Abstract
Cells from many tumors produce transforming growth factor (TGF)-βwhich facilitates their escape from control by the immune system. We previously reported that non-immunogenic cells from either of two transplantable mouse tumors became effective as therapeutic tumor vaccines after lentivirus-mediated shRNA interference to ‘silence’the TGF-β1 gene. We now show that cells from in vitro cultured human ovarian carcinomas (OvC) make large amounts of TGF-β1 and that this can be prevented by ‘silencing’ the TGF-β1 gene. We further show that in vitro sensitization of peripheral blood mononuclear cells (PBMC) in the presence of either mitomycin-treated OvC cells whose TGF-β1 gene was silenced or in vitro matured dendritic cells (DC) which had been pulsed with homogenates from OvC cells with silenced TGF-β1 generated a stronger Th1/Tc1 immune response to the respective WT OvC and also to the OvC antigens mesothelin and HE4 as measured by ELIspot assays. The percentage of interferon (IFN)-γ and tumor necrosis factor (TNF)-α-producing CD4+ and CD8+ T cells increased while there were fewer cells expressing markers characteristic for regulatory T cells or myeloid derived suppressor cells. Similar results were obtained when PBMC from a patient with OvC were sensitized to DC pulsed with homogenate from autologous TGF-β1-silenced tumor cells, and a cytolytic lymphocyte response was generated to autologous OvC cells. Our results support clinical evaluation of TGF-β1-silenced tumor vaccines for immunotherapy of OvC.
Keywords: TGF-β, Immunosuppression, Tumor vaccine, Dendritic cells
Introduction
Although most patients with advanced OvC can be brought into clinical remission, relapses are frequent. Since preclinical studies have shown that therapeutic tumor vaccines are often effective against micrometastases, immunization against antigens expressed by OvC cells may provide a beneficial addition to conventional therapy by destroying remaining small nests of cancer cells. The high mutation level in cancer cell populations makes it unlikely that a vaccine against one or two targets would be effective and favors the use of vaccines that encompass many tumor antigens.1 This need may be met by immunizing with modified whole tumor cells2-6 or with dendritic cells (DC) that have been pulsed7 or transfected8 with tumor material so as to engage a large part of the patient's immunological repertoire. However, the efficacy of tumor cell based vaccines has, as a rule, been low.
Many tumors,9-13 including most OvC,14, 15 make large amounts of TGF-β, which is a potent suppressor of the immune system with broad activity on T cells, natural killer (NK) cells, monocytes/macrophages, and DC.16-20 Active TGF-β binds to the TGF-βreceptors of tumor-infiltrating lymphocytes, including CD4+, CD8+, or NK cells and inhibits their proliferation, differentiation and cytokine secretion.21 Tumor-infiltrating DC secrect TGF-β and respond to TGF-β and IL-10 with markedly down-regulated expression of the costimularoty molecules CD80, CD86, and CD40, and decreased secretion of TNF-α, IL-12, and CCL5/RANTES.22 In addition, a DC subpopulation (CD4-CD8-DC) secrecting TGF-βhas been implicated in the generation and effector function of CD4+FoxP3+ regulatory T cells (Treg),23 which deliver inhibitory signals to CD4+, CD8+ cytolytic T cells, and NK cells to induce host tolerance to tumor.24 TGF-β is thus a key factor in the suppression of antitumor immunity and its action needs to be counteracted to improve the efficacy of immunotherapy.25
The SW1 clone of the K1735 mouse melanoma does not normally respond to immunotherapy. It makes large amounts of TGF-β1 and ‘silencing’of the TGF-β1 gene via lentivirus-mediated shRNA interference can make SW1 cells effective as a therapeutic vaccine.26 We hypothesized that production of TGF-β1 by OvC cells, likewise, contributes to their limited clinical efficacy for cell-based tumor vaccination. Applying an approach similar to that taken for SW1 we now show that OvC cells with a silenced TGF-β1 gene are more effective immunogen for in vitro sensitization, both when whole cells are used as a source of antigen or mature DC are pulsed with material from OvC cells with a silenced TGF-β1 gene.
