Abstract
Previous studies have shown that early life trauma induced by maternal separation or colonic irritation leads to hypersensitivity to colorectal distension in adulthood. We tested the hypothesis that repetitive colorectal distension in neonates leads to abnormalities in colonic permeability and smooth muscle function in the adult rat. In neonatal rats, repetitive colorectal distension was performed on days 8, 10, and 12. As adults, stool consistency was graded from 0 (formed stool) to 3 (liquid stool). Colonic tissue was isolated for histology and myeloperoxidase levels. The colonic mucosa was placed in modified Ussing chambers for measurements of permeability and short-circuit current responses to forskolin, electrical field stimulation, and carbachol. Segments of colonic musculature were placed in organ baths and contractile response to potassium chloride, electrical field stimulation, and carbachol were determined. In adult rats that experienced neonatal colonic irritation, no significant changes in colonic histology or myeloperoxidase activity were observed; however, stool consistency scores were increased. Mucosal permeability, measured as an increase in basal conductance, was significantly increased but no changes in short-circuit current responses were observed. In adulthood, rats that underwent colorectal distension as neonates exhibited an elevated smooth muscle contractile response to potassium chloride, but no changes in response to electrical field stimulation or carbachol. In summary, neonatal colonic irritation, shown previously to produce colonic hypersensitivity, leads to significant alterations in colonic mucosal and smooth muscle function characterized by loose stools, increased mucosal permeability, and increased smooth muscle contractility in the absence of colon inflammation in adulthood.
Keywords: Early life trauma, Irritable Bowel Syndrome, Mucosal permeability, Contractility
1. Introduction
Irritable Bowel Syndrome (IBS) is a common functional gastrointestinal (GI) disorder with chronic symptoms including abnormal bowel habits, bloating, and abdominal cramping due at least in part, to changes in visceral sensation manifested as hypersensitivity to visceral stimuli (Mayer and Gebhart, 1994; Whitehead et al., 1990). Although no definite causative factors have been identified for the development of visceral hypersensitivity associated with IBS, psychosocial interactions such as physical and emotional stress or anxiety may play a significant role in the pathophysiology of IBS (Gaynes and Drossman, 1999; Blanchard and Scharff, 2002; Drossman, 1999). Specifically, traumatic events during the neonatal period, a time of neural maturation and development, can produce long-term alterations in visceral perception. A history of early life stress or trauma has been associated with the development of IBS in adulthood (Ladd et al., 2000; Talley et al., 1994). Furthermore, rodent models of early life trauma such as maternal separation (MS), unpredictable electrical stimulation, or neonatal colonic irritation (nCI) via repetitive colorectal distension (CRD) lead to visceral hypersensitivity in adult rats (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003; Chung et al., 2007; Ren et al., 2007; Tyler et al., 2007; Coutinho et al., 2002). Previous studies have shown that repetitive CRD in neonatal rats alters the development of nociceptive pathways, thereby resulting in heightened sensitivity to nociceptive stimulation in adulthood (Barreau et al., 2007). Specifically, studies have indicated that early life stress in the form of a transient neonatal challenge induced by noxious repetitive distension of the colon serves to increase central and peripheral sensitivity to CRD, thereby mimicking visceral hypersensitivity associated with IBS (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003). Moreover, in addition to increased visceral sensitivity, neonatal stress from MS has been shown to have profound effects on colonic function in adulthood including altered colonic motility and an increase in colonic permeability. MS enhances colonic motility in response to acute restraint stress and causes changes in epithelial transport of the adult rat reflected as a marked increase in conductance and permeability of macromolecules across the colon (Gareau et al., 2007; Schwetz et al., 2005; Söderholm et al., 2002). Furthermore, neonatal MS leads to a heightened inflammatory response following colonic insult in adult rats, suggesting an enhanced immune response of the colonic mucosa (Barreau et al., 2004b).
Although much is known about the effects of MS on the colon, the influence of nCI on colonic function and bowel habits in adulthood has not been characterized. Understanding the effects of nCI on colonic function is important because nCI and MS are valid models of early life trauma but they induce neonatal stress through distinctly different mechanisms. MS models psychological neonatal stress through disruption of mother–offspring interactions, whereas nCI is a model of physical trauma in the neonatal period and requires exposure to colonic pain in the neonatal period (Al-Chaer et al., 2000). Given the variant types of early life trauma induced by these models, differential effects in colonic function may be observed in adulthood. Therefore, the goal of this study was to investigate whether mechanical irritation of the neonatal colon has an effect on colonic mucosal and smooth muscle function. To address this goal, rats were exposed to nCI and, after stool characterization, colonic tissue was harvested for (i) histology, (ii) MPO analysis, and (iii) functional characterization of the mucosa and smooth muscle in adulthood. In the current study, we found that colonic irritation in neonatal rats leads to long-term changes in adulthood including loose stools, increased epithelial conductance, and smooth muscle hyper contractility, all of which occurred in the absence of any histological or inflammatory changes of the colon. Results were previously published in abstract form(Rao et al., 2008).
