Abstract
Massive intra-operative bleeding is not an infrequent occurrence in obstetrics. Worldwide obstetric bleeding remains a major cause of morbidity and mortality. Conventional management of this bleeding consists of resuscitation with fluids, blood, surgical maneuvers, and embolisation of feeding blood vessels. But in most of cases, these measures appear to be ineffective in controlling bleeding. Recently, the ‘off-label’ use of the recombinant activated factor VII (rFVIIa) concentrate has emerged as promising treatment for such bleeding when conventional measures fail. We came across a similar scenario in which a young lady was admitted with per-vaginal bleeding due to abruptio placentae. In spite of usual surgical and medical interventions, she continued to bleed. rFVIIa was administered as a desperate measure to avoid hysterectomy and the bleeding could be stopped. She recovered successfully without any complication. Thus, the timely use of rFVIIa, hence, can be used to save life and fertility in cases of intractable obstetric bleeding.
Keywords: Disseminated intravascular coagulation, intractable obstetric bleed, recombinant activated factor VII concentrate
INTRODUCTION
In spite of currently available management, intractable bleeding in obstetrics still remains a major cause of maternal morbidity and mortality.[1] Though the use of recombinant activated factor VII (rFVIIa) (NovoSeven®) is currently approved for patients with haemophilia, an inhibitor of factor VIII and XI,[1–8] but recently it has been used in patients without any preexisting coagulopathy to treat intractable bleeding in various surgical procedures and trauma cases (‘off-label’use).[2–10] This indicates that rFVIIa can be used for the management of intractable obstetric bleeding which is often complicated with disseminated intravascular coagulation (DIC) as an adjunct to conventional management.[1,3–5,8,9] But there are very few reports of its use in intractable obstetric bleeding.[1,5–8]
CASE REPORT
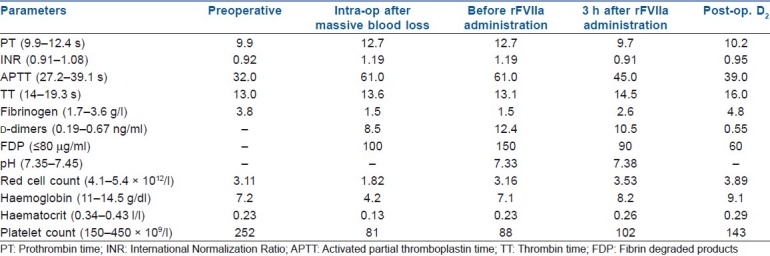
A pregnant (19 weeks) patient (27 years old) presented with per-vaginal bleeding and was diagnosed to have abruptio placentae. She did not have any prior medical illness or coagulopathy. The placenta was removed manually by the traction of the cord and digital separation under general anaesthesia, but she continued to have profuse bleeding in spite of maximal oxitotic treatment as per our hospital protocol (oxytocin intravenous infusion 40 IU, methyl-ergometrin 0.5 mg intramuscular, misoprostole 1000 μg per rectal). She became haemodynamically unstable in spite of resuscitation. She was immediately taken for emergency laparotomy under general anaesthesia for surgical control of bleeding after discussing all risks and benefits with the patient and relatives and taking a written consent. Generalized oozing was found mostly at the lower part of the uterus. Bleeding could be temporarily controlled with suture and packing in an attempt to preserve fertility. She lost about 3 L of blood which was replaced with eight units of packed red blood cells and fresh frozen plasma, six units of platelets and Cryoprecipitate. There was no hypothermia and the Acid base status of the patient was also corrected, and empirically calcium (1 g) was also given. But despite all our efforts, the patient started to bleed again. She was then shifted to the radiology room for uterine artery embolisation, but no active bleeding was found. Even after bilateral uterine artery embolisation, she continued to bleed. While obstetricians were considering hysterectomy, we decided to use the rFVIIa concentrate after discussing the risks and benefits with relatives. Bleeding reduced within few minutes after receiving a single dose of rFVIIa (90 μg/kg)[1–3,5–7,9] and she became vitally stable and her laboratory parameters normalised [Table 1]. Finally, she was shifted to ICU where she was extubated on the next day. She was put on pneumatic stocking for thrombo-prophylaxis. She had an uneventful recovery without any thromboembolism (TE) or allergic complication. She was discharged after 7 days.
Table 1.
