Abstract
Long-term treatment of hydrocephalus continues to be dismal. Shunting is the neurosurgical procedure more frequently associated with complications, which are mostly related with dysfunctions of the shunting device, rather than to mishaps of the rather simple surgical procedure. Overdrainage and underdrainage are the most common dysfunctions; of them, overdrainage is a conspicuous companion of most devices. Even when literally hundreds of different models have been proposed, developed, and tested, overdrainage has plagued all shunts for the last 60 years. Several investigations have demonstrated that changes in the posture of the subject induce unavoidable and drastic differences of intraventricular hydrokinetic pressure and cerebrospinal fluid (CSF) drainage through the shunt. Of all the parameters that participate in the pathophysiology of hydrocephalus, the only invariable one is cerebrospinal fluid production at a constant rate of approximately 0.35 ml/min. However, this feature has not been considered in the design of currently available shunts. Our experimental and clinical studies have shown that a simple shunt, whose drainage capacity complies with this unique parameter, would prevent most complications of shunting for hydrocephalus.
Keywords: Cerebrospinal fluid production, hydrocephalus, hydrocephalus treatment, intraventricular pressure, shunt overdrainage, siphon effect, ventriculoperitoneal shunts
INTRODUCTION
Most patients with hydrocephalus are treated by an extracranial bypass of cerebrospinal fluid (CSF) to the abdominal cavity; the prevention of overdrainage and underdrainage by this bypass is the obvious priority. Most shunts for hydrocephalus are based on a comprehensive variety of valve systems aimed to function according to intracerebral variations of hydrostatic pressure.[1,5,14,26,44,45,61] However, the middle term of viability for most shunting devices, including the most expensive, is about 2 years after surgical implantation; also, occasional surgical review for shunt dysfunction is common, even with devices that remain functional for longer periods.
Modern shunts for hydrocephalus use sophisticated mechanisms of valve opening and closing in accordance with variations of intracranial pressure, and some are equipped with ingenious anti-siphon devices.[5,28,30,33,38,40] Nevertheless, even with them, the phenomenon of overdrainage is still common.[3,11,14,23,32,35,46,55] Results of countless studies make apparent that the classical hydrokinetic parameters that have long been taken as reference for the theoretical design of ventriculoperitoneal shunts are not reliable for a system designed to drain CSF through an artificial pathway, which runs parallel to the cerebrospinal axis.
For the last 60 years, most devices used for relief of hydrocephalus have depended on the same physiological grounds however, the experience has shown that we are still far away from an ideal shunt.[2,8,9,18,25,26,37,43,51] It might be assumed that if, for several years, all attempts for technical solution of hydrocephalus have proved unsatisfactory, perhaps it is time to review the framework used for technological research, rather than continue designing devices with improvements in mechanisms but identical in theoretical framework.[3,9,14,20,32]
HYDROKINETIC CHARACTERISTICS OF VENTRICULOPERITONEAL SHUNTING
Most shunts used for the treatment of hydrocephalus communicate the ventricular cavity with the peritoneal cavity.[5,12,24,34,45] Several factors participate in their drainage capacity; however, some hydrokinetic characteristics of this peculiar pathway may not have been adequately considered in the design of currently used shunting devices.
When humans rise to the erect posture, there is a gravity gradient within the cerebrospinal axis that runs along a virtual line that measures in adults approximately 55 ± 5 cm from the floor of the lateral ventricle (where the proximal tip of a ventriculoperitoneal shunt for relief of hydrocephalus is placed) to the periumbilical peritoneal area (where the distal tip of the shunt is also placed). This virtual line is constantly moving according to the posture of the subject; it varies from the horizontal plane when the subject is lying down in supine position to the vertical plane when the subject is standing or sitting straight [Figure 1]; slight variations occur according to the multiple positions that the subject can adopt along the day, producing constant movements of this hypothetical line, in consonance with constant activity.[12,13,15] In general terms, with some inclined variations, humans maintain the ventriculoperitoneal line in the vertical position two-thirds of the day (while standing or sitting) and the other third is maintained in the horizontal position (while sleeping). An artificial bypass (such as any shunting device) between the ventricles and the peritoneum that intends the drainage of fluid from the former into the later is subject to the gravity force acting upon the flow according to the position of the ventriculoperitoneal line in regard to the earth's surface. The hydrostatic pressure gradient of the catheter is not dependent on its length, but rather on the vertical distance between the inlet and the outlet. The gravity force is so important that the so-called “siphon” effect, described in countless reports,[38,40,42,49,56] corresponds, by far, to the most intense hydrokinetic force acting upon the velocity of flow, and therefore upon the amount of fluid drained. In the laboratory, under experimental conditions, when a container (simulating the ventricles) is connected to a recipient (simulating the peritoneum) by a catheter 1 m long and of 1 mm internal diameter (ID), the following changes occur; if the container and the recipient are located at the same level and the connecting catheter lies horizontal, there is absence of flow from one to the other; in contrast, if the container is elevated 55 cm between the tips of the catheter in a vertical position, there is a flow of 80 ml/min to the recipient (115 l/day). This huge increase of flow rate is exclusively due to the suction effect caused by the gravitational attraction or siphon effect [Figure 1].
