Abstract
Background:
General anaesthesia is currently the conventional technique used for surgical treatment of breast lump. Paravertebral block (PVB) has been used for unilateral procedures such as thoracotomy, breast surgery, chest wall trauma, hernia repair or renal surgery.
Methods:
We compared unilateral thoracic PVB with general anaesthesia (GA) in 60 consenting ASA physical status I and II female patients of 18–65 years age, scheduled for unilateral breast surgery. Patients were randomly assigned into two groups, P (n=30) or G (n=30), to receive either PVB or GA, respectively.
Results:
The average time to first post-operative analgesic requirement at visual analogue scale score≥4 (primary endpoint) was significantly longer in group P (303.97±76.08 min) than in group G (131.33±21.36 min), P<0.001. Total rescue analgesic (Inj. Tramadol) requirements in the first 24 h were 105.17±20.46 mg in group P as compared with 176.67±52.08 mg in group G (P<0.001). Significant post-operative nausea and vomiting requiring treatment occurred in three (10.34%) patients of the PVB group and eight (26.67%) patients in the GA group.
Conclusion:
The present study concludes that unilateral PVB is more efficacious in terms of prolonging post-operative analgesia and reducing morbidities in patients undergoing elective unilateral breast surgery.
Keywords: Unilateral breast surgery, anaesthetic technique, unilateral thoracic paravertebral block, general anaesthesia, post-operative analgesia, multiple-injection technique
INTRODUCTION
Breast cancer is perhaps the most common cancer in women that requires frequent surgical intervention.[1] Nearly 40% of post-operative breast surgery patients experience significant acute postoperative pain, with a pain score above five reflecting inadequacy of conventional pain management.[2] Most of the responses of the human body to post-surgical pain have been proven to be detrimental to the patient's homeostasis and recovery. Moreover, the incidence of chronic postoperative pain in breast surgery patients is as high as 50%, and inadequate analgesia is considered as an independent risk factor.[3] Hence, a number of therapeutic measures have been accepted as a part of the “multimodal” approach to post-operative pain control.
General anaesthesia (GA) is the conventional technique used for surgical treatment of breast lump. However, the side-effects and complications of GA, such as post-operative pain, nausea and vomiting, increase morbidity for most patients undergoing breast surgery. Since the last two decades, there is a search for optimal regional techniques for operative procedures on the breast and axilla, which would reduce post-operative nausea and vomiting (PONV) and also provide prolonged post-operative sensory block, minimising narcotic requirements.
It has been proposed that injection of local anaesthetic drug into the thoracic paravertebral space could easily lead to the establishment of a block appropriate for the breast surgeries without any significant side-effects. Paravertebral block (PVB) can uniquely eliminate the cortical responses to thoracic dermatomal stimulation. It is associated with a decreased need for opioids for controlling postoperative pain,[4] decreased PONV, improved patient outcome, lowered postoperative pulmonary complications and, finally, decreased duration of post-anaesthesia care unit (PACU) stay.[5] PVB has also been used effectively in other unilateral surgeries like thoracotomy, herniorrhaphy and cholecystectomy.[6–8]
Despite a plethora of manuscripts in the current literature on PVB, comprehensive evidence is still lacking probably due to heterogeneity of clinical studies, various study designs and smaller sample sizes. In the present study, an effort has been made to compare the efficacy of thoracic PVB as an anaesthetic procedure for elective breast lump surgeries with GA, duration of post-operative pain relief being the primary end point.
METHODS
After obtaining Institutional ethics committee approval, 60 consenting female, ASA physical status I and II, aged 18–65 years, scheduled for a unilateral breast surgerywithout axillary clearance, were enrolled in this randomised observer blinded prospective clinical study. Patients who refused to participate, less than 18 years of age, ASA physical status 3 or more, body mass index >35, known pregnancy, lactating mothers, bleeding disorders, allergy to any of the study drugs, patients having any contraindication to placement of PVB, kyphoscoliosis, presence of acute herpes zoster, chronic pain syndrome, chronic analgesic use and psychiatric disease were excluded from this study. Considering a 30% increase in the duration of post-operative analgesia to be clinically relevant, with a power of 80% (β=0.2) at 0.05 level of significance (α=0.05), we required 25 patients in each group. We took 30 patients in each group considering the possibility of dropouts. Patients were randomised, by a sealed envelope technique on the day of surgery, into group P (n=30) and group G (n=30) to receive either thoracic PVB or GA, respectively.