2. Experimental procedures
2.1. Animals
Preweanling male Sprague-Dawley neonatal rats were obtained from Harlan Sprague-Dauley Inc. (Indianapolis, IN) and received neonatal colonic irritation at the University of Arkansas. At 2 months of age, rats were transferred to the Veteran’s Affairs Medical center animal facility located at the University of Oklahoma Health Sciences Center for organ bath recordings, stool scoring, and Ussing chamber recordings. Following transfer, animals were allowed to acclimate to the novel environment for a period of 3 weeks prior to experimentation. Rats were group-housed with ad libitum food and water at 23 °C in a 12:12 h light–dark controlled room at both facilities. All experiments were approved by the Institutional Animal Care and Use Committee.
2.2. Neonatal colonic irritation
Rats were randomly assigned to either the colorectal distension treatment group (n = 7) or to the control group (n = 7). At the University of Arkansas, the treatment group received colorectal distension on postnatal days 8, 10, and 12. Balloon distensions were performed at a pressure of 60 mmHg for 1 min and repeated two additional times per day with an interval of 30 min between distensions. The control group did not receive any colonic manipulation but were separated from their mothers for the same length of time as the treatment group and gently touched on the perineal area on postnatal days 8, 10, and 12.
2.3. Evaluation of stool character
Before in vitro experimentation, adult animals were weighed and stool samples were collected for evaluation. Specifically, the stool was scored as 0 for normal, 1 for soft with formed pellets, 2 for soft with no pellet formation, and 3 for watery diarrhea. Average stool scores were calculated for each experimental group. Following scoring, the stool was immediately weighed then baked for 24 h at 37 °C. The dried fecal pellets were weighed again, and the stool wet to dry ratio was calculated (wet weight of stool/dry weight of stool).
2.4. Ussing chamber recordings
Tissues were obtained between 3 and 4 months of age following euthanasia by decapitation after heavy sedation with 5% isoflurane. Immediately following euthanasia, a midline incision was made, and the colon was isolated and excised. The colonic segment was cut longitudinally and placed in ice-cold oxygenated Krebs buffer. The segments were then stripped of the external muscle layer and myenteric plexus and clamped between two Perspex chambers (volume 10mL, area of opening 0.7 cm2) which are part of the Ussing Chamber System (World Precision Instruments, Florida, USA). Each mucosal sheet was bathed with Krebs buffer maintained at pH 7.4 and 37 °C and gassed with 95% oxygen and 5% carbon dioxide. Two pairs of agar–salt bridge electrodes were connected to an automatic voltage clamp apparatus (DCV4000, World Precision Instruments, Sarasota, FL) to measure both potential difference (PD) and short-circuit current (Isc). Baseline values were obtained 30 min after tissue mounting. Baseline transepithelial conductance (G) was then calculated according to Ohm’s law(V = IR) using the baseline Isc and PD (G = Isc/PD). Isc was continuously monitored as a measure of net electrolyte transport during experiments and recorded using a MacLab data acquisition system (AD Instruments Ltd., Castle Hill, Australia). The effect of forskolin (10 µM) in the presence of tetrodotoxin (TTX; 1 µM), carbachol (CCh; 0.01–50 µM), and electrical field stimulation (EFS; 1–32 Hz) were measured as an increase in basal Isc. Data was normalized for the area of the Ussing chamber opening (µA/cm2).
2.5. Organ bath recordings
Circular muscle strips were isolated from the mucosa and mounted vertically in 10-mL organ baths with one end fixed and the other attached to an isometric force transducer (Radnoti Glass Technology Inc., Monrovia, CA). The baths were filled with Krebs buffer maintained at 37 °C and aerated with 95% O2 and 5% CO2. Each muscle strip was allowed to equilibrate for 20 min at zero tension before consecutive loading with 0.2 g force increments until a level of optimal resting tension was achieved. Optimal resting tension was achieved when the contractile response to 80 mM KCl ceased to increase with loading. All experiments were performed at optimal tension and isometric contractions were recorded using a MacLab data acquisition system (AD Instruments Ltd., Castle Hill, Australia). Contractions induced by potassium chloride (KCl; 80 mM), CCh (0.1–6 µM), or EFS (0.5–16 Hz) were measured as changes in basal tension (mN) and normalized per gram wet weight of the tissue.