Materials and Methods
Reagents
A neutralizing mouse anti-human TGF-β1 (clone T0438) monoclonal antibody (mAb)27 and control mAb (clone MOPC21) and mitomycin (MMC) were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human GM-CSF, IL-4 and TNF-αwere purchased from Promocell (Heidelberg, Germany). HLA-A2-restrcited mesothelin A2(531-539) peptide VLPLTVAEV or HIV-gag A2(75-83) peptide SLYNTVATL were synthesized by Biosynthesis (Lewisville, TX) and dissolved in 100% DMSO (Sigma). Recombinant human mesothelin28 and HE4 protein29 were purified by immunoaffinity chromatography from supernatants of cultured CHO-DG44 cells which had been stably transfected with genes encoding human mesothelin or HE4. Recombinant HIV-gag p24 protein was purchased from Bioclone Inc (San Diego, CA).
Cell Lines and PBMC
OvCar3 cells were obtained from ATCC (Manassas, VA) and 8 OvC lines (He109, He207, He235, H3750, H3907, H3909, H4007 and H4020) had been established in our laboratory from patients with stage III/IV OvC using published techniques.30 According to flow cytometry performed as previously described,28 47% of OvCar3 cells, 43% of He207 cells and 38% of He235 cells express mesothelin. ELISA assays demonstrated that OvCar3 cells secrete HE429 antigen in culture supernatants (1342pM/1 million cell/24 h), while He207 and He235 cells do not (data not shown); we also confirmed by real-time PCR that all 3 OvC lines express mesothelin while only OvCar3 also expresses HE4 (data not shown). T2 cells are human B and T lymphoblast hybrid that only expresses the HLA-A*0201 allele; they were obtained from ATCC.31 All cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Atlanta Biological, Norcross, GA), penicillin and streptomycin. PBMC were isolated from buffy coats from 4 healthy adult volunteers and from 1 patient #He235 with advanced ovarian cancer by Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) density gradient centrifugation and used immediately or after they had been cryopreserved in liquid nitrogen. All PBMC samples were HLA-A2-positive as determined by flow cytometry using HLA-A2-specific antibody (BB7.2).
Lentivirus Construction and Preparation
We used an approach similar to one applied to silence the TGF-β1 gene in mouse tumor cells,26 applying a web-based program, BLOCK-iT RNAi Designer (Invitrogen, Carlsbad, CA) for designing shRNA targets for human TGF-β1. Sense and antisense oligonucleotides for TGF-β1 and control were synthesized by Sigma and ligated into pENTR/U6 to express gene-specific shRNA under the U6-RNA promoter. The sequences of the oligonucleotides were as follows: control, which doesn't have any homologue in human genome, sense oligonucleotides, 5′-CACCCAGTCGCGTTTGCGACTGGCGAA CCAGTCGCAAACGCGACTG-3′, and antisense oligonucleotide, 5′-AAAACAGTCGC GTTTGCGACTGGTTCGCCAGTCGCAAACGCGACTG -3′; TGF-β1, sense oligonucleotides, 5′-CACCGGCAGCTGTACATTGACTTCC (1754-1774) CGAAGGAAGTCAATGTACAGCTGCC-3′, and antisense oligonucleotide, 5′-AAAAGGCAGCTGTA CATTGACTTCCTTCGGGAAGTCAATGTACAGCTGCC-3′. The resulting entry vectors were termed pENTR/sh control and pENTR/sh TGF-β1, respectively. The LR recombination reaction was completed between the pENTR/sh control (or pENTR/sh TGF-β1) vector and the pLenti6/BLOCK-iTTM-DEST construct to generate pLenti6/BLOCK-iTTM expression constructs according to the manufacturer's instructions. Lentivirus were produced by cotransfecting 293FT cells, which stably express the SV40 large T antigen from the pCMVSPORT6TAg.neo plasmid, with the lentivirus expression plasmid and packaging plasmids using the Lipofectamine 2000 method (Invitrogen).