During the pre-operative visit on the day before surgery, patients were thoroughly explained about the procedures to be undertaken and the risks and benefits associated. They were made well conversant with the visual analogue scale (VAS) for post-operative pain. Patients were advised preoperative fasting for a period of 6 h and premedicated with tab diazepam (10 mg) the night before surgery.
On arrival to the operation theatre (OT) complex, patients of group P were taken to a monitored block room where the PVBs were performed. Intravenous infusion of lactated Ringer's solution as maintenance fluid was started. Prior to both the procedures, all necessary equipment for GA and resuscitation were kept ready in case of a block failure or any complication. Baseline vital parameters like pulse rate, non-invasive blood pressure (NIBP), respiratory rate and peripheral arterial oxygen saturation (SpO2)were noted. The patients were shifted to the OT after surgical anaesthesia was achieved. Time to perform the blocks and time to surgical anaesthesia were noted. Monitoring was continued throughout the operative procedure, recorded at 15-min interval in the intraoperative and at 1-h intervals in the post-operative period.
Patients assigned to receive thoracic PVB were given incremental doses of iv midazolam (up to a maximum dose of 0.06 mg/kg) in the block room before block placement to decrease anxiety and discomfort during the procedure while maintaining a meaningful patient contact. Fentanyl (2 mcg/kg) was given as pre-emptive analgesic for block placement. The classic technique, which essentially includes loss of resistance, was used to identify the paravertebral space. With the patient in lateral decubitus with operative site non-dependant, relevant anatomical landmarks were identified and marked with a sterile permanent skin marker. Similarly, points corresponding to 2.5 cm lateral to the upper border of spinous processes of the T3 to T6 vertebrae were marked as needle insertion sites, and each space was infiltrated with 2 mL of 1% lignocaine [Figure 1]. An 18-gauge Tuohy needle with depth label on its shaft was introduced perpendicular to the skin in all planes to touch the transverse process of the lower vertebra up to a maximum depth of 4 cm initially [Figure 2]. In case of non-contact with bone, it was presumed that the needle was in between two transverse processes. Then, the needle was withdrawn to the subcutaneous tissue and placed with a cephalic or caudal direction to the same depth of 4 cm. If bone was still not encountered, the needle was advanced 1 cm further and the above stated method was repeated until the transverse process was spotted. After identification of the transverse process, the needle was walked off the superior surface of the transverse process and slowly advanced 1–1.5 cm until a loss of resistance to saline was obtained with a 5-mL glass syringe. Loss of resistance is said to occur while needle pierced superior costotransverse ligament to enter into the thoracic paravertebral space.[9]
Figure 1.
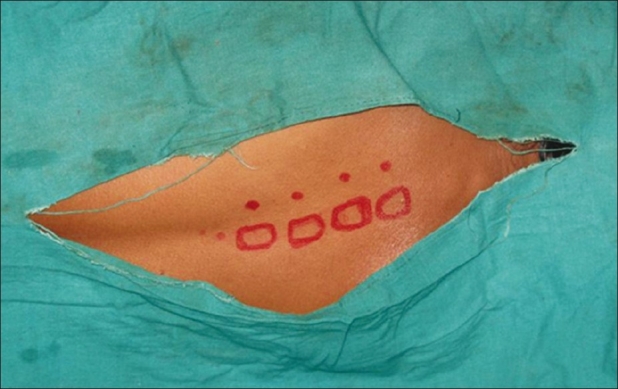
Needle insertion points at vertebral levels T3-T6
Figure 2.