2.6. Assessment of the colonic mucosa: histology
Distal colonic tissue was collected, placed in formalin, and sent for histologic processing. The sections of colonic tissue were chosen to correspond with the area of balloon distension during nCI. A cross-section of the colon was fixed in formalin and embedded in paraffin. Slide-mounted specimens were stained with hematoxylin and eosin. Each slide was then reviewed for inflammation. Mucosal thickness was also measured.
2.6.1. Myeloperoxidase (MPO) assay
MPO is an enzyme expressed by neutrophils and is considered amarker for tissue inflammation. One full-thickness segment from the midcolon, within the region of nCI, of each rat was collected, frozen in liquid nitrogen, and stored at −80 °C. MPO activity was assayed simultaneously for the whole set of experiments. Homogenization and extraction of MPO from the homogenate was carried out in hexadodecyl-trimethylammonium bromide (HTAB) phosphate buffer with pH of 6. MPO activity was tested in 10-µL samples using 3,3′,5,5′-tetramethylbenzidine (TMB) Microwell Peroxidase Substrate System (Sigma–Aldrich Co., St. Louis, MO) and horseradish peroxidase as a relative standard. MPO activity was expressed as equivalent to the activity of the relative standard converting the same amount of 3,3′,5,5′-TMB substrate for 10 min at room temperature. The data are expressed in nanograms per gram wet weight of the tissue.
2.7. Solution and drugs
Krebs buffer (mmol L−1): NaCL 120, KCl 6, MgCl2 1.2, NaH2PO4 1.2, CaCl2 2.5, NaHCO3 14.4, glucose 10. Carbamoylcholine (carbachol), tetrodotoxin, forskolin, and KCl were obtained from Sigma Chemical Co. and were dissolved in distilled water.
2.8. Statistics
Results are expressed as mean ± standard error of the mean (SEM). Statistical significance of differences was determined using a two-tailed Student’s t-test. Differences of p < 0.05 were considered to be significant. In the text, n refers to number of mucosal and muscular preparations unless otherwise stated. Statistical analysis and curve fitting of concentration–response data were performed using Prism software (GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Fecal analysis
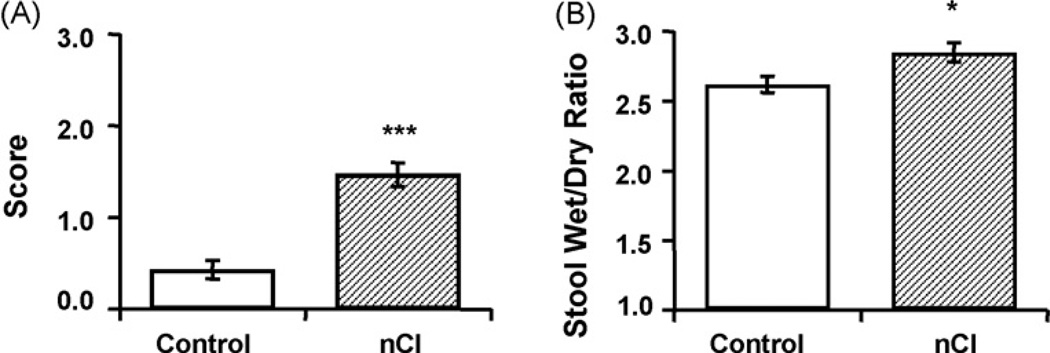
Repetitive CRD at a distension pressure of 60 mmHg on postnatal days 8, 10, and 12 led to significant changes of stool character in adult rats. Using a validated stool consistency score where 0 represents formed stool and 3 represents watery stool, we found that neonatally stressed rats exhibited more unformed and watery stools in adulthood compared to untreated and age-matched controls (1.47 ± 0.13 vs. 0.43 ± 0.10, respectively, p < 0.001) (Fig. 1). Additionally, nCI animals displayed a higher stool wet/dry ratio than controls (2.85 ± 0.07 vs. 2.62 ± 0.06, respectively, p < 0.05), thereby confirming that neonatal colonic insult increases stool water content.
Fig. 1.
Neonatal colonic irritation (nCI) led to the development of loose, watery stools in adult rats. nCI caused a higher stool consistency score, indicating more watery stools (A) and a higher stool wet/dry ratio than controls (B). n = 7 rats/group; *p < 0.05, ***p < 0.001.