Figure 1.
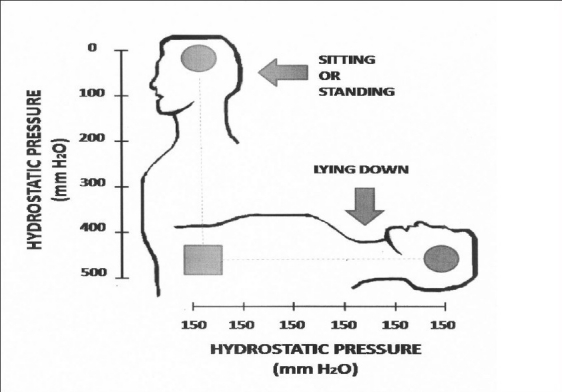
Physiological differences of hydrostatic pressure within the ventriculosubarchanoid axis according to the posture of the individual. When sitting or standing, a gradient of pressure develops in which there is absence of pressure in the ventricular cavity  but a maximal pressure of 500 ± 50 mm H2O in the lumbar area
but a maximal pressure of 500 ± 50 mm H2O in the lumbar area  . In contrast; when the subject lies down, the pressure is evenly distributed along the ventriculosubarachnoid axis with an identical mean value of 150 ± 50 mm H2O anywhere from the ventricular cavities to the lumbar area
. In contrast; when the subject lies down, the pressure is evenly distributed along the ventriculosubarachnoid axis with an identical mean value of 150 ± 50 mm H2O anywhere from the ventricular cavities to the lumbar area
The internal fluid pressure within the ventriculosubarachnoid space is highly variable according to the position of the subject: When the subject is lying down, the internal pressure is identical throughout the ventriculosubarachnoid axis at a mean pressure of 150 mm H2O (100–200 mm H2O) [Figure 1]. In contrast, the pressure changes to a differential gradient when the subject stands up; there is a pressure of zero mm H2O or even negative pressure at the top of the vertical axis (within the lateral ventricles) and simultaneously a maximal pressure of 500 ± 50 mm H2O at the bottom (within the lumbar area) [Figure 1]. This gradient develops as soon as the subject stands up or sits, moving the axis to its vertical position.[43,57] These differences can be demonstrated when, in the same individual, a lumbar puncture is made either in the supine posture or while he is sitting. The gravity force greatly modifies the topographical pressure of the fluid inside the ventriculosubarachnoid space when the subject stands; in sharp contrast, the pressure is evenly distributed and identical anywhere inside the space when the subject lies horizontally [Figure 1].
Another independent hydrokinetic force acting upon the flow is produced by the internal pressure generated by the equilibrium of production/absorption of CSF. The absorption takes place in large areas of the subarachnoid space. The capacity of these histological structures to absorb the CSF under normal circumstances largely exceeds its production rate, which takes place mostly at the choroid plexus, inside the ventricular system. This circumstance explains the fair homeostatic equilibrium achieved physiologically between production and absorption of CSF, which is mostly maintained by the combination of a constant rate of production and a vast capacity of fluid absorption. Hydrocephalus develops only after most sites of absorption have been blocked (communicating hydrocephalus) or a mechanical obstruction of CSF transit prevents the passage of CSF from the production sites in the choroid plexus to the absorption sites in the subarachnoid membrane (non-communicating hydrocephalus). Of all acting forces within the neural axis that influence fluid dynamics, the only steady parameter is the production of CSF,[7,10,14] whose output in humans is constant at a rate of 0.35 ml/min, for a total daily production of approximately 500 ml, with minimal variations under most physiological conditions.
DYSFUNCTIONS OF VENTRICULOPERITONEAL SHUNTS
Under normal circumstances, the ventriculosubarachnoid axis is a closed cavity. The insertion of a ventriculoperitoneal shunting device opens this space on its upper site and drains the fluid downward, directly into the peritoneum. This artificial circumstance greatly modifies the natural mechanisms of CSF dynamics. Excessive shunt drainage, the most frequent complication of shunting, generates intracranial hypotension accompanied by slit ventricles, which result from an unavoidable shift in the functioning of the shunt during postural changes of the patient. This shift goes from the effectively controlled flow when the subject is supine, in which the valve of the shunt maintains a ventricular pressure of 150 ± 50 mm H2O, as settled by the producer of the shunting device, to an abnormally high drainage induced by uncontrolled suction as soon as the upright position is assumed.[40,41] Although in the upright position the ventricular pressure might be zero mm H2O or even negative, the suction force produced by the siphon effect opens the valve and drains CSF regardless of ventricular hydrokinetic pressure. Theoretically, in the absence of intraventricular pressure, the valve of the shunt should be closed; however, the intense siphon effect produced by the gravity force acting upon the ventriculoperitoneal catheter in the vertical position exerts a negative suction force of –550 mm H2O,[57] sufficient by far to open the valve, whose manufacture is usually settled to open at a positive pressure of around 100 mm H2O. Once the valve is opened by the suction effect, the CSF is pulled down, the ventricular cavity is emptied, and the ventricles might collapse, thus inducing intracranial hypotension in which the slit ventricle syndrome could develop.[21,22,31]