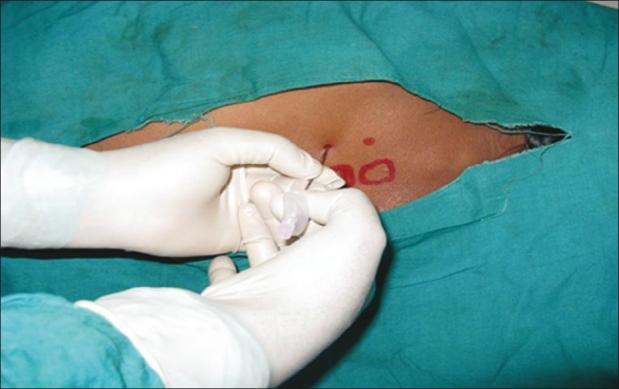
Tuohy needle placed into the paravertebral space
Five milliliters of 0.5% bupivacaine solution was injected at each level after repeated negative aspiration for blood or cerebrospinal fluid, whether or not paraesthesia was elicited. We assessed the onset of unilateral pinprick discrimination at 5 min and every 5 min thereafter up to 30 min. A block was considered as “unsuccessful” if onset of pinprick discrimination was not evident within 15 min or failure to achieve adequate sensory block (T2-T6) within a maximum time of 30 min. If the block was considered as failed, the patient was administered GA and the case excluded from the study. Numbers of dermatomes having complete loss of sensation to pinprick were noted. Intraoperatively, patients received an iv infusion of propofol (30–70 mcg/kg/min) titrated to light sleep with easy arousability. Intermittent doses of fentanyl 25 mcg and propofol 10 mg were given for supplemental sedation if heart rate or Mean Arterial Pressure (MAP) increased more than 20% of the baseline value.
The control group underwent GA with endotracheal intubation. Premedication was done with intravenous (iv) midazolam (0.04–0.06 mg/kg) and analgesia with iv fentanyl (2 μg/kg). The induction of anaesthesia was done with I.V. propofol (2 mg/kg) followed by iv atracurium (0.5 mg/kg) to facilitate tracheal intubation. Inj. Diclofenac sodium 75 mg intramuscular was given after induction. Maintenance of anaesthesia was achieved with nitrous oxide 66% and oxygen 33%. The patients received top-ups of iv atracurium (0.1 mg/kg) at regular intervals and iv fentanyl (1 μg/kg) at 1-h intervals if the surgery extended beyond 1 h. Heart rate and MAP were maintained within 20% of the baseline values by giving additional bolus doses of fentanyl 25 mcg and propofol 10 mg. At the end of surgery, all patients were reversed from muscle relaxation with iv neostigmine (40–70 μg/kg) and iv glycopyrrolate (7–15 μg/kg) titrated to clinical effect, as per the usual protocol.
Induction time (time to surgical anaesthesia) was defined in group G as the time gap between preoxygenation and successful intubation. In group P, it was defined as the time gap between the completion of local anaesthetic injection to unilateral pinprick discrimination of at least three segments. Duration of surgery was defined as the time between surgical incision and application of adhesive bandage after closure of the wound in both the groups. Recovery time was defined in group P as the time between discontinuation of propofol infusion and eye opening on verbal command. In group G, it was defined as the time between administration of appropriate dose of neostigmine–glycopyrrolate combination and eye opening on verbal command. In group P, the patients were shifted to the OT after surgical anaesthesia was achieved in the monitored block room. In both the groups, the total OT time was defined as the time between the entry of the patient to the OT to the transfer of the patient to the recovery room.
Post-operatively, all patients were monitored in the recovery room for the first 24 h. Patients were assessed for pain and nausea and vomiting just after shifting to recovery from OT by a resident not involved in the study. Thereafter, in the recovery room, data were collected at 2, 4, 6, 12 and 24 h, calculated from the time of block placement by the same resident. Patients’ anaesthesia records were not available to the residents at recovery for the first 24 h. Post-operative pain was assessed with a VAS score of 0–10 (0=no pain and 10=worst imaginable pain). VAS scores ≥4 were treated with rescue analgesic tramadol in boluses of 50 mg iv, repeated if necessary after 15 min. If analgesia was still inadequate after 30 min, inj. diclofenac sodium 75 mg intramuscular was administered as a backup analgesic. The total doses of administered tramadol and diclofenac during the first 24-h period were recorded. Time to the first analgesic requirement was noted. Duration of postoperative analgesia was defined as the time between the last suture application and the request for first rescue analgesic at VAS score ≥4. Number of patients experiencing PONV were accounted for and treated accordingly. Apart from these, patients were monitored throughout the study period for any evidence of complications.
During surgery, the surgeon assessed the quality of anaesthesia following a numeric rating scale (NRS) of 0-100. At the time of discharge, patients were asked to mention about their satisfaction of the respective anaesthetic procedure (NRS 0–100).