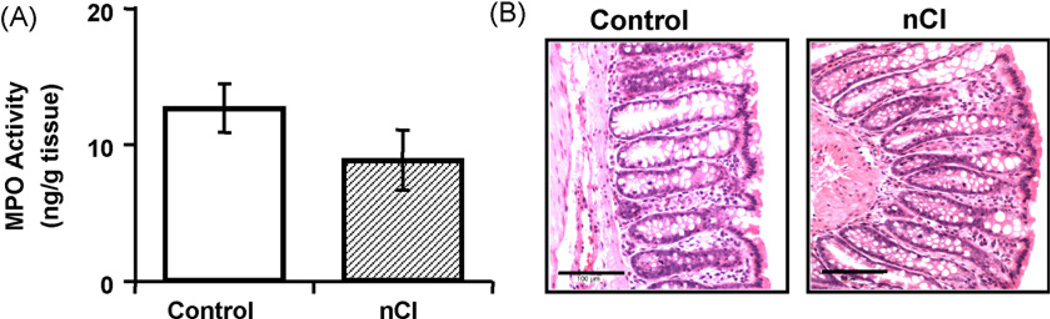
3.2. Assessment of colonic histology and MPO analysis
As illustrated in Fig. 2, we did not observe any marked changes in the colonic tissue taken from neonatally treated rats compared to controls. Specifically, in colonic tissue from treated and untreated rats, there was no evidence of inflammation as assessed histologically, or changes in inflammatory infiltrate as measured via MPO analysis (nCI 8.91 ± 2.18 ng/g tissue; control 12.67 ± 1.80 ng/g tissue, p > 0.05). Additionally, we found through direct measurements that there was no statistically significant difference (p > 0.05) in colonic mucosal thickness between neonatally stressed rats (284.45 ± 9.68 µm) compared to untreated and age-matched controls (249.32 ± 10.58 µm).
Fig. 2.
