Abstract
Objective:
To assess brain development and brain injury in neonates with cyanotic and acyanotic congenital heart disease (CHD).
Methods:
The study included 52 term infants with CHD who were divided into two groups: Cyanotic (n=21) and acyanotic (n=31). Fifteen healthy neonates of matched age and sex were enrolled in the study as controls. Three-dimensional proton magnetic resonance spectroscopic imaging and diffusion tensor imaging were used to assess brain development and injury. We calculated the ratio of N-acetylaspartate (NAA) to choline (which increases with maturation), average diffusivity (which decreases with maturation), fractional anisotropy of white matter (which increases with maturation), and the ratio of lactate to choline (which increases with brain injury).
Results:
As compared with control neonates, those with CHD had significant decrease in NAA/choline ratio (P<0.001), significant increase in lactate/choline ratio (P<0.0001), significant increase in average diffusivity (P<0.0001), and significant decrease of white matter fractional anisotropy (P<0.001). Neonates with cyanotic CHD had significant less brain development and more brain injury than those with acyanotic CHD (P<0.05).
Conclusions:
Newborn infants with cyanotic and acyanotic CHD are at high risk of brain injury and impaired brain maturity.
Keywords: Brain development, brain injury, congenital heart disease, neonates
INTRODUCTION
Congenital heart disease (CHD) has been reported to occur in 5 to 8 per 1000 live births.[1] Brain injuries occur with high frequency in newborns with CHD.[2] Although most forms of CHD are now amenable to early surgical repair, deficits that impair widespread neurodevelopmental domains are identified in up to half of childhood survivors. It is not until school age that the full extent of neurological sequelae becomes apparent and the rapid pace of innovation in neonatal cardiac surgery prevents timely evaluation of changes in care.[3]
Although magnetic resonance imaging (MRI) shows focal brain injuries acquired before or after surgery,[4,5] the extent of these lesions may not account for global impairments in development that are seen later in childhood.[6,7] In normal neonates, N-acetylaspartate (NAA), a marker of neuronal integrity, increases with increasing maturity, whereas deceased NAA indicates impaired cerebral integrity or function.[4] Cerebral lactate, a marker of anaerobic metabolism, increases after hypoxic ischemic brain injury in newborn infants, thus suggesting its usefulness in detecting early brain injury.[8,9] Quantitative MRI is emerging as a powerful tool for the investigation of early brain development. Three-dimensional proton magnetic resonance spectroscopic imaging (MRSI) with specialized lactate-editing overcomes the limitation of conventional, single-voxel MRS with the use of a point-resolved spectroscopic sequence to acquire spatially resolved MRS data over most of the brain with a spatial resolution of 1 cm.[10–12] The lactate-editing MRSI technique detects and localizes the distributions of cerebral metabolites (N-acetylaspartate (NAA), choline (Ch), and lactate) through newborn's brain. Diffusion tensor imaging (DTI), an advanced MRI technique, characterizes the three-dimensional spatial distribution of water diffusion in each voxel of MRI scan, providing a sensitive measure of regional microstructural development.[13] With increasing brain maturity, average diffusivity decreases,[14] owing to a decrease in water content and to the development of membranes in neuronal and glial cells.[15] Fractional anisotropy, a measure of the directionality of water diffusion, increases with the maturation of white matter.[16,17] The aim of this study was to assess brain development and brain injury in neonates with cyanotic and acyanotic CHD with the use of MRSI.
MATERIALS AND METHODS
A prospective study was conducted at our neonatal intensive care unit between October 2008 and September 2010. The study was approved by the ethics committee of the hospital. During the study period, 52 term (> 37 weeks gestational age) infants with CHD were enrolled in the study. They were divided into two groups: cyanotic (group I, n=21) and acyanotic (group II, n=31). Moreover, fifteen healthy term neonates of matched age and sex were included in the study as controls. The control group was recruited at a well-baby newborn nursery and with an Apgar score of 8-10 at 5 min, a birth weight appropriate for age, and no history of prenatal or perinatal complications. Written informed consent was obtained from all parents of patients and controls before the study. Neonates were excluded if they had intrauterine growth retardation, suspected congenital infection, genetic malformation syndrome, multiple congenital malformations associated with cardiac defects, perinatal asphyxia or CNS malformations.