Intracranial hypotension due to hydrostatic negative suction (siphon effect) is indeed the most relevant peril to any patient who receives a ventriculoperitoneal shunt, regardless of the etiology of hydrocephalus.[29,46,58] To prevent this unavoidable effect, countless anti-siphon devices or gravity-controlled valves have been designed.[2,4,19,20] Their function is the interruption of CSF flow when the hydrostatic suction exceeds the minimal intracranial pressure. Thus, these devices are directed to stop the transit of CSF when intraventricular pressure lowers below the setting value of the valve (100 mm H2O). However, they are not designed considering that in the erect posture the normal intraventricular pressure is well below 100 mm H2O and the valve might be opened not by the positive ventricular pressure but by the negative suction effect produced by gravity. In fact, under experimental simulations made in our laboratory, all valves settled to open at 100 mm H2O of positive pressure indeed are opened at the negative suction effect generated when the connecting catheter is lowered more than 100 mm H2O of vertical distance from the upper container (which in real conditions would be within the ventricular cavity) to the lower recipient (which in real conditions would be inside the peritoneal cavity).[52,53] Thus, it becomes evident that anti-siphon devices that close the shunting valve when the subject is erect[19,31] do not comply with the natural mechanisms of CSF circulation and present an unnatural separation from physiological parameters of fluid dynamics within the ventriculosubarchanoid axis.[22,27,48]
In addition to the above complications is the fact that the whole daily production of CSF in adults is about 500 ml. This amount of fluid can be promptly drained through any of the currently used shunt devices if the valve is maintained opened just a few minutes. Thus, under no circumstance, the fluid transit can be steadily constant for long periods through any of the currently used devices; most of the time the fluid is static inside the shunt and the actual flow occurs just only for brief moments, promptly returning to fluid stasis until new CSF is produced, accumulated, and expeditiously drained again, closing this unfavorable cycle. These long periods of fluid stasis might induce clots inside the catheters, particularly in cases where the CSF contains a high level of proteins.[50]
According to the experience gathered with the use of valvular shunting devices for the last 50 years and the list of complications common to most of them, various unnatural conditions of fluid generated by the switch of fluid transit and drainage through these devices may be blamed as the main source of shunt dysfunctions.
-
The valvular mechanism of all shunts produces an on/off phenomenon of fluid passage that is not seen in the physiology of CSF circulation. This fact indicates that the actual transit of CSF through common shunts is frequently interrupted and fluid stasis occurs during long periods, which in turn would favor shunt obstruction.
The catheter connecting the ventricular cavity with the peritoneal cavity usually has an ID of approximately 1 mm. The amount of fluid that can be drained through this catheter is very large indeed and largely exceeds the natural amount of CSF production in humans. However, the valvular mechanism and the constant extenuation of ventricular CSF interrupt this flow. The discrepancy between excessive drainage capacity of the shunt and the limited drainage requirement of the subject (a maximum of 500 ml/day) causes long periods of fluid stasis within the shunt, which might occlude the catheter by clots or facilitate retrograde bacterial contamination.[18]
-
The intraventricular pressure, which has been settled as the core parameter for the functioning of all currently used shunting devices, varies widely, under normal circumstances, from negative values (when upright) to a positive pressure of 150 ± 50 mm H2O (when supine) according to changes in the posture of the subject.[11,15,17,39] In sharp contrast, at the lumbar level, the CSF pressure also varies widely under normal circumstances; but in this case, it might vary from a pressure of 150 ± 50 mm H2O (when supine) to a pressure of 500 ± 50 mm H2O (when upright), according to changes in the posture of the subject.
The valve mechanism is supposed to control adequate drainage based exclusively on intraventricular pressure. However, when the subject is upright, the ventricular pressure is normal at zero mm H2O, but when he lies down, it is normal at 150 mm H2O.[44,46] Nonetheless, the inverse situation, i.e. a ventricular pressure of zero mm H2O when he lies down or 150 mm H2O when he is upright, both are abnormal. Of course, the mechanical valve cannot differentiate between these two rather different physiological situations.
The above considerations indicate that intraventricular pressure should not be considered as the core parameter for the functioning of a device whose only goal is to prevent the accumulation of CSF and to divert the excess of fluid that cannot be disposed or absorbed through the natural channels.[2,9,35,42,55]
CSF PRODUCTION: A SINGULAR PARAMETER
From all parameters that participate in the physiology of CSF, only one, i.e. the production of CSF, is constant and remains unaltered under most pathologies that induce hydrocephalus.[10,47] The only condition that has been reported to increase the amount of CSF production is papilloma of the choroid plexus. However, even in this case, hydrocephalus is rarely induced due to the very large capacity for CSF absorption common to all individuals.[2] Thus, it seems reasonable that if the only steady parameter in the rather complex physiology and dynamics of CSF is its production, at a constant rate of 0.35 ml/min, for an approximate amount of 500 ml/day, this singular value should be considered for the design of a device that intends to prevent its intracranial accumulation. This principle would apply for all pathologies that induce hydrocephalus. Surprisingly, there is no shunt device whose core mechanism is based in the enduring drainage of CSF according to the stable and predictable parameter of CSF production.