RESULTS
The study was conducted over a 17-month period (February ‘09 to June ’10). One patient in the paravertebral group was converted to GA due to inadequate block and was also excluded from the study. Therefore, data from 59 patients were available for analysis; group P (n=29) and group G (n=30).
Discrete categorical data are presented as n (%) and median; continuous data are given as mean±SD. Differences in demographic, surgical, anaesthetic and post-operative data were tested by independent Student's t-test (continuous data) or by Pearson Chi-square test and Fisher's exact test as appropriate (categorical data). For descriptive purposes, P value differences <0.05 are noted in the tables. All analyses were conducted using SPSS for Windows (version 12.0; SPSS Inc., Chicago, IL, USA).
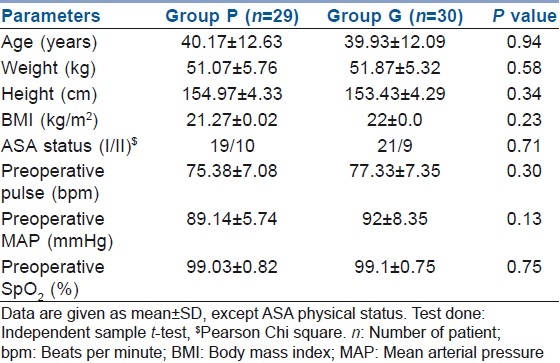
Demographic patterns and pre-operative vital parameters were similar when the two groups were compared [Table 1].
Table 1.
Demographic and pre-operative parameters
Durations of surgery were 78.72±22.98 and 80.56±19.54 min in group P and group G, respectively, the values being comparable (P>0.05). Total time spent in the OT was also similar in the two groups; 92.24±24.94 min in group P and 94.28±19.74 min in group G (P>0.05). Time for inducing anaesthesia was significantly longer in group P (19.17±4.84 min) compared with group G (6.20±1.65 min). As the PVBs were performed in the block room and the patient was transferred to the OT after surgical anaesthesia was achieved, the total OT time was not significantly prolonged in group P. Intraoperative vital parameters were comparable in the two groups (P>0.05). The requirement of fentanyl during the surgery was significantly lower in group P (107.76±11.77 mcg) as compared with group G (150.83±26.65 mcg), P<0.0001 [Table 2, Figures 1 and 3]. The total dose of propofol was higher in group P (219.82±74.48 mg) than in group G (122.67±15.07 mg), P<0.0001, as would be expected for any regional procedure to maintain immobility.
Table 2.
Intraoperative characteristics
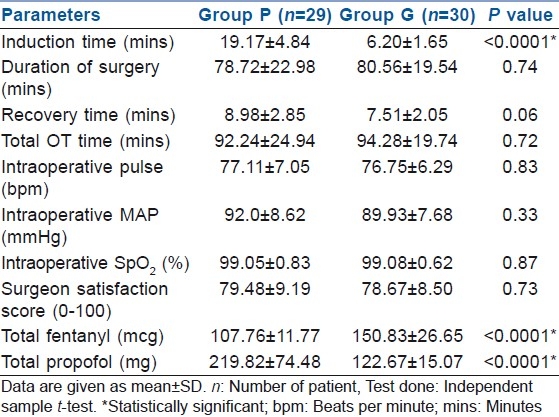
Figure 3.