Different laboratory parameters
DISCUSSION
Obstetric bleeding may be caused by combined utero-placental pathology, surgical and/or acquired coagulopathic insult (DIC, defective thrombin generation) which is common in abruptio placentae.[1,6–9] Bleeding due to surgical insult can be corrected by medical, surgical interventions (arterial ligation, hysterectomy) or arterial embolization.[5,7–9] However, acquired coagulopathic bleeding is more difficult to control especially when it is associated with acidosis, hypothermia, thrombocytopaenia and hypofibrinogenaemia.[8–10] Conventional treatments for massive intra-operative bleeding include replacement of fluids, blood and blood products.[3,5,7,9] However, resuscitation with large volumes of fluids can worsen the bleeding due to dilutional and acquired coagulopathy (DIC).[1,6,8,10] Also thrombin burst and inhibition of fibrinolysis are required to achieve haemostasis in such cases.[9] Thus, uncontrolled intra-operative bleeding still remains a major cause of morbidity and mortality in spite of aggressive management.[1,6,9,10] Furthermore, massive transfusion may lead to transfusion-related complications adding to the increased morbidity and mortality.[2,8,10] These problems of replacement therapy demand need for pro-haemostatic agents as an adjunct to conventional treatments.[2,8,10] Several pro-haemostatic agents have been used to control surgical bleeding, but none is found to be beneficial in controlling massive intra-operative coagulopathic bleeding.[8]
rFVIIa plays a vital role in haemostasis.[2,6] It is now considered as a leading pro-haemostatic agent based on current insight into its role in the coagulation cascade.[1,6,8] rFVIIa acts by binding with the tissue factor locally at the site of the injury and generates a ‘thrombin burst’ which in turn activates platelets and forms fibrin.[2–9] The clot thus formed is further stabilised by the inhibition of fibrinolysis by rFVIIa.[3–9] rFVIIa does not usually lead to systemic activation of coagulation and the fibrin formation remains restricted to the injured area.[3,7–9] Also, its performance is not affected by the presence of anti-thrombin and other inhibitors.[9] This forms the basis of its use in severe uncontrolled surgical and acquired coagulopathic bleeding.[3–9]
The rFVIIa concentrate is the emerging alternative when conventional therapy becomes useless and is shown to reduce transfusion requirement and improve survival considerably.[1–3,5–9] But the rFVIIa concentrate is effective if given timely before the occurrence of massive transfusion-related complications and without severe hypothermia, acidosis, thrombocytopaenia or fibrinogen deficiency as further delay may compromise the prognosis and life of the patient.[5–10] Although the rFVIIa concentrate is safe with regard to the transmission of infection,[3] but there is some risk of hypersensitivity in susceptible patients[3] and TE (0–10%)[1–4,6–9] when used for ‘off-label’ indications. A routine ‘off-label’ use thus is not recommended for this higher incidence of TE and cost (about $2000 per 2.4 mg vial).[3,6,8] However, TE is found to be statistically significant only for arterial TE in higher doses (>120 μg/kg) in elderly patient especially when other haemostatic agents are used, but not for venous TE.[2,3,9] Thus in a dire situation, it can be used to save the life of younger patients without any history of TE after the failure of standard management.[1–5,8] Even institutional guidelines need to be published for this ‘off-label’ use.[3,4,8] Thus the present recommendation needs revision based on newer large-scale randomised trials.[3,4,6,7,9]
We used the rFVIIa concentrate as a desperate measure just to avoid hysterectomy in a young patient.[1,5,7] We tried all other feasible conventional measures, but they were of no avail.[5,6] Fresh whole blood may have been a good option in this case. But in our hospital, whole blood is no more available and only component products are now used as per standard international guidelines.[9] So we opted for rFVIIa matching the guideline published by Martinowitz et al. and the patient had a successful recovery.[7] Also rFVIIa helped in our case which was complicated by DIC [Table 1].[1,4,6–8] Earlier, DIC was considered as a contraindication for the use of rFVIIa, but recently it has been successfully used to control bleeding complicated by DIC.[1,3,6,7] In most of the reported cases, rFVIIa was used for the control of postpartum haemorrhage in cesarean section and many of them required hysterectomy along with rFVIIa.[1,7] In our case, we managed the bleeding successfully with single dose of rFVIIa which helped us to avoid hysterectomy.[1,5,7] Though a single case does not have much impact on the medical fraternity, but the purpose of this case report is to recommend that the timely use of the rFVIIa concentrate in cases of severe intractable obstetric bleeding complicated with DIC can save lives and avoid more drastic surgery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.von Heymann C, Jonas S, Spies C, Wernecke KD, Ziemer S, Janssen D, et al. Recombinant activated factor VIIa for the treatment of bleeding in major abdominal surgery including vascular and urological surgery: A review and meta-analysis of published data. Crit Care. 2008;12:R14. doi: 10.1186/cc6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M, Levy JH, Anderson HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 3.Ickx BE. rFVIIa (Novoseven): The new panacea? Acta Anaesthesiol Belg. 2003;54:333–5. [PubMed] [Google Scholar]
- 4.Hamaekers AE, van Mook WN, Offermans JP, Marcus MA. Successful use of recombinant factor VIIa for treatment of severe postpartum hemorrhage. Am J Crit Care. 2006;15:399–401. [PubMed] [Google Scholar]
- 5.Price G, Kaplan J, Skowronski G. Use of recombinant factor VIIa to treat life-threatening non-surgical bleeding in a post-partum patient. Br J Anaesth. 2004;93:298–300. doi: 10.1093/bja/aeh196. [DOI] [PubMed] [Google Scholar]
- 6.Franchini M, Manzato F, Salvagno GL, Lippi G. Potential role of recombinant activated factor VII for the treatment of severe bleeding associated with disseminated intravascular coagulation: A systematic review. Blood Coagul Fibrinolysis. 2007;18:589–93. doi: 10.1097/MBC.0b013e32822d2a3c. [DOI] [PubMed] [Google Scholar]
- 7.Martinowitz U, Michaelson M. Israeli Multidisciplinary rFVIIa Task Force.Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: A report by the Israeli Multidisciplinary rFVIIa Task Force. J Thromb Haemost. 2005;3:640–8. doi: 10.1111/j.1538-7836.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 8.Hedner U, Erhardtsen E. Potential role for rFVIIa in transfusion medicine. Transfusion. 2002;42:114–24. doi: 10.1046/j.1537-2995.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 9.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–9. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 10.Surbek D, Huber A, Alberio L, Meyer-Wittkopf M, Raio L. The role of recombinant factor VIIa in the treatment of severe postpartum hemorrhage: Can fertility be preserved? Am J Obstet Gynecol. 2006;195(Suppl 1):S92. [Google Scholar]