TGF-β1 Gene Silencing
OvCar3, He207 or He235 OvC cells (106) were plated in 6-well tissue culture plates (Corning Inc., Corning, NY) 1 day before infection and incubated with 1 mL medium containing lentivirus encoding shRNA specific for TGF-β1 or a control gene. After 24 h, the lentivirus containing medium was replaced with fresh medium and the cells were cultured in the presence of 10 μg/mL blasticidin for 5-7 days before assaying TGF-β1 production by ELISA. Cells transfected to silence the TGF-β1 gene were referred to as OvCar3-TGF-β1, He207-TGF-β1 and He235-TGF-β1 respectively, while the corresponding control cell lines were called OvCar3-control, He207-control and He235-control.
Measurement of TGF-β1 Secretion
The level of TGF-β1 was measured in culture supernatants using a commercially available TGF-β1 ELISA kit (R&D Systems, Minneapolis, MN). Wild type (WT) OvC cells, as well as OvC cells transfected with a lentivirus vector specific for TGF-β1 or control were seeded into 6 well plates with 106 cells per well at 37°C in 2 mL DMEM containing 2% FBS. The supernatants were harvested after 48 hours and assayed for TGF-β1 secretion following the manufacturer's instructions. Results were expressed as pg/mL after subtracting the amount of TGF-β1 in medium alone.
In Vitro Sensitization with MMC Treated Tumor cells
MMC-treated tumor cells (TGF-β1 silenced or control) were plated at a density of 5 ×105/well in 6-well tissue culture plates containing 2 mL complete PRMI-1640 medium (Invitrogen) with 10% human AB serum (Valley Biomedical, Winchester, VA), 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. 5 ×106 PBMC (encompassing T and B cells as well as monocytes as a source of DC) were added to each well, and the cultures were maintained for 7 days with the addition of 20 U/mL IL-2 on days 3 and 5. On the 7th day, T cells were gently removed, purified and tested with ELIspot assays to evaluate responses to WT ovarian cancer cells. In order to evaluate the effect of TGF-β1 antibody during the sensitization, 10 μg/mL anti-TGF-β1 was added into the culture containing PBMC with MMC treated WT ovarian cancer cells. After 7 days, PBMC were purified by Ficoll-Hypaque density gradient centrifugation and tested for IFN-γproduction by ELIspot assay.
Preparation of Mature DC and Tumor Cell Lysates
Monocytes were purified from PBMC by positive selection with CD14 microbeads according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Monocytes (>95% pure) were cultured for 6 days with medium change every 2 days in complete RPMI-1640 medium with 2% human AB serum in the presence of 800 U/mL GM-CSF and 500 U/mL IL-4. After 6 days, the loosely adherent or non-adherent cells displayed typical DC morphology and surface markers (CD11c, CD80, CD86, HLA-DR). For maturation, DC were plated at a density of 106/mL and incubated with 10-50 ng/mL TNF-α for 48 h.
We cultured OvC cells with control or TGF-β1 silence for 2 weeks before lysate preparation. Tumor cells (107) were washed in PBS and subjected to five freeze (liquid nitrogen) and thaw (37 °C water bath) cycles to obtain a crude lysate. After removal of large particles by centrifugation (2000 g for 10 min at 4 °C), the lysate was aliquoted and stored at −80 °C until use.
In Vitro Sensitization of PBMC with Lysate-Pulsed DC
1 ×106 DC in one mL were incubated for 18 h with TGF-β1-silenced or control tumor cell lysates at 3:1 cell equivalent ratio. After washing 3 times with medium, the pulsed DC were added to 10 ×106 monocyte-depleted autologous PBMC at the ratio of 1:10 and cultured in complete RPMI-1640 medium containing 20 U/mL IL-2 at 37 °C for 6-7 days.
For treating cells with anti-TGF-β1 antibody, we cultured OvCar3 cells in the presence of 10 μg/mL anti-TGF-β1 or control antiobdy for 2 weeks with media/antibody change every 3 days. The antibody-treated OvCar3 cells were then subjected to intracellular phospho-smad2/smad3 analysis by flow cytometry and lysate preparation by repeated freeze and thaw.