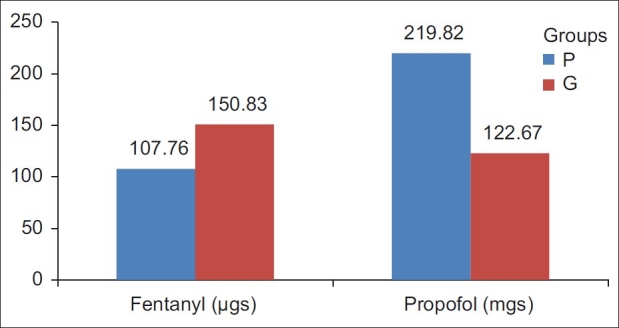
Intraoperative propofol and fentanyl requirements
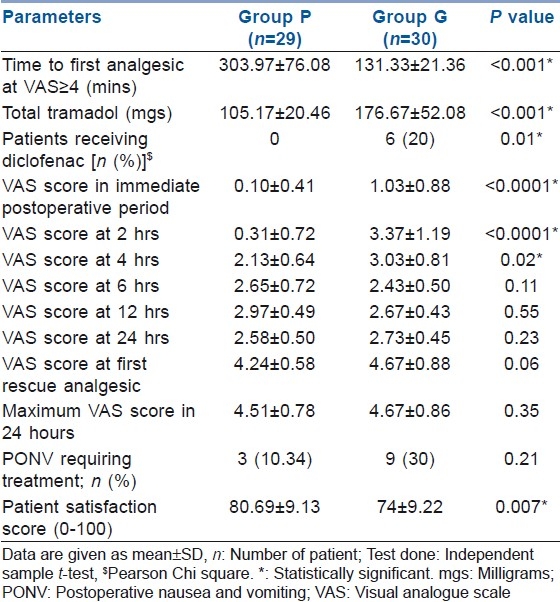
Time to request for analgesic for the first time was considered as the duration of postoperative analgesia [Table 3]. It ranged from 135 to 456 min in group P and from 86 to 170 min in group G. The mean duration of post-operative analgesia was 303.97±76.08 min in group P and 131.33±21.36 min in group G, the difference being statistically significant (P<0.001). Total dose of tramadol as rescue analgesic during the first 24 h was 105.17±20.46 mg in group P as compared with 176.67±52.08 mg in group G (P<0.001). Back-up analgesic in the form of intramuscular diclofenac sodium had to be used in six patients (20%) in group G as compared with none in group P (P=0.01). The VAS scores in the immediate post-operative period and after 2 and 4 h in the post-operative period were significantly higher in group G (P<0.05). The VAS scores at 6, 12 and 24 h were comparable in the two groups, but at the expense of higher analgesic consumption in group G. VAS score at request for first rescue analgesic was comparable in both the groups (4.24±0.58 vs 4.67±0.88 in group P and group G, respectively, P=0.06). Maximum VAS score in 24 h also did not differ between the groups [Table 3]. The incidence of PONV requiring treatment was 10.34% in group P and 30% in group G. Patients had higher satisfaction scores in group P (80.69±9.13) as compared with group G (74±9.22), P=0.007. In the present study, the surgeon satisfaction score was found to be comparable (79.48±9.19 vs 78.67±8.50 in group P and group G, respectively, P=0.73).
Table 3.
Post-operative analgesia and PONV
We have not observed any incidence of direct epidural spread, inadvertent intravascular injection, haemodynamic instability or persistent pain after the block procedure. One patient had experienced moderate pain during the block injection (PVB) in spite of fentanyl (2 mcg/kg) used as preemptive analgesic for block placement. This was managed with further administration of local anaesthetics, fentanyl bolus iv and redirection of the needle. In six patients, multiple pass of needle with single prick was required in one of the multiple injections of the block. No patient showed bilateral spread of sensory block. Significant bleeding was not observed in any patient. No inadvertent pleural puncture was observed.
DISCUSSION
Regional anaesthesia using thoracic PVB has been described as an ideal alternative to GA for selected breast surgery patients.[5] Benefits include prolonged post-operative pain relief, a reduction of PONV and the potential for ambulatory discharge. The means to assess post-operative pain control was the time to first analgesic consumption, the total amount of analgesic consumed in the first 24 h period after surgery and the VAS scores at different times in the first post-operative day. The time to first analgesic in the post-operative period was significantly greater in group P than in group G. This is due to the longer duration of post-operative analgesia achieved with PVB. Mean requirement of tramadol (rescue analgesic) in the first 24 h was also lesser in group P as compared with group G [Table 3]. No patient in group P required injection diclofenac (back-up analgesic) in contrast to six patients in group G. The unavailability of a patient-controlled analgesia (PCA) device at the time of this study resulted in more fluctuation of the target VAS score, and a difference of VAS score in the early post-operative period (up to 4 h) can thus be explained. The delayed onset and prolonged duration of action of tramadol and diclofenac resulted in better post-operative analgesia subsequently in the later part of the post-operative period. This was reflected in the comparative values of the VAS scores at 6, 12 and 24 h post-operatively, but at the expense of higher analgesic consumption in group G.