VENTRICULOPERITONEAL SHUNT DEVOID OF VALVULAR MECHANISMS
Our studies have tested the theoretical framework of a shunt devoid of valvular mechanisms whose drainage capacity would function according to the constant rate of CSF production (±0.35 ml/mm). Clinical and experimental results have been favorable.[3,34,52,53] In these studies, we eliminated all valvular mechanisms and the functioning of a ventriculoperitoneal bypass was dependent on the drainage capacity and fluid resistance generated solely by the peripheral catheter that goes from the skull to the peritoneum.
The usual ID of common catheters for ventriculoperitoneal shunts is about 1–2 mm; we substituted this measure for another, medical degree catheter made of Tygon (S-50-HL medical and surgical catheter, Saint-Gobain.com/USA), identical in length, but with a precise ID of 0.51 mm (0.021 inches).[56,57] This unique catheter was directly connected, in the traditional way, to a common ventricular catheter; no further mechanisms were introduced in this rather simple connection. Performance of this shunt in long-term studies in the experimental laboratory and in patients with hydrocephalus has been superior than in controls with valvular shunts.[3,53,54,57] Initial laboratory studies under hydrokinetic simulations of physiological conditions[52,53,57] tested several variations of ID of the connecting catheter as unique mechanism for fluid resistance; the precise measure of 0.51 mm ID was shown to comply, under all physiological variations, with the desired drainage capacity. The connecting catheter of very thin ID instead of the usual catheters of wide ID showed in the laboratory to have a daily drainage capacity of approximately 500 ml under conditions that simulated common parameters of intraventricular pressure and siphon effect in humans. This rather peculiar catheter takes advantage of the two principal hydrokinetic forces acting in the ventriculospinal axis of humans according to posture [Figure 2]. When the subject stands, the suction effect that the gravity force imposes constitutes the principal hydrokinetic force; it generates an uninterrupted flow range between 0.30 and 0.50 ml/min for a mean of 0.38 ± 0.08 ml/mm. In contrast, when the subject lies down, the principal hydrokinetic force acting upon the shunt is the intraventricular pressure and the mean flow through the catheter is 0.35 ± 0.06 ml/min [Figure 2]. The flow resistance provided by the narrow diameter of the catheter generates two advantages: first, a continuous flow; second, it prevents overdrainage as the daily amount of CSF that passes through the shunt leads to a daily drainage close to 500 ml/day. The negative suction force of siphon effect on the erect posture during two-thirds of the day plus the positive intraventricular pressure during the other third of the day, when the subject lies in the horizontal posture, accomplishes the total daily drainage goal of about 500 ml through this rather simple subcutaneous catheter with an ID of half-a-millimeter width. A remarkable fact was that neither overdrainage nor shunt occlusion was seen in our patients despite the thin ID of the catheter.[3,57] The parametric values obtained in the laboratory under experimental conditions simulate human physiology which might slightly vary according to modifications of intraventricular hydrokinetic conditions; if the ventricular pressure diminishes, the amount of drainage decreases and vice versa. Thus, under limited variations of intraventricular pressure, the amount of fluid drained through the open shunt would be compensated by slight and temporary increase or decrease of flow rate, still maintaining an uninterrupted flow. The cerebrospinal flow through this open bypass maintains the homeostasis of CSF production and allows the endurance of whatever remnant mechanisms of fluid absorption are still viable in an individual case because after most experimental variations, the shunt maintains a constant flux, although the amount varies according to flow velocity. The slim ID of the catheter prevents a large or sudden flow, even in cases of positive intraventricular pressure and active siphoning effect. Although the peritoneal cavity, where outlet tip of the shunt is located, maintains a negative pressure which exerts a slight suction effect, it is not significant upon the amount of CSF drainage. We think that this feature aids in the constant flow achieved through this ventriculoperitoneal catheter. In long-term studies and under special circumstances, like hydrocephalus secondary to tumors of the posterior fossa, the open shunt functioned far better than valvular commercial shunts and no case of excessive drainage was observed.[3,6,34,57] This open shunt is not useful in newborn patients with congenital hydrocephalus as the vertical posture is not constant and most of the time the baby remains in decubitus. In these cases, this shunt will be adequate when the child maintains long periods of erect posture (approximately at the age of 3 years). However, our experience has been limited to adult subjects; due to their short stature, infants and small children do not siphon much and the length of the catheter might be shortened, thus diminishing flow resistance that would increase the amount of drainage. Nonetheless, this circumstance in small children remains to be studied. The results have shown that a simple shunt consisting on a peripheral subcutaneous catheter of approximately 80 cm length with a highly precise measure of 0.51 mm (0.021 inches) ID, connecting the ventricular with the peritoneal cavities, combines the two hydrokinetic forces that participate in an alternative manner, depending on the posture of the subject, in a shunt that connects the ventricular cavity with the peritoneal cavity. The gravity effect acts as a negative suction force and the intraventricular pressure acts as a positive injecting force. In our experiments, the precise measure of ID was so important that minimal variations led, under physiological simulations, either to underdrainage or to overdrainage. Minimal variations of ID in the catheter, from the optimal measure of 0.51 mm to either 0.45 or 0.60 mm ID, produced a total amount of flow that was significantly minor or larger (350 and 720 ml/day, respectively) than the physiologically ideal amount of 500 ml/day.