ELIspot Assays
T cell responses to OvC cells or mesothelin or HE4 were determined by ELIspot assays as previously described.26 For responses to tumor cells, MMC-treated WT OvC cells were used as antigen-presenting cells (APC) at ratio of 1:10. For T cell responses to the OvC antigens, autologous mature DC pulsed with 10 μg/mL of recombinant mesothelin, HE4 or irrelevant HIV-gag protein (as control) were used as APC at ratio of 1:20. Sensitized PBMC (50,000/well) were cocultured with APC in triplicate wells of ELIspot assay plates (R&D Systems), which were coated with rat IgG1 anti-human IFN-γ mAb. After incubating for 40 hours at 37 °C, 5% CO2, the plate was washed with PBS/Tween 20, incubated with biotinylated rat IgG1 anti-human IFN-γ mAb for 2 h at room temperature, washed with PBS, incubated with avidin-peroxidase complex for 1 h at room temperature, washed, incubated with substrate 3-amino-9-ethylcarbazole (AEC; BD Bioscience, San Diego, CA) at room temperature and spot development stopped by distilled water rinse. Plates were dried and counted by AID ELIspot Reader System (Autoimmun Diagnostika GmbH, Strassberg, Germany). Results were expressed as the mean spot-forming cells (SFC)/105 PBMC of triplicates.
CTL Assays
We used OvC cells (allogeneic or autologous to the PBMC donor as indicated in Results) or T2 cells pulsed with either 10 μg/mL HLA-A2-restricted mesothelin,32 HLA-A2-restricted HE4 (H.Wei, data to be published) or an antigenically irrelevant HIV-gag peptide as target. Experiments were initially performed using the CytoTox 96®Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI) to determine target cell killing, following the manufacturer's instructions. In brief, target cells were incubated with varying numbers of effector cells for about 4 h, and supernatants were then analyzed for lactate dehydrogenase release. After obtaining clearance to work with 51Cr, we shifted to label target cells with 100 μCi Na251CrO4 in cell culture medium containing 10% FCS for 60 min at 37°C. The cells were washed twice in culture medium. After 4 to 6 h of incubation, 25 μL of the assay supernatant were placed into a 96-well Lumaplate (Perkin-Elmer, San Jose, CA). Scintillant (100 μL) was added and radioactivity was counted using a Microbeta scintillation counter (Perkin-Elmer). The results are expressed as percent specific lysis, calculated as (experimental release −spontaneous release / total release −spontaneous release) ×100. The two assays gave similar results.
Flow Cytometry Analysis
The sensitized PBMC were preincubated with human Fc receptor binding inhibitor for 10 min prior to staining with antibodies of CD4, CD8, CD56, CD14, B220, CD11b, HLA-DR, and CD33 (eBioscience, San Diego, CA) for 30 min. For the intracellular staining of Foxp3 or phospho-smad2 (Ser465/467)/smad3 (Ser423/425), the cells were fixed and permeabilized with the Cytofix/Cytoperm reagent (BD Bioscience) for 30 minutes at 4°C. This was followed by two washings with PermWash buffer and staining with anti-human Foxp3 (eBioscience) or anti-phospho-Smad2/Smad3 (D6G10; Cell signaling) at 4°C for 30 minutes. For intracelluar cytokine staining, sensitized PBMC were restimulated with 1 × PMA and ionomycin in the presence of brefeldin A and monensin (Cell stimulation cocktail with transporter inhibitors; eBioscience) for 5 h, then stained with antibodies of CD4, CD8 and CD56 for 30 min at 4 °C. Thereafter, the cells were fixed and permeabilized as described above and stained with anti-human IFN-γ and anti-human TNF-α (BD Bioscience). The flow cytometry was performed using FACSCalibur (Becton Dickinson, Mountain View, CA) and data were analyzed by FlowJo software.