nCI did not cause colonic inflammation in adult rats. MPO activity showed no difference in infiltration of neutrophiles within the colon between controls and the nCI group (n = 7 rats/group; p > 0.05) (A). Representative histological cross-sections of the distal colon revealed no structural damage or inflammation due to nCI (B).
3.3. Mucosal function
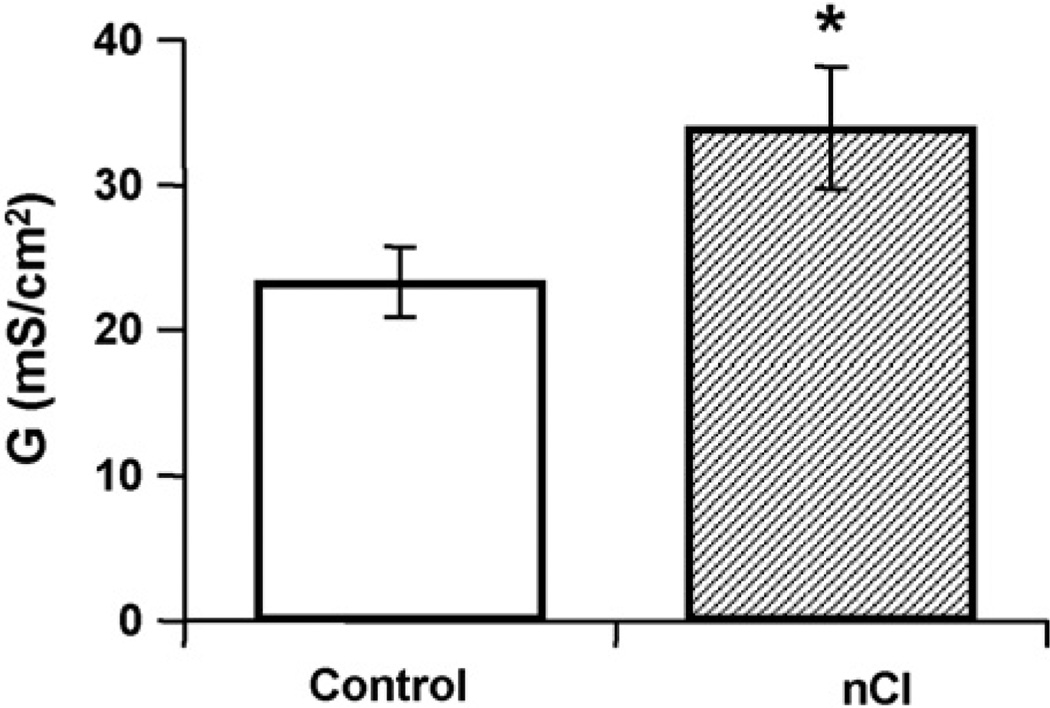
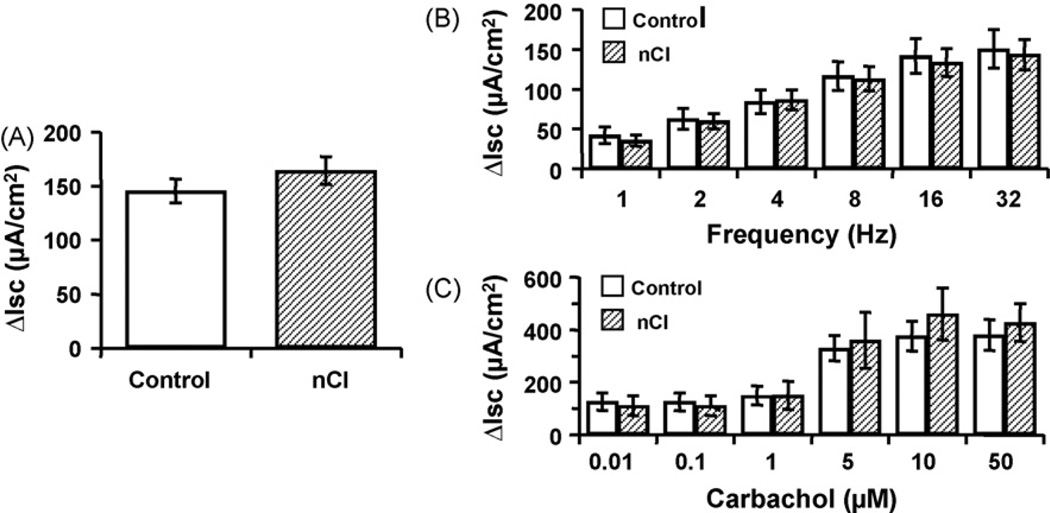
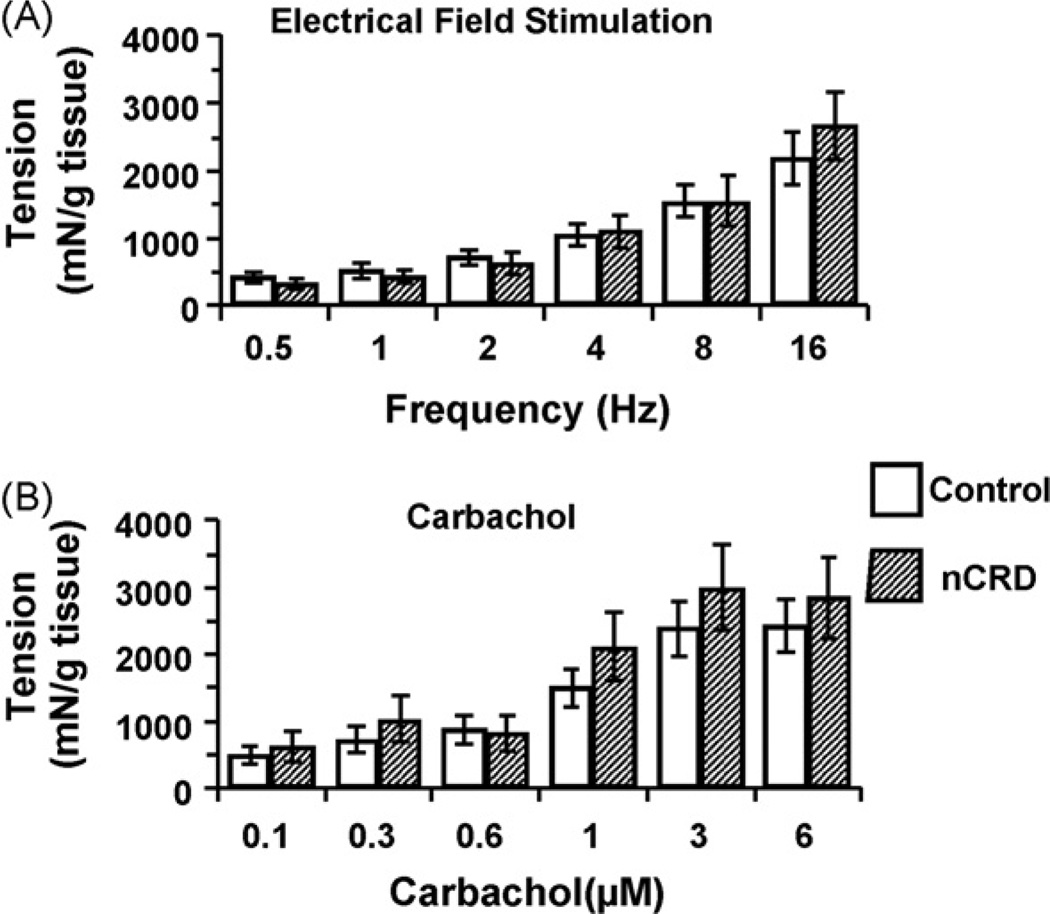
In adult rats that received repetitive CRD as neonates, basal epithelial conductance was significantly increased compared to untreated and age-matched controls (nCI 33.87 ± 4.24; control 23.25 ± 2.44 mS/cm2, p < 0.05) (Fig. 3). However, increases in active ion transport as assessed through measures of Isc in response to EFS (1–32 Hz), CCh (0.01–50 µM) or forskolin (10 µM) in TTX-treated tissue (1 µM) were similar in both nCI and control rats (p > 0.05) (Fig. 4).