Figure 2.
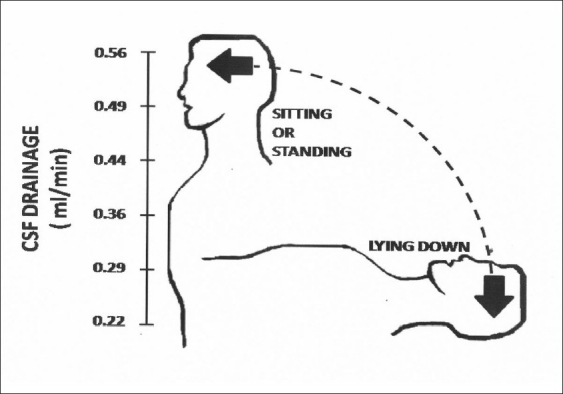
Variable flow through a peritoneal catheter 800 mm long and of 0.51 mm (0.021 inches) internal diameter, connecting subcutaneously the ventricular (V) with the peritoneal (P) cavities. When the subject lies down, the main draining power is the intraventricular pressure, whereas the gravitational force (siphon effect) is absent. In contrast, when the subject is erect, the main draining power is the gravitational force, whereas the intraventricular pressure is minimal. With their combination, a mean of 500 ml of cerebrospinal fluid is daily drained (Sotelo J. et al. Surg Neurol 2005;63:197-203, with permission)
This rather simple shunt provides various advantages; it does not induce excessive drainage; it generates uninterrupted flow that complies with the physiological circulation of CSF; it is devoid of mechanical intricacies, like valvular mechanisms. The peripheral catheter, crucial for the functioning of the shunt, can be easily replaced or substituted as it is subcutaneously inserted and connected in the skull to a common ventricular catheter. This “shunt” (which actually is a medical catheter) was developed initially for hydrocephalus secondary to cysticercosis; however, after the initial results, it was tested in a comprehensive variety of hydrocephalus in adults and proved to be very effective.[2,3,52,53,54,56,57] The pathophysiology of hydrocephalus due to cysticercosis is very severe (mean survival after diagnosis 1.8 years) and the CSF shows indeed a high content of cells and proteins. That is why all common shunts are obstructed soon after surgery. This “shunt” was not occluded because it did not induce retrograde passage of CSF from the subarachnoid space (high proteins and cells) to the ventricles (low proteins and cells, even in severe cases of cysticercosis), which is a common feature in cases of overdrainage.[50] As documented, overshunting was not seen with the use of this “shunt” (catheter?).[52,53] It is important to stress that this manuscript does not promote any shunt; it mostly gives arguments that provide potential explanations for the failure of all available shunts.
The catheter used in these studies is commercially available and commonly used in countless medical applications (Tygon S-50-HL, medical and surgical catheter; Saint-Gobain.com/USA) with an ID of 0.021 inches. Moreover, the shunt can be assembled with any high-performance catheter of medical degree with the precise ID of 0.021 inches (0.51 mm). I think that no company would be interested in its commercialization (including us) because it would cost something around 25 USD (vs. about 2300 USD of some new shunts).[3,6,56,57] I believe that the industry around modern medicine is not attracted by this kind of investments. Nonetheless, the intention of this article is to make a proposal for research (a shunt based on CSF production, rather than on intraventricular pressure) and use our experience to support this proposal.
A PROPOSAL FOR THE DEVELOPMENT OF A NOVEL SHUNT FOR HYDROCEPHALUS
Considering that all shunting devices whose functioning is based on a valve that responds to internal hydrostatic pressure have failed after six decades of countless designs and that in the opinion of most experts, we are still far from achieving an ideal shunt,[2,6,16,23,25,32,42,51,59,60,62] it might be time to reconsider the fundamental principles upon which all technological research for shunts has been grounded and adopt a novel stratagem. An innovative model could be based on CSF production,[10,47] rather than on hydrostatic pressure.[6,53] My proposal would be a shunting device, non-dependent on variations of hydrostatic pressure, with drainage capacity near 0.35 ml/min, to produce an uninterrupted flow, regardless of variations of posture of the patient or ventricular hydrostatic pressure. In cases of non-communicating hydrocephalus, such as in patients with aqueduct stenosis, this hypothetical shunt would be inserted into the ventricular cavity, whereas in cases of communicating hydrocephalus such as chronic arachnoiditis or fibrosis, the shunt could be inserted anywhere in the ventriculosubarachnoid axis; for instance, it could be placed in the lumbar area eluding the introduction of a ventricular catheter through the brain tissue.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/40/94292.