Echocardiographic studies
Echocardiography was performed by a pediatric cardiologist. A Philips Sonos 5500 with 7.5-MHz transducer echo machine was used. Echo was done within 6 h after birth concerning morphological findings, anatomical defects, echo Doppler and color-flow mapping. M mode study was performed concerning cardiac dimensions and left ventricular systolic functions. Neonates proved to have persistent pulmonary hypertension of the newborn were excluded. Electrocardiogram (ECG) was done at the same time of echocardiographic examination. ECG included all standard leads was performed using a direct writing Hewlett Packard machine.
MRI studies
MRI studies were performed as soon as the baby could be safely transported to MRI scanner with the use of a specialized MRI-compatible isolette, which included a dedicated neonatal head coil.[18] Images were obtained after feeding without use of sedation. A radiologist who was unaware of all clinical information except for age and cardiac diagnosis scored each MRI scan for focal, multifocal, or global changes.
Three-dimensional proton magnetic resonance spectroscopic imaging
Magnetic resonance spectroscopic imaging (MRSI) was done and spectra were analyzed off-line using MRUI software with voxels (1 cm3) centered bilaterally on white matter (frontal, posterior, perirolandic, and optic radiation) and gray matter (basal ganglia, thalamus, and calcarine region). These regions were chosen because of their previously demonstrated sensitivity to hypoxic ischemic injury in neonates.[4] The lactate-editing MRS scheme provided the accurate detection of lactate as well as choline and NAA.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) was performed with the use of a sequence for the same regions assessed by MRS with fractional anisotropy calculated from white matter regions. Images were acquired in 4.8min with the use of multi-repetition, single-shot echo planner sequence with six gradient directions, with a diffusion weighting of 700 s/mm2(b value) and an image without diffusion weighting. The diffusion tensor describes an ellipsoid in space, with size, shape, and orientation given by the “maximum”, “intermediate”, and “minimum” eigenvalues and their corresponding eigenvectors. The maximum eigenvalue reflects axial diffusion, such as that parallel to organized white-matter tracts. In contrast, the intermediate and minimum eigenvalues reflect radial diffusion, perpendicular to white-matter tracts. Average diffusivity reflects the mean of these eigenvalues, expressed as 10−3mm2/s, whereas fractional anisotropy reflects their variance (higher fractional anisotropy with increasing variance). Given the high spatial resolution, same regions of interest were smaller than those used for MRSI to separate white and gray matter as much as possible.[19]
Statistical analysis
Quantitative data were expressed as mean and standard deviations (SD). Student “t” test was used to compare mean values of two groups. The significance of comparison between more than two groups was performed by ANOVA for parametric continuous variables. Simple Pearson correlation coefficient was calculated to quantify the correlation between continuous variables. Qualitative data were expressed as number and percentage and were tested by Chi-square (χ2) and Fisher exact tests. P value < 0.05 was considered as significant. Statistical analysis was performed using SPSS software.
RESULTS
A total of 52 newborn infants with CHD were included in the study. Patients were subdivided into 2 groups: Group I included 21 neonates with cyanotic CHD and group II included 31 neonates with acyanotic CHD. Group I comprised 8 patients (38.1%) with transposition of great arteries, 6 (28.6%) with Fallot's tetralogy, 4 (19%) diagnosed as total anomalous pulmonary venous return and 3 (14.3%) had tricuspid atresia. In group II, 15 neonates (48.4%) had VSD, 8 (25.8%) had critical pulmonary stenosis 5 (16.1%) had isolated PDA, and 3 (9.7%) were diagnosed as aortic stenosis. We calculated the overall severity of illness in newborns with congenital heart disease with the use of the Score for Neonatal Acute Physiology-Perinatal Extension (SNAP-PE), in which scores range from 0 to 70, with higher scores indicating a greater severity of illness.[20] The clinical characteristics of patients and controls are shown in Table 1.
Table 1.