Fig. 3.
nCI increased basal conductance across the colon. n = 26–27/group; *p < 0.05.
Fig. 4.
The neonatal challenge caused no significant change compared to controls in Isc in adulthood in response to forskolin (A), EFS (B), or carbachol (C). n = 26–27/group; p > 0.05.
3.4. Smooth muscle function
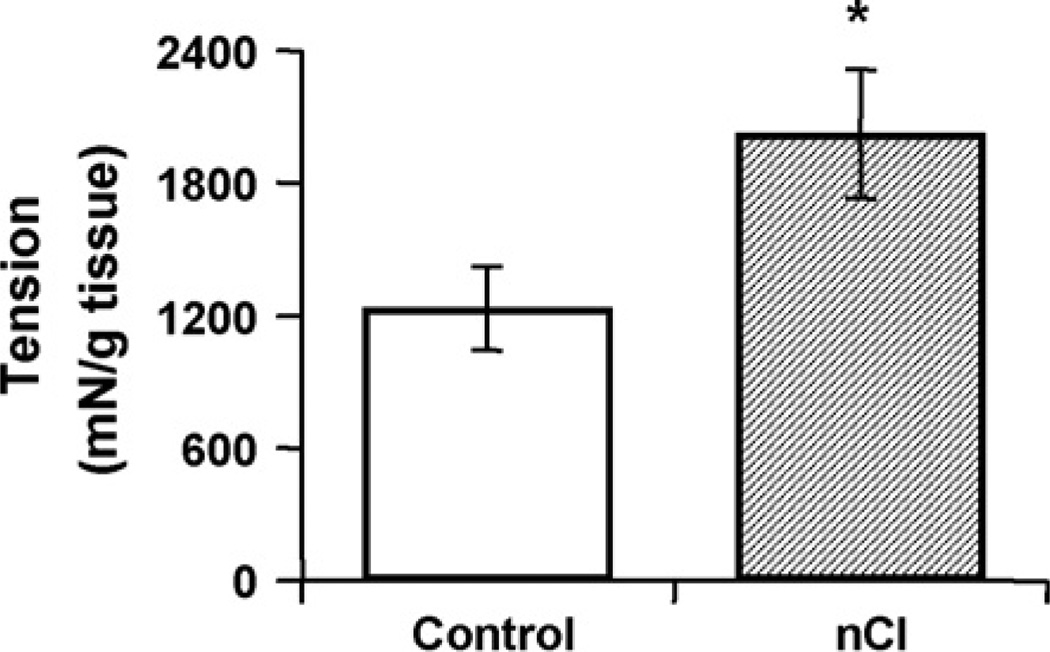
Colonic tissue from adult rats that had undergone repetitive neonatal CRD showed no differences in smooth muscle contractility in response to EFS (0.5–16 Hz) or CCh (0.1–6 µM) compared to untreated controls (Fig. 5; p > 0.05). However, the maximal contraction of smooth muscle produced by KCl (80 mM) was significantly elevated in the colon from adults that underwent the neonatal challenge (Fig. 6; nCI 2023.58 ± 294.07; control 1233.48 ± 796.95 mN/g tissue, p < 0.05)
Fig. 5.
There was no difference in the change in contractile response of colonic circular muscle following EFS (A) or carbachol (B) between adult nCI animals and controls. n = 26–27/group; p > 0.05.