REFERENCES
- 1.Aihara Y, Kawamata T, Mitsuyama T, Hori T, Okada Y. Novel method for controlling cerebrospinal fluid flow and intracranial pressure by use of a tandem shunt valve system. Pediatr Neurosurg. 2010;1:12–8. doi: 10.1159/000314052. [DOI] [PubMed] [Google Scholar]
- 2.Arriada N, Sotelo J. Review: Treatment of hydrocephalus in adults. Surg Neurol. 2002;58:377–84. doi: 10.1016/s0090-3019(02)00894-7. [DOI] [PubMed] [Google Scholar]
- 3.Arriada N, Sotelo J. Continuous-flow shunt for treatment of hydrocephalus due to lesions of the posterior fossa. J Neurosurg. 2004;101:762–6. doi: 10.3171/jns.2004.101.5.0762. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff A, Kremer P, Fruh K, Hashemi B, Kunze S. Orbis-sigma valve.Results of 4 long-term-tested exemplars and a critical comment on the concept of the socalled flow-controlled valves. Child Nerv Syst. 1994;10:474–5. [Google Scholar]
- 5.Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22:67–93. doi: 10.1007/s101430050035. [DOI] [PubMed] [Google Scholar]
- 6.Ausman JI. Editorial. Surg Neurol. 2005;63:193–4. [Google Scholar]
- 7.Bergsneider M. Evolving concepts of cerebrospinal fluid physiology. Neurosurg Clin N Am. 2001;36:631–8. [PubMed] [Google Scholar]
- 8.Blount JP, Campbell JA, Haines SJ. Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin N Am. 1993;4:633–56. [PubMed] [Google Scholar]
- 9.Bonkowski J, Drake JM, Sainte-Rose C, Tenti G, Portnoy HD, Foltz L. Shunts: Are they flow-controlled, pressure-controlled, or neither? Surg Neurol. 1999;51:105–9. [Google Scholar]
- 10.Bradbury MW. Anatomy and physiology of cerebrospinal fluid. In: Schurr PH, Polkey CE, editors. Hydrocephalus. New York: Oxford University Press; 1993. pp. 19–47. [Google Scholar]
- 11.Czosnyka M, Czosnyka Z, Whitehouse H, Pickard JD. Hydrodynamic properties of hydrocephalus shunts: United Kingdom shunt evaluation laboratory. J Neurol Neurosurg Psychiatry. 1997;62:43–50. doi: 10.1136/jnnp.62.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czosnyka Z, Czosnyka M, Richards HK, Pickard J. Posture-related overdrainage: Comparison of the performance of 10 hydrocephalus shunts in vitro. Neurosurgery. 1998;42:327–34. doi: 10.1097/00006123-199802000-00069. [DOI] [PubMed] [Google Scholar]
- 13.De Stefani A, De Risio L, Platt SR, Matiasek L, Lujan-Feliu-Pascual A, Garosi LS. Surgical technique, postoperative complications and outcome in 14 dogs treated for hydrocephalus by ventriculo/peritoneal shunting. Vet Surg. 2011;40:183–91. doi: 10.1111/j.1532-950X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 14.Drake JM, Sainte-Rose C, editors. Cambridge: Blackwell Science; 1995. The shunt book. [Google Scholar]
- 15.Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76:207–11. doi: 10.3171/jns.1992.76.2.0207. [DOI] [PubMed] [Google Scholar]
- 16.Foltz EL, Blanks JP. Symptomatic low intracranial pressure in shunted hydrocephalus. J Neurosurg. 1988;68:401–8. doi: 10.3171/jns.1988.68.3.0401. [DOI] [PubMed] [Google Scholar]
- 17.Frim DM, Goumnerova LC. In vivo intracranial pressure dynamics in patients with hydrocephalus treated by shunt placement. J Neurosurg. 2000;92:927–32. doi: 10.3171/jns.2000.92.6.0927. [DOI] [PubMed] [Google Scholar]
- 18.Fulkerson DH, Vachhrajani S, Bohnstedt BN, Patel AJ, Fox BD, Jea A, et al. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2011;7:147–51. doi: 10.3171/2010.11.PEDS10244. [DOI] [PubMed] [Google Scholar]
- 19.Gruber RW, Roehrig B. Prevention of ventricular catheter obstruction and slit ventricle syndrome by the prophylactic use of the Integra antisiphon device in shunt therapy for pediatric hypertensive hydrocephalus: A 25-year follow-up study. J Neurosurg Pediatr. 2010;5:4–16. doi: 10.3171/2008.7.17690. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Gonzalez R, Rivero-García M, Marquez-Rivas J. Ventriculovascular shunts via the femoral vein: A temporary feasible alternative in pediatric hydrocephalus. J Pediatr Surg. 2010;45:2274–7. doi: 10.1016/j.jpedsurg.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Haberl EJ, Messing-Juenger M, Schuhmann M, Eymann R, Cedzich C, Fritsch MJ, et al. Experiences with a gravity-assisted valve in hydrocephalic children: Clinical article. J Neurosurg Pediatr. 2009;4:288–93. doi: 10.3171/2009.4.PEDS08204. [DOI] [PubMed] [Google Scholar]