Characteristics of newborns with congenital heart disease and controls
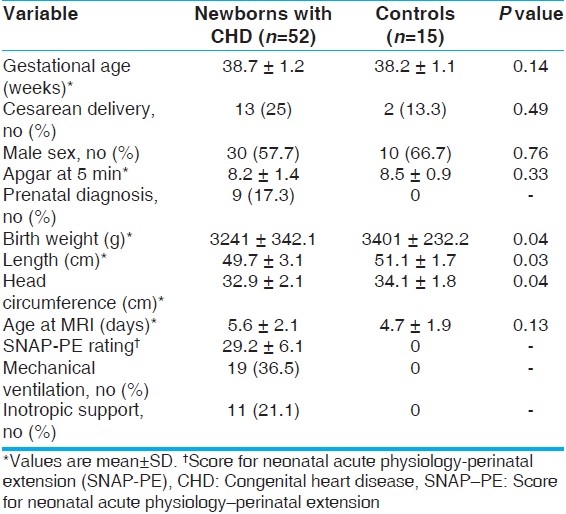
NAA/Ch mean ratio in three-dimensional MRS averaged across all of the brain regions in cyanotic patients compared to acyanotic and controls was significantly lower. Also, the ratio in acyanotic was significantly lower than in controls. These findings were detected across all studied segments in both white and gray matters [P<0.001, Table 2].
Table 2.
NAA/Ch metabolite ratio from threedimensional MRS for the studied groups*
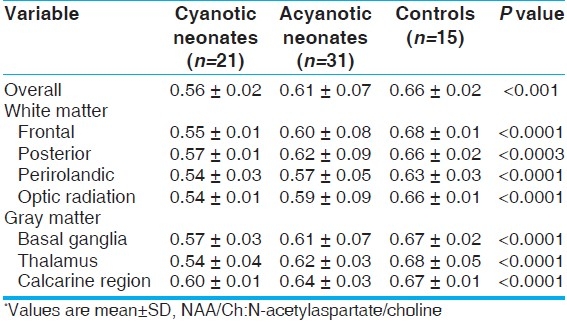
The mean ratio of lactate/Ch metabolite averaged across all of the brain regions was significantly higher in cyanotic neonates when compared to other studied groups (P<0.0001). The same results were observed in acyanotic patients when compared to controls. Both white and gray matter studied segments showed the same results [Table 3].
Table 3.
Lactate/Ch metabolite ratio from threedimensional MRS for the studied groups*
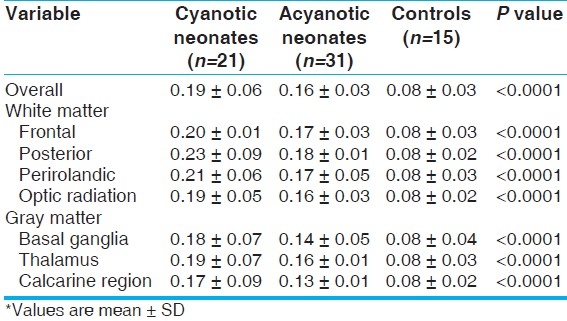
The mean value of average diffusivity from DTI across all the brain regions and both white and gray matter segments were significantly higher in cyanotic patients when compared to other groups (P<0.0001). The same results were observed in acyanotic neonates when compared to controls [Table 4].
Table 4.
Average diffusivity from DTI for the studied groups*
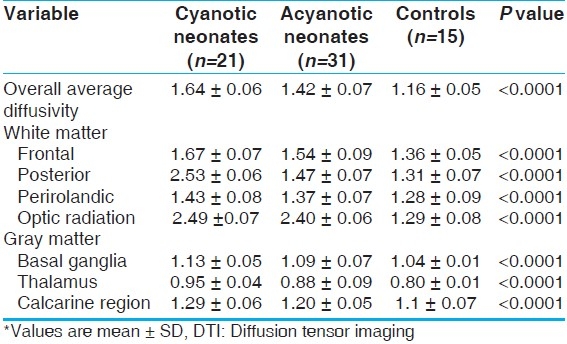
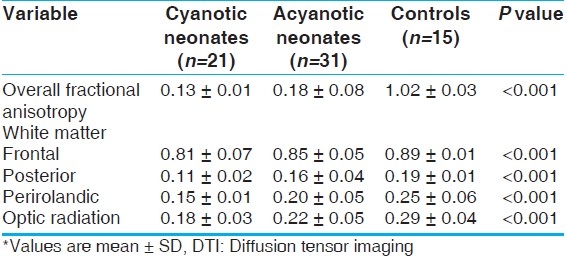
The mean value of white matter fractional anisotropy was significantly lower in cyanotic neonates than in both acyanotic neonates and controls either allover white matter or as regarding isolated studied segments (P<0.001). Acynotic neonates showed the same results when compared to controls [Table 5]. Brain injury (lactate/Ch ratio) correlated positively with SNAP-PE rating (r=0.61, P<0.05). No significant correlation between brain maturity (NAA/Ch, average diffusivity, fractional anisotropy) and SNAP-PE rating was detected.
