a major procedure such as a pancreaticoduodenectomy at the initial operation could have also undergone an additional right hemicolectomy in the same sitting, via the same incision, without substantially increasing the morbidity of the procedure.
Table 1.
Previous reports of sporadic synchronous ampullary and colorectal malignancies
To conclude, sporadic double malignancies are rare but should be kept in mind while evaluating a patient with cancer. Simultaneous resection is the preferred approach in most, however a staged procedure may be done in select high-risk surgical patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 2.Esposito I, Friess H, Büchler MW. Carcinogenesis of cancer of the papilla and ampulla: Pathophysiological facts and molecular biological mechanisms. Langenbecks Arch Surg. 2001;386:163–71. doi: 10.1007/s004230100232. [DOI] [PubMed] [Google Scholar]
- 3.Minni F, Casadei R, Marrano N, Guerra E, Piccoli L, Pagogna S, et al. Second tumours in patients with malignant neoplasms of the digestive apparatus. A retrospective study on 2406 cases. Ann Ital Chir. 2005;76:467–72. [PubMed] [Google Scholar]
- 4.Björk J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: Cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127–35. doi: 10.1053/gast.2001.28707. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER. Vogelstein B: A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 6.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, et al. Loss of expression of DPC4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–6. [PubMed] [Google Scholar]
- 7.Lagarde S, Dauphin M, Delmas C, Vitry F, Bouché O, Thiéfin G, et al. Increased risk of colonic neoplasia in patients with sporadic duodenal adenoma. Gastroenterol Clin Biol. 2009;33:441–5. doi: 10.1016/j.gcb.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Das A, Neugut AI, Cooper GS, Chak A. Association of ampullary and colorectal malignancies. Cancer. 2004;100:524–30. doi: 10.1002/cncr.11943. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida J, Morisaki T, Yamaguchi K, Itoh T, Yokohata K, Kishikawa H, et al. Carcinoma in adenoma of the ampulla of Vater synchronous with cancer of the sigmoid colon. Dig Dis Sci. 1990;35:271–5. doi: 10.1007/BF01536776. [DOI] [PubMed] [Google Scholar]
- 10.González Sánchez JA, López-Ríos Fernández F, González Conde R, Miguel Velasco JE, Casas Pinillos MS, Larrauri Martínez J. Carcinoma of the Vater's ampulla and colonic carcinoma with synchronous onset. Rev Esp Enferm Dig. 1993;83:459–61. [PubMed] [Google Scholar]
- 11.Kunkel D, Moreau X, de Jaureguiberry JP, Pouderoux P. Ampullar, peri-ampullar and colonic tumors: An association not to be disregarded? Apropos of 3 cases. Gastroenterol Clin Biol. 1990;14:512–3. [PubMed] [Google Scholar]
- 12.Eriguchi N, Aoyagi S, Tamae T, Nishimura K, Hamada S, Kawabata M, et al. Carcinoma of the ampulla of Vater associated with other organ malignancies. Kurume Med J. 2001;48:255–9. doi: 10.2739/kurumemedj.48.255. [DOI] [PubMed] [Google Scholar]
- 13.Schneider AR, Seifert H, Trojan J, Stein J, Hoepffner NM. Frequency of colorectal polyps in patients with sporadic adenomas or adenocarcinomas of the papilla of vater-an age matched, controlled study. Z Gastroenterol. 2005;43:1123–7. doi: 10.1055/s-2005-858628. [DOI] [PubMed] [Google Scholar]