- 22.Kaestner S, Kruschat S, Nitzsche N, Deinsberger W. Gravitational shunt units may cause under-drainage in bedridden patients. Acta Neurochir (Wien) 2009;151:217–21. doi: 10.1007/s00701-009-0215-7. [DOI] [PubMed] [Google Scholar]
- 23.Kang JK, Lee IW. Long-term follow-up of shunting therapy. Child Nerv Syst. 1999;15:711–7. doi: 10.1007/s003810050460. [DOI] [PubMed] [Google Scholar]
- 24.Kelley R, Duong DH, Locke GE. Characteristics of ventricular shunt malfunctions among patients with neurocysticercosis. Neurosurgery. 2002;50:757–62. doi: 10.1097/00006123-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kestle J, Milner R, Drake J. The shunt design trial: Variation in surgical experience did not influence shunt survival. Pediatr Neurosurg. 1999;30:283–7. doi: 10.1159/000028812. [DOI] [PubMed] [Google Scholar]
- 26.Korinek AM, Fulla-Oller L, Boch AL, Golmard JL, Hadiji B, Puybasser L. Morbidity of ventricular cerebrospinal fluid shunt surgery in adults: An 8-year study. Neurosurgery. 2011;68:985–95. doi: 10.1227/NEU.0b013e318208f360. [DOI] [PubMed] [Google Scholar]
- 27.Kurtom KH. Magram Gary.Siphon regulatory devices: Their role in the treatment of hydrocephalus. Neurosurg Focus. 2007;22:1–7. doi: 10.3171/foc.2007.22.4.6. [DOI] [PubMed] [Google Scholar]
- 28.Lavinio A, Harding S, Van Der Boogaard F, Czosnyka M, Smielewski P, Richards HK, et al. Magnetic field interactions in adjustable hydrocephalus shunt. J Neurosurg Pediatr. 2008;2:222–8. doi: 10.3171/PED/2008/2/9/222. [DOI] [PubMed] [Google Scholar]
- 29.Lee P, DiPatri AJ., Jr Evaluation of suspected cerebrospinal fluid shunt complications in children. Clin Pediatr Emerg Med. 2008;9:76–82. [Google Scholar]
- 30.Lee WC, Seo DH, Choe IS, Ha YS, Lee KC. A comparative result of Ventriculo/peritoneal shunt, focusing mainly on gravity-assisted valve and programmable valve. J Korean Neurosurg Soc. 2010;48:251–8. doi: 10.3340/jkns.2010.48.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima-Maldonado I, Valery CA, Boch AL. Shunt dependence: Myths and facts. Acta Neurochir (Wien) 2010;152:1449–54. doi: 10.1007/s00701-009-0587-8. [DOI] [PubMed] [Google Scholar]
- 32.Lo P, Drake J. Shunt malfunctions. Neurosurg Clin N Am. 2001;36:695–701. [PubMed] [Google Scholar]
- 33.Lollis SS, Mamourian AC, Vaccaro TJ, Duhaime AC. Programmable cerebrospinal fluid shunt valves: Radiographic identification and interpretation. Am J Neuroradiol. 2010;31:1343–6. doi: 10.3174/ajnr.A1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-González MA, Benita-Bordes A, Izurrieta-Ulloa M, Sotelo-Morales J. Análisis preliminar de un nuevo sistema de derivación para el tratamiento de hidrocefalia. Arch Neurosci Mex. 1998;3:25–30. [Google Scholar]
- 35.McGirt MJ, Leveque JC, Wellons JC, Villavicencia At, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: A seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–55. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 36.Meier U, Kiefer M, Sprung C. Evaluation of the Miethke dual-switch valve in patients with normal pressure hydrocephalus. Surg Neurol. 2004;61:119–28. doi: 10.1016/j.surneu.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Miller JP, Fulop SC, Dashti SR, Robinson S, Cohen AR. Rethinking the indications for the Ventriculoperitoneal shunt tap. J Neurosurg Pediatr. 2008;1:435–8. doi: 10.3171/PED/2008/1/6/435. [DOI] [PubMed] [Google Scholar]
- 38.Momani L, Al-Nuaimy W, Al-Jumaily M, Mallucci C. A mechatronic valve in the management of hydrocephalus: Methods and performance. Med Biol Eng Comput. 2011;49:121–32. doi: 10.1007/s11517-010-0716-9. [DOI] [PubMed] [Google Scholar]
- 39.Munshi I, Lathrop D, Madsen JR, Frim DM. Intraventricular pressure dynamics in patients with ventriculopleural shunts: A telemetric study. Pediatr Neurosurg. 1998;28:67–9. doi: 10.1159/000028623. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima K, Oishi A, Itokawa H, Fujimoto M. Effect of magnetic fields from home-use magnetic induction theraphy apparatuses on adjustable cerebrospinal fluid shunt valves. No Shinkei Geka. 2010;38:725–9. [PubMed] [Google Scholar]
- 41.Notarianni C, Vannemreddy P, Caldito G, Bollam P, Wylem E, Willis B, et al. Congenital hydrocephalus and ventriculoperitoneal shunts: Influence of etiology and programmable shunts on revisions. J Neurosurg Pediatr. 2009;4:547–52. doi: 10.3171/2009.7.PEDS08371. [DOI] [PubMed] [Google Scholar]