Table 5.
White matter fractional anisotropy from DTI for the studied groups*
DISCUSSION
Advances in cardiac surgical techniques and perioperative intensive care have led to improved survival in babies with congenital heart disease. While it is true that the majority of children with CHD today will survive, many will have impaired neurodevelopmental outcome across a wide spectrum of domains.[21] Advanced MRI can quantify brain development and injury at a time when intervention for brain protection may be possible, allowing for incorporation of these data into the development and assessment of new clinical interventions for this population. The discovery of abnormal brain microstructure and metabolism shortly after birth in newborns with congenital heart disease is consistent with mounting evidence that these newborns have impaired brain development in utero, possibly related to impaired cerebral oxygen and substrate delivery prenatally.[22,23] Information regarding brain maturation may be important in considering when to perform these interventions.[24]
In the present study, newborn infants with CHD had brain abnormalities as evidenced by altered brain metabolism and microstructure even in the absence of visible injury on MRI and in uninvolved regions. MRSI showed that the mean ratio of NAA/Ch, across all of brain regions was significantly lower in neonates with cyanotic and acyanotic CHD compared to the controls. However, the mean ration of lactate/Ch across all brain regions was significantly higher. These findings are consistent with that reported by previous studies.[25,26]
As the brain develops, membranes in neuronal and glial cells usually develop with more barriers to water diffusing through tissues, which means that diffusivity decreases with maturity.[27] In the current study, the mean value of average diffusivity across all brain regions was significantly higher in neonates with CHD than in controls, indicating that less brain maturity and development among patients with CHD. Our results showed that white-matter fractional anisotropy was significantly lower in patients with CHD than in the control group. Abnormal brain development and brain injury were observed more in neonates with cyanotic than those with acyanotic CHD.
Approximately 3% of systemic blood flow returns to the left heart without perfusing the systemic vascular bed. In cyanotic CHD, this amount may increase up to 30 - 40% of the systemic blood flow which causes under perfusion of the systemic circulation affecting all organs including the brain.[2] In acyanotic CHD, abnormal brain development and brain injury may be attributed to decreased systemic blood flow and potentially cerebral blood flow.[28] Van Houten and co-workers,[29] reported that newborns with coarctation of aorta and ventricular septal defects had a high incidence (71%) of cranial ultrasound abnormalities. The findings of lower ratios of NAA/Ch, higher average diffusivity, and lower white-matter fractional anisotropy in newborns with congenital heart disease are similar to findings in premature newborns approximately one month before full term age. This similarity to the preterm brain led the authors to suggest that infants with CHD have abnormal brain development in utero.[12,14] The pattern of white-matter injury in premature newborns is attributed to cell populations that are vulnerable to ischemia, inflammation, and oxidative stress.[30] Though predominant injury to neurons would be the expected response to these insults in term newborns with congenital heart disease,[31] white-matter injury, the pattern of injury that is typical in premature newborns, occurs frequently.[4] Our findings suggest that white-matter vulnerability in term newborns with congenital heart disease is related to impaired brain development that is detected shortly after birth. The increase in white-matter radial diffusion (perpendicular to axon tracts) in newborns with congenital heart disease, as in premature newborns, suggests an abnormality of cells associated with axons forming white-matter tracts, such as oligodendrocyte progenitors or glia.[32]
Newborns with cyanotic and acyanotic CHD have evidence of cerebral metabolites (NAA, choline, lactate) abnormalities indicating immaturity of their brain, together with brain injuries early in their neonatal period, and these findings could be significant risk factors for later neurodevelopmental impairment.
The total number of patients enrolled in this study was small; therefore, further controlled, multicenter studies with larger sample sizes should be undertaken.
In the future, fetal management and intervention strategies for specific defects may ultimately play a role to improve in utero hemodynamics and increase cerebral oxygen delivery to enhance brain maturity and improve early neurodevelopment.
CONCLUSIONS
Newborn infants with either cyanotic or acyanotic congenital heart disease are at high risk of brain injury and impaired brain maturity. Early identification of infants at risk permits the initiation of early intervention programs to enhance the outcome of survivors. The state of brain maturation and the pattern of brain injury suggest that new and specific neuroprotective strategies may be needed in this population. Larger, controlled studies are warranted.