Results of our study did not show post-operative analgesia with PVB to be as prolonged as shown in the study by Klein and colleagues,[10] who found reduced pain scores even at 72 h post-operatively. A higher patient satisfaction score was observed in group P (80.69±9.13) than in group G (74±9.22). This is in accordance with most of the earlier studies.[10–14] This inconsistency in duration of post-operative pain relief may depend on various factors like pre-emptive use, the drug and its dose, presence of additive, single or multiple injections, continuous infusion or bolus injection, ultrasound or neurostimulation guidance, use of PCA in the post-operative regimen and age of the patient. High speed of injection and patient positioning can promote contralateral spread. Previous studies,[10,15,16] report that multiple injection does improve the duration and quality of analgesia, with a higher probability of procedural complications. On the other hand, single injection provides more patient comfort and lowers the need for sedation during performance of the PVB, thereby improving patient satisfaction.[5,11] Small dose of multiple injections are supposed to provide better consistency in optimal spread of the injectate.[17] On the contrary, a single-injection technique has been reported to produce a safe but unpredictable block.[18,19] Ultrasound or neurostimulation guidance, either alone or in combination, seem to increase the safety and success of the technique compared with the “loss of resistance to saline” technique. But, current evidence is not sufficient enough so as to support the use of these gadgets as these are not likely to change the outcome.[20,21] Addition of clonidine or fentanyl may have a modest effect on the overall VAS score, but no robust evidence is available that favours the addition to local anaesthetics.[22]
It was found that multiple PVBs (T3-T6) permitted effective surgical manipulation for breast surgeries without axillary clearance. However, a block level of T1 to T6 has provided adequate anaesthesia for successful breast surgery with axillary clearance.[14] Despite multiple punctures, the patients were satisfied with this procedure due to the combined use of midazolam, fentanyl and local infiltration of skin and subcutaneous tissue before the block placement, and also for the prolonged period of pain relief. In a recently performed study,[12] it was concluded that most elective outpatient breast cancer surgeries, including mastectomy, full axillary dissections, expander or implant reconstruction, could be performed reliably with considerable safety under PVB regional anaesthesia.
In the present study, 27 (90%) of the patients in group P completed the surgery with PVB and light sedation. Two patients (6%) in group P had to be supplemented with local anaesthetic infiltration for adequate anaesthesia as a result of inconsistent block of the targeted dermatomes.[13] One patient (3%) had to be converted to GA due to failed block. Technical difficulty in locating the paravertebral space has been cited as the reason for failure by authors. Failure rates with PVB are consistent with that of other regional techniques. Multiple-injection PVB decreases the chance of inconsistent block associated with a single-injection technique.[17] In a review of eight randomized controlled trials of the use of PVB in breast surgeries and hernia repairs, the overall failure rates of PVB were not >13%.[13]
The general risk of PONV in women undergoing breast surgery under GA is appreciably high. Perioperative opioid administration compounds the problem further. One systemic review showed a statistically significant lesser risk of PONV of PVB over GA.[13] Significant PONV requiring treatment occurred in three (10.34%) patients of group P and in nine (30%) patients of group G. Despite a lack of statistical significance between the groups, which may be due to the relatively small sample size, the overall nausea and vomiting was found to be less in group P [Table 3]. The reason for this may be the better post-operative pain control and lesser opioid consumption in group P.