Fig. 6.
Circular muscle strips from the nCI group had an increased contractile response to KCl in adulthood as compared to controls, suggesting that long-term changes resulting in smooth muscle hypercontractility do not involve alterations in enteric nerve-mediated mechanisms. n = 26–27/group; *p < 0.05.
4. Discussion
Previous investigations into the effects of nCI on visceral sensitivity have shown an increase in central and peripheral sensitization resulting in visceral hypersensitivity in adulthood (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003). The results of the current study further characterize this model of early life trauma by showing that nCI leads to longlasting abnormalities in colonic function in the adult rat including loose stools, increased epithelial conductance, and smooth muscle hypercontractility which occurred in the absence of any histological or inflammatory changes to the colon. Therefore, this model induces alterations not only in peripheral and central processing of pain, but global changes in the function of the GI tract that are reflective of those observed in IBS patients.
In the present study, we found that neonatally challenged rats exhibit more unformed and watery stools in adulthood. This is in concordance with a subgroup of rats from a previous study where 13.6% of nCI animals had an increase in daily percent water content as compared to controls (Wang et al., 2008). However, this study had more variable results with 61.4% of animals within the control range and 25% with a decrease in stool water content. Additionally, although there was a significant difference of p < 0.05 between nCI and control animals in stool wet/dry ratio, this data must be interpreted with caution given the substantial overlap in the 95% confidence interval.
Importantly, the changes observed in the current study are not accompanied by inflammation of the colon as assessed histologically and via MPO analysis, which is consistent with previous results and confirms that the alterations in colonic function observed as a result of the transient neonatal challenge are not influenced or caused by the existence of inflammatory mediators at the level of the colon (Al-Chaer et al., 2000; Wang et al., 2008). In contrast, MS has been associated with an increase in colonic MPO activity, mast cell number, and an altered cytokine profile indicative of a low-grade inflammation at maturity (Gareau et al., 2006; Barreau et al., 2004a,b). The observed differences in adult immune response between nCI and MS suggest that the changes in colonic function and visceral pain perception are mediated by distinct mechanisms that are unique to each model.
In the current study, adult rats previously exposed to colonic irritation via CRD as neonates had significantly increased basal ionic transport across the colonic mucosa as compared to control animals. Enhanced ionic transport was also found in a previous study involving MS (Gareau et al., 2006) and suggests a similarity between the effects of nCI and MS on basal ionic conductance. The observed increase in ionic transport may directly cause increased luminal water content in animals previously subjected to nCI. Therefore, this heightened basal ionic transport may serve as a mechanism for the existence of more loose, watery stools in nCI animals as compared to controls. In this study, we did not observe a difference between the nCI group and controls in the change in short-circuit current across the colonic mucosa after addition of forskolin (a direct cAMP activator) in tissue treated with TTX. TTX is a potent neurotoxin that selectively blocks voltage-gated sodium channels, thereby removing the neural regulation of the enterocyte (Narahashi et al., 1964; Narahashi, 1974). Therefore, it can be concluded that the active, cAMP-mediated function of the enterocyte independent of neuronal output is not altered in response to nCI. The neurally-mediated secretory response was investigated by EFS of the enteric nerves. EFS induced frequency-dependent responses in both groups; however, there was no significant difference in the magnitude of the responses in the neonatally stressed animals, compared to controls thereby showing that neural modulation of colonic permeability is not affected by nCI. Carbachol, a cholinergic agonist, induced a dose-dependent secretory response that was similar in both groups, suggesting that the cholinergic component of the secretory response is not altered following nCI. This finding is in contrast to previous work involving MS where it was found that MS induces an enhanced cholinergic component which contributes to the development of increased epithelial permeability (Gareau et al., 2007). These differences give further evidence that MS and nCI induce changes in colonic functioning by distinctly unique mechanisms.
To investigate potential changes in colonic muscle contractility following nCI, circular muscle strips were isolated in adulthood and exposed to CCh, EFS, and KCl. Although the nCI group had an increased contractile response after exposure to KCl in adulthood as compared to controls, there was no difference between nCI and controls in the neurally-mediated response as induced by EFS or CCh. KCl induces a contractile response within the muscle as a result of direct depolarization and subsequent Ca2+ influx that is not mediated by neuronal communication (Bolton, 1979). Therefore, the observed differences in response to KCl exposure coupled with the lack of changes in response to EFS or CCh suggests that nCI causes long-term changes that result in smooth muscle hypercontractility independent of enteric nerve-mediated mechanisms. The smooth muscle hypercontractility may influence the existence of loose, watery stools in animals exposed to nCI, particularly when combined with the observed increase in basal ionic transport across the colonic mucosa. However, in vivo motility patterns were not assessed in this study and would be of interest for further investigation.