ACKNOWLEDGEMENTS
The authors acknowledge the major contribution of colleagues at radiology department and at the neonatal intensive care unit.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Boneva RS, Botto DL, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: Trends and racial disparities, 1979-1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 2.Scallan MJ. Brain injury in children with congenital heart disease. Paediatr Anaesth. 2003;13:284–93. doi: 10.1046/j.1460-9592.2003.00996.x. [DOI] [PubMed] [Google Scholar]
- 3.Sherlock RL, McQuillen PS, Miller SP. aCCENT. Preventing brain injury in newborns with congenital heart disease: Brain imaging and innovative trial designs. Stroke. 2009;40:327–32. doi: 10.1161/STROKEAHA.108.522664. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I-109–14. [PubMed] [Google Scholar]
- 5.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–5. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 6.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, Newton N, Ferriero DM, Partridge JC, Glidden DV, Barnwell A, et al. Predictors of 30-month outcome after perinatal depression: Role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52:71–7. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Azpurua H, Alvarado A, Mayobre F, Salom T, Copel JA, Guevara-Zuloaga F. Metabolic assessment of the brain using proton magnetic resonance spectroscopy in a growth-restricted human fetus: Case report. Am J Perinatol. 2008;25:305–9. doi: 10.1055/s-2008-1076603. [DOI] [PubMed] [Google Scholar]
- 9.Wolfberg AJ, Robinson JN, Mulkern R, Rybicki F, Du Plessis AJ. Identification of fetal cerebral lactate using magnetic resonance spectroscopy. Am J Obstet Gynecol. 2007;196:e9–11. doi: 10.1016/j.ajog.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 11.Star-Lack J, Spielman D, Adalsteinsson E, Kurhanewicz J, Terris DJ, Vigneron DB. In vivo lactate editing with simultaneous detection of choline, creatine, NAA, and lipid singlets at 1.5 T using PRESS excitation with applications to the study of brain and head and neck tumors. J Magn Reson. 1998;133:243–54. doi: 10.1006/jmre.1998.1458. [DOI] [PubMed] [Google Scholar]
- 12.Vigneron DB, Barkovich AJ, Noworolski SM, von dem Bussche M, Henry RG, Lu Y, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22:1424–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–32. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 15.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - A technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 16.Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, et al. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: Diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27:579–86. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 17.McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12:1237–43. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 18.Dumoulin CL, Rohling KW, Piel JE, Rossi CJ, Giaquinto RO, Watkins RD, et al. Magnetic resonance imaging compatible neonate incubator. Magn Reson Eng. 2002;15:117–28. [Google Scholar]
- 19.Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, et al. Diffusion tensor imaging: Serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–14. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Fleisher BE, Murthy L, Lee S, Constantinou JC, Benitz WE, Stevenson DK. Neonatal severity of illness scoring systems: A comparison. Clin Pediatr (Phila) 1997;36:223–7. doi: 10.1177/000992289703600407. [DOI] [PubMed] [Google Scholar]
- 21.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–7. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, et al. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatr Cardiol. 2003;24:436–43. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- 23.Jouannic JM, Benachi A, Bonnet D, Fermont L, Le Bidois J, Dumez Y, et al. Middle cerebral artery doppler in fetuses with transposition of the great arteries. Ultrasound Obstet Gynecol. 2002;20:122–4. doi: 10.1046/j.1469-0705.2002.00756.x. [DOI] [PubMed] [Google Scholar]
- 24.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, et al. Aortic valvuloplasty in the fetus: Technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–9. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 25.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 26.Shedeed SA, Elfaytouri E. Brain maturity and brain injury in newborns with cyanotic congenital heart disease. Pediatr Cardiol. 2011;32:47–54. doi: 10.1007/s00246-010-9813-7. [DOI] [PubMed] [Google Scholar]
- 27.Miller G, Tesman JR, Ramer JC, Baylen BG, Myers JL. Outcome after open-heart surgery in infants and children. J Child Neurol. 1996;11:49–53. doi: 10.1177/088307389601100112. [DOI] [PubMed] [Google Scholar]
- 28.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. 1999;103:402–8. doi: 10.1542/peds.103.2.402. [DOI] [PubMed] [Google Scholar]
- 29.van Houten JP, Rothman A, Bejar R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13:47–53. doi: 10.1055/s-2007-994202. [DOI] [PubMed] [Google Scholar]
- 30.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia ischemia. J Neurosci. 2003;23:3308–15. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, et al. Developmental changes in diffusion anisotropy coincide with i`mmature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–97. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