To conclude, in view of excellent analgesia in the early post-operative period, requirement of significantly lesser amount of postperative analgesics, decrease in the occurrence of PONV and low rate of serious complications, along with potential for early ambulation and home discharge, thoracic PVB can be used as a suitable alternative to GA as the anaesthetic procedure in elective breast surgeries without axillary clearance. Probability of inconsistent block leaves a few gray areas in its implication as the ideal alternative to GA, although use of nerve stimulation and ultrasound guidance, either alone or in combination, can be used to further enhance block consistency with optimal drug delivery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: A review of current American Cancer Society Guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 2.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain. 2006;7:626–34. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. J Am Med Assoc. 2009;302:1985–92. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 4.Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, et al. A systematic review of randomized trials evaluating regional techniques for post-thoracotomy analgesia. Anesth Analg. 2008;107:1026–40. doi: 10.1213/01.ane.0000333274.63501.ff. [DOI] [PubMed] [Google Scholar]
- 5.Dabbagh A, Elyasi H. The role of paravertebral block in decreasing postoperative pain in elective breast surgeries. Med Sci Monit. 2007;13:CR464–7. [PubMed] [Google Scholar]
- 6.Hadzic A, Kerimoglu B, Loreio D, Karaca PE, Claudio RE, Yufa M, et al. Paravertebral blocks provide superior same-day recovery over general anesthesia for patients undergoing inguinal hernia repair. Anesth Analg. 2006;102:1076–81. doi: 10.1213/01.ane.0000196532.56221.f2. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya P, Mandal MC, Mukhopadhyay S, Das S, Pal PP, Basu SR. Unilateral paravertebral block: An alternative to conventional spinal anaesthesia for inguinal hernia repair. Acta Anaesthesiol Scand. 2010;54:246–51. doi: 10.1111/j.1399-6576.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- 8.Fibla JJ, Molins L, Mier JM, Siera A, Vidal G. A prospective study of analgesic quality after a thoracotomy: Paravertebral block with ropivacaine before and after rib spreading. Eur J Cardiothorac Surg. 2009;36:901–5. doi: 10.1016/j.ejcts.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 9.Karmakar MK, Ho AM. Thoracic and lumbar paravertebral block. In: Hadzic A, editor. Textbook of regional anesthesia and acute pain management. 1st ed. NewYork: McGraw-Hill; 2007. pp. 583–97. [Google Scholar]
- 10.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–5. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004;99:1837–43. doi: 10.1213/01.ANE.0000136775.15566.87. [DOI] [PubMed] [Google Scholar]
- 12.Kitowski NJ, Landercasper J, Gundrum JD, De Maiffe BM, Chestnut DH, Bottcher ML, et al. Local and paravertebral block anesthesia for outpatient elective breast cancer surgery. Arch Surg. 2010;14:592–4. doi: 10.1001/archsurg.2010.77. [DOI] [PubMed] [Google Scholar]
- 13.Thavaneswaran P, Rudkin GE, Cooter RD, Moyes DG, Perera CL, Maddern GJ. Paravertebral Block for Anesthesia: A systematic review. Anesth Analg. 2010;110:1740–4. doi: 10.1213/ANE.0b013e3181da82c8. [DOI] [PubMed] [Google Scholar]
- 14.Buckenmaier CC, Kwon KH, Howard RS, McKnight GM, Shriver CD, Fritz WT, et al. Double-blinded, placebo-controlled, prospective randomized trial evaluating the efficacy of paravertebral block with and without continuous paravertebral block analgesia in outpatient breast cancer surgery. Pain Med. 2010;11:790–9. doi: 10.1111/j.1526-4637.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 15.Terheggen MA, Wille F, Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94:335–9. doi: 10.1097/00000539-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: A randomized double-blind study. Anesth Analg. 2007;105:1848–51. doi: 10.1213/01.ane.0000286135.21333.fd. [DOI] [PubMed] [Google Scholar]
- 17.Naja ZM, El-Rajab M, Al-Tannir MA, Ziade FM, Tayara K, Younes F, et al. Thoracic paravertebral block: Influence of the number of injections. Reg Anesth Pain Med. 2006;31:196–201. doi: 10.1016/j.rapm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Cheema S, Richardson J, McGurgan P. Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space. Anaesthesia. 2003;58:684–711. doi: 10.1046/j.1365-2044.2003.03189_1.x. [DOI] [PubMed] [Google Scholar]
- 19.Cotter JT, Nielsen KC, Guller U, Steele SM, Klein SM, Greengrass R, et al. Increased body mass index and ASA physical status IV are risk factors for block failure in ambulatory surgery—an analysis of 9,342 blocks. Can J Anaesth. 2004;51:810–6. doi: 10.1007/BF03018454. [DOI] [PubMed] [Google Scholar]
- 20.Liu SS, Ngeow JE, Yadeau JT. Ultrasound-guided regional anesthesia and analgesia: A qualitative systematic review. Reg Anesth Pain Med. 2009;34:47–59. doi: 10.1097/AAP.0b013e3181933ec3. [DOI] [PubMed] [Google Scholar]
- 21.Schnabel A, Reichl SU, Kranke P, Pogatzkie-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: A meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105:842–52. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 22.Kotze A, Scally A, Howell S. Efficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: A systematic review and metaregression. Br J Anaesth. 2009;103:626–36. doi: 10.1093/bja/aep272. [DOI] [PubMed] [Google Scholar]