- 42.O’Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Christopher Wallace M. Shunt-dependent hydrocephalus after aneurismal subarachnoid hemorrhage: Incidence, predictors and revision rates – Clinical article. J Neurosurg. 2009;111:1029–35. doi: 10.3171/2008.9.JNS08881. [DOI] [PubMed] [Google Scholar]
- 43.Park J, Kim GJ, Hwang SK. Valve inclination influences the performance of gravity-assisted valve. Surg Neurol. 2007;68:14–8. doi: 10.1016/j.surneu.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 44.Pollack I, Albright L, Adelson PD. A randomized controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Neurosurgery. 1999;45:1399–411. doi: 10.1097/00006123-199912000-00026. [DOI] [PubMed] [Google Scholar]
- 45.Pudenz RH. The surgical treatment of hydrocephalus: An historical review. Surg Neurol. 1981;15:15–26. doi: 10.1016/s0090-3019(81)80084-5. [DOI] [PubMed] [Google Scholar]
- 46.Pudenz RH, Foltz EL. Hydrocephalus: Overdrainage by ventricular shunts.A review and recommendations. Surg Neurol. 1991;35:200–12. doi: 10.1016/0090-3019(91)90072-h. [DOI] [PubMed] [Google Scholar]
- 47.Rekate H, Olivero W. Current concepts of CSF production and absorption. In: Scott M, editor. Hydrocephalus. Baltimore: Williams and Wilkins; 1990. pp. 11–22. [Google Scholar]
- 48.Rekate HL. Antisiphon device. J Neurosurg Pediatr. 2010;5:1–3. doi: 10.3171/2009.8.00249. [DOI] [PubMed] [Google Scholar]
- 49.Rohde V, Haberl EJ, Ludwig H, Thomale UW. First experiences with an adjustable gravitational valve in childhood hydrocephalus: Clinical article. J Neurosurg Pediatr. 2009;3:90–3. doi: 10.3171/2008.11.PEDS08154. [DOI] [PubMed] [Google Scholar]
- 50.Rubalcava MA, Sotelo J. Differences between ventricular and lumbar Cerebrospinal Fluid in hydrocephalus secondary to cysticercosis. Neurosurgery. 1995;37:668–72. doi: 10.1227/00006123-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Stein SC, Guo W. A mathematical model of survival in a newly inserted ventricular shunt. J Neurosurg (Suppl Pediatrics) 2007;107:448–54. doi: 10.3171/PED-07/12/448. [DOI] [PubMed] [Google Scholar]
- 52.Sotelo J. A new Ventriculoperitoneal shunt for treatment of hydrocephalus.Experimental results. Eur J Biomed Tech RBM. 1993;15:257–62. [Google Scholar]
- 53.Sotelo J, Rubalcava MA, Gomez-Llata S. A new shunt for hydrocephalus that relies on cerebrospinal fluid production rather than on ventricular pressure: Initial clinical experiences. Surg Neurol. 1995;43:324–32. doi: 10.1016/0090-3019(95)80057-n. [DOI] [PubMed] [Google Scholar]
- 54.Sotelo J. Update: The new ventriculoperitoneal shunt at the institute of neurology of Mexico. Surg Neurol. 1996;46:19–20. doi: 10.1016/0090-3019(96)00098-5. [DOI] [PubMed] [Google Scholar]
- 55.Sotelo J. Shunts: Which one, and why? Surg Neurol. 1998;49:8–13. doi: 10.1016/s0090-3019(97)00439-4. [DOI] [PubMed] [Google Scholar]
- 56.Sotelo J, Izurieta M, Arriada N. Treatment of hydrocephalus in adults by placement of an open ventricular shunt. J Neurosurg. 2001;94:873–9. doi: 10.3171/jns.2001.94.6.0873. [DOI] [PubMed] [Google Scholar]
- 57.Sotelo J, Arriada N, Lopez MA. Ventriculoperitoneal shunt of continuous flow vs valvular shunt for treatment of hydrocephalus in adults. Surg Neurol. 2005;63:197–203. doi: 10.1016/j.surneu.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 58.Toledo E, Eynan N, Shalit M. Intracranial hypotension: An iatrogenic complication of vacuum drainage systems. Acta Neurochir (Wien) 1980;52:55–9. doi: 10.1007/BF01400947. [DOI] [PubMed] [Google Scholar]
- 59.Trost HA. Is there a reasonable differential indication for different hydrocephalus shunt systems? Child Nerv Syst. 1995;11:189–92. doi: 10.1007/BF00277652. [DOI] [PubMed] [Google Scholar]
- 60.Vassiyadi M, Tataryn ZL, Alkherayf F, Udjus K, Ventureyra EC. The necessity of shunt series: Clinical article. J Neurosurg Pediatr. 2010;6:468–73. doi: 10.3171/2010.8.PEDS09557. [DOI] [PubMed] [Google Scholar]
- 61.Weinzierl MR, Rohde V, Gilsbach JM, Korinth M. Management of hydrocephalus in infants by using shunts with adjustable valves. J Neurosurg Pediatr. 2008;2:14–8. doi: 10.3171/PED/2008/2/7/014. [DOI] [PubMed] [Google Scholar]
- 62.Williams MA, McAllister JP, Walker Ml, Kranz DA, Bergsneider M, Del Bigio MR, et al. Priorities for hydrocephalus research: Report from a National Institutes of Health sponsored workshop. J Neurosurg. 2007;107(Suppl 5):S345–57. doi: 10.3171/PED-07/11/345. [DOI] [PubMed] [Google Scholar]