Statistics
The results presented in the study were analyzed by 2-way ANOVA or student's t test using Prism 5.0 software. P < 0.05 was considered significant.
Results
Cultured OvC Cells Release TGF-β1 into Supernatants Which Can be Prevented by Silencing the TGFβ1 Gene
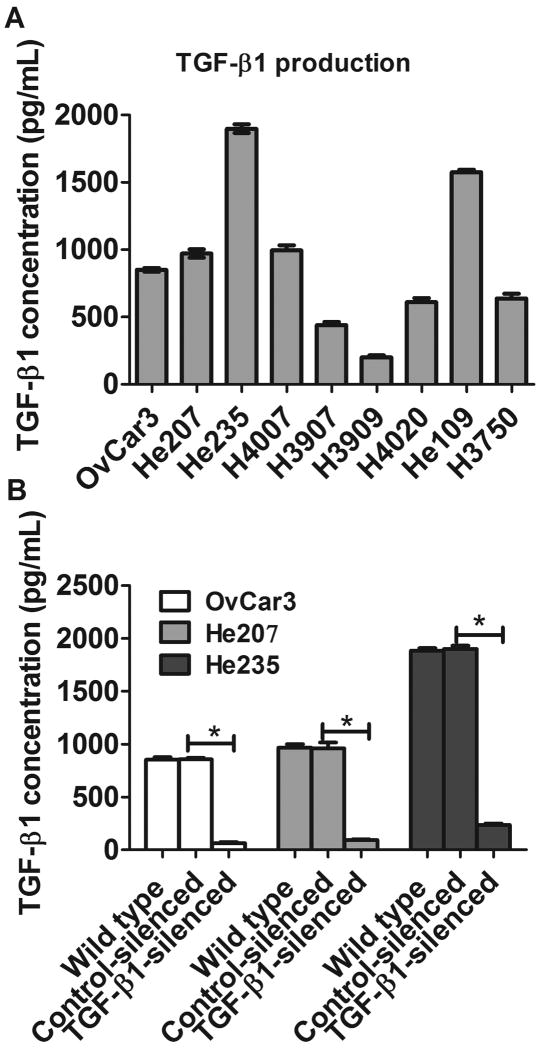
As shown in Fig. 1A, all of 9 human OvC lines released TGF-β1 into culture supernatants, although there was a substantial variation between individual lines. The experiment was repeated twice with similar results. Supernatants from OvCar3, He207 and He235 cells contained high levels of TGF-β1 and we selected these three cell lines for the studies described below.
FIGURE 1.
TGF-β1 production by cultured ovarian cancer cell lines. A, TGF-β1 production in supernatants from 9 ovarian cancer cell lines was determined by ELISA. B, TGF-β1 production in supernatants from TGF-β1 silenced or control ovarian cell lines. Result is representative of three experiments. *, p<0.001, silence vs control cell lines.
We next tried to silence the TGF-β1 gene using lentivirus-mediated shRNA interference. Fig. 1B shows that TGF-β1 was almost completely absent from supernatants of cultured OvCar3-TGF-β1 cells, He207-TGF-β1 or He235-TGF-β1 cells, while supernatants of cells from the respective tumors that had been transfected with the control lentivirus produced as much TGF-β1 as the WT cells. The experiment was repeated twice with similar results. We also confirmed the TGF-β1 silence using real-time PCR (data not shown).