Several mechanisms may result in the observed changes in colonic function following nCI. Macromolecular permeability was not assessed in this series of experiments but has shown to be enhanced following MS (Söderholm et al., 2002; Barreau et al., 2004a,b; Gareau et al., 2006; Gareau et al., 2007) and in IBS patients (as reviewed in Barbara, 2006). Mucosal barrier dysfunction in the form of increased macromolecular permeability permits enhanced uptake of antigens, luminal bacteria, and inflammatory mediators which can lead to sensitization of primary afferent neurons and promote subsequent visceral hypersensitivity. Therefore, given the connection between mucosal barrier dysfunction and visceral afferent sensitization, macromolecular permeability will be of interest in future studies and may provide a mechanism for the previously reported peripheral afferent sensitization in response to nCI (Lin et al., 2003). Since changes in luminal compliance as a result of nCI were not directly measured, this cannot be ruled out as a potential contributor to the observed effects in adulthood. However, previous results using this protocol of nCI have reported colonic appearance within the normal range with no gross colon enlargement or anal sphincter relaxation, which suggests little to no changes in luminal compliance secondary to the neonatal colonic insult (Wang et al., 2008).
5. Conclusion
The results of this study confirm that neonatal CRD serves as an appropriate model for diarrhea-predominant IBS, having characteristics of both visceral hypersensitivity (Al-Chaer et al., 2000; Lin and Al-Chaer, 2003) and altered colonic function in the form of watery, unformed stool consistency, increased epithelial ionic permeability, and colonic smooth muscle hypercontractility in adulthood without significant signs of inflammation or histological changes within the colon.
Abbreviations
- CRF
corticotrophin releasing factor
- CRD
colorectal distension
- EFS
electrical field stimulation
- GI
gastrointestinal
- IBS
Irritable Bowel Syndrome
- Isc
short-circuit current
- KCl
potassium chloride
- MS
maternal separation
- nCI
neonatal colonic irritation
- PD
potential difference
- SEM
standard error of the mean
- TMB
tetramethylbenzidine
- TTX
tetrodotoxin
- VMR
visceromotor response
References
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Barbara G. Mucosal barrier defects in irritable bowel syndrome. who left the door open? Am. J. Gastroenterol. 2006;101(6):1295–1298. doi: 10.1111/j.1572-0241.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004a;127(2):795–804. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004b;53(4):501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr. Res. 2007;62(3):240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Scharff L. Psychosocial aspects of assessment and treatment of irritable bowel syndrome in adults and recurrent abdominal pain in children. J. Consult. Clin. Psychol. 2002;70(3):725–738. doi: 10.1037//0022-006x.70.3.725. [DOI] [PubMed] [Google Scholar]
- Bolton TB. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 2007;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282(2):G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Do psychosocial factors define symptom severity and patient status in irritable bowel syndrome? Am. J. Med. 1999;107(5A):41S–50S. doi: 10.1016/s0002-9343(99)00081-9. [DOI] [PubMed] [Google Scholar]
- Gareau M, Jury J, Perdue M. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am. J. Physiol. Gastiintest. Liver Physiol. 2007;293(1):G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr. Res. 2006;59(1):83–88. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Drossman DA. The role of psychosocial factors in irritable bowel syndrome. Baillieres Best Pract. Res. Clin. Gastroenterol. 1999;13(3):437–452. doi: 10.1053/bega.1999.0038. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RH, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–97. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971(1):73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107(1):271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol. Rev. 1974;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Rao A, Al-Chaer ED, Greenwood-Van Meerveld B. Repetitive colorectal distension in neonatal rats induces colonic mucosal and muscular dysfunction in adulthood. Gastroenterology. 2008;134(4):A-114. [Google Scholar]
- Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(3):G849–G856. doi: 10.1152/ajpgi.00400.2006. [DOI] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Taché Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289(4):704–712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Söderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283(6):G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107(4):1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Tyler K, Moriceau S, Sullivan RM, Greenwood-Van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol. Motil. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gu C, Al-Chaer ED. Altered behavior and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav. Brain Funct. 2008;4:28. doi: 10.1186/1744-9081-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Holtkotter B, Enk P, Hoelzl R, Holmes AK, Shabsin H, Schuster M. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]