TGF-β1-Silenced OvC Cells Have Increased Immunogenicity
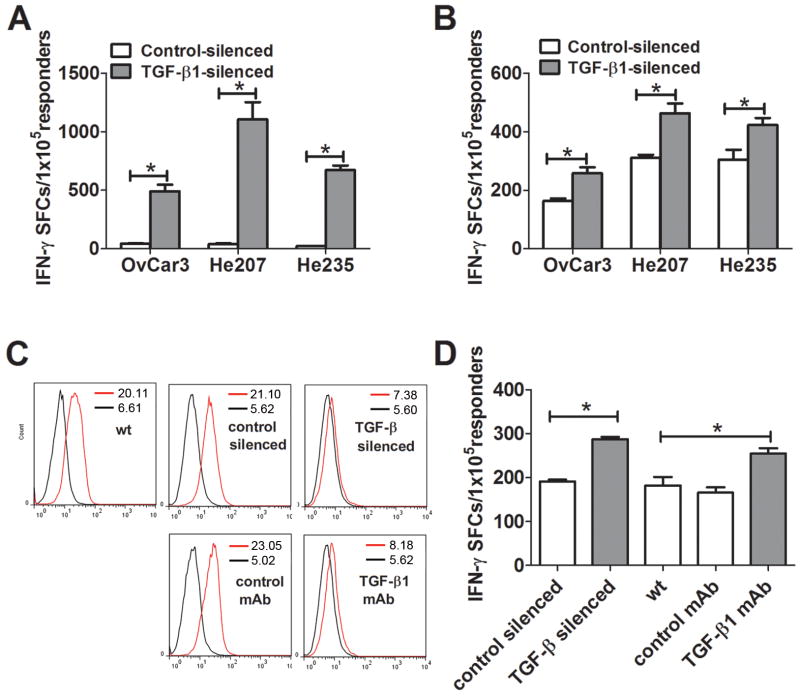
To explore whether knockdown of TGF-β1 expression in OvC cells enhances their immnogenicity, we utilized two different protocols for in vitro sensitization of PBMC to OvC cells. We first cocultivated PBMC from each of 3 healthy donors for 7 days with MMC-treated cells from TGF-β1-silenced or the corresponding control-silenced and WT OvC lines, after which we measured IFN-γ production by the sensitized cells in ELISPOT assays using MMC-treated WT cells as stimulators. In all of three independent experiments, sensitization against MMC-treated OvCar3, He207 or He235 OvC cells whose TGF-β1 gene had been silenced was significantly more effective than sensitization to cells whose TGF-β1 gene had not been silenced (Fig. 2A). No difference was observed in PBMC sensitized with control-silenced cells and WT cells (data not shown).
FIGURE 2.
TGF-β1-silenced OvC cells have increased immunogenicity. A, PBMC from 3 healthy donors were cocultured with MMC-treated TGF-β1 silenced or control-silenced OvC cells at ratio of 10:1 in 6-well plates for 7 days. The sensitized PBMC were assayed for IFN-γ production by ELISPOT using MMC-treated WT OvC cells as stimulators. B. PBMC were sensitized with autologous DC pulsed with homogenates from TGF-β1-silenced or control-silenced OvC cells at ratio of 10:1 in 6-well plates for 7 days. The sensitized PBMC were assayed for IFN-γproduction by ELISPOT using MMC-treated WT OvC cells as stimulators. C. OvCar3 WT cells were cultured in the presence of 10 μg/mL anti-TGF-β1 or control mAb for 2 weeks with media change every 3 day. The OvCar3 WT cells, OvCar3-control, OvCar3-TGF-β1 and antibody-treated OvCar3 cells were intracellularly stained with anti-phospho-Smad2 (Ser465/467)/Smad3 (Ser423/425) followed by flow cytometric analysis. Black and red lines denote, respectively, control and specific staining, and the number in the histogram denotes the geometric mean of fluorescence intensity. D. PBMC were sensitized against autologous DC which had been pulsed with homogenates from OvCar3-TGF-β1 silenced or corresponding control OvCar3 cells or from OvCar3 WT cells that had been grown for 14 days without added mAb, in the presence of a control mAb or with a mAb specific for TGFβ1 as described in Materials and Methods. PBMC and pulsed DC were cocultivated in 6 well plates for 7 days at a ratio of 10:1. and the sensitized PBMC were assayed for IFN-γ production by ELISPOT using MMC-treated WT OvC cells as stimulators. Data represented as mean ± SD of triplicate cultures. *, p<0.001, **, p<0.01, silence vs control cell lines.
Next we generated mature DC from CD14+ monocytes dervived from PBMC from 3 healthy donors, after which we pulsed them with homogenates from OvC cells which had an intact or silenced TGF-β1 gene and used them to sensitize the respective autologous monocyte-depleted PBMC. As shown in Fig. 2B, sensitization against DC pulsed with homogenates from TGF-β1-silenced OvC cells induced a significantly higher ELIspot response than seen with DC pulsed with homogenates from the respective control cells. It is noteworthy that the responses, as measured by ELIspots, were lower than when the PBMC had been sensitized by cocultivation with MMC-treated cells, and also that the differences between PBMC sensitized to DCs which had been pulsed with homogenates from OvC cells with silenced versus intact TGF-â1 gene were smaller, albeit still statistically significant.
We could recapitulate these results by cultivating OvC cells in the presence of a TGF-β1 neutralizing mAb (10 μg/mL) for 2 weeks before they were used to prepare homogenates for pulsing DC and sensitizing PBMC. Intracellular staining of phosphorylated SMAD2/3 demonstrated that the antibody treatment inhibited the TGF-β -associated signaling pathway comparable to that in cells whose TGF-β1 gene was silenced (Fig. 2C). As shown Fig. 2D, sensitization against DC pulsed with homogenates from anti-TGF-β mAb treated OvC cells induced an increased ELIspot response similar to that seen in the same experiment with DC pulsed with homogenates from cells with a silenced TGF-β1 gene and significantly higher than with DC pulsed with homogenate from cells grown in the presence of a control mAb. However, if the anti-TGF-β1 mAb was added to PBMC during the in vitro sensitization against MMC-treated OvCar3 WT cells or to homogenates from OvCar3 WT cells used to pulse DC we did not observe any increased IFN-γ release (data not shown).
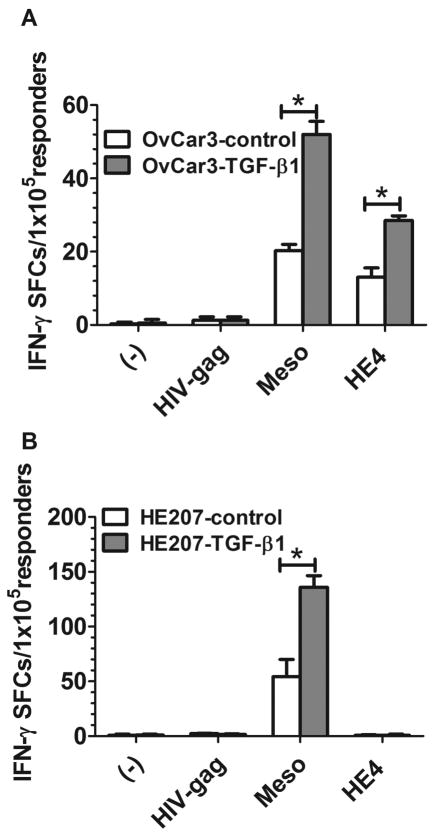
Sensitization against TGF-β1-Silenced OvC Cells Induces an Immune Response to Mesothelin and HE4, Two Ovarian Cancer Antigens
We sensitized lymphocytes obtained from PBMC with autologous DC that were pulsed with homogenates from TGF-β1-silenced or control-silenced OvCar3 or He207 cells and evaluated, in ELIspot assays, the sensitized lymphocytes for an immune response to autologous DC which had been pulsed with mesothelin or HE4 as stimulators; OvCar3 cells express both mesothelin and HE4 while He207 cells only express mesothelin. As shown in Fig. 3A, sensitization of PBMC from a healthy donor with OvCar3-TGF-β1 lysate-pulsed autologous DC induced a significantly increased IFN-γ response to both mesothelin and HE4. In contrast, lymphocytes that had been sensitized to DC that had been pulsed with He207-TGF-β 1 lysate only gave significant ELIspots to mesothelin (Fig. 3B), i.e. the data were consistent with the antigen expression profiles of the two cell lines. No IFN-γ production was observed when tumor homogenate-sensitized PBMC were restimulated with DC which had not been pulsed with antigen or were pulsed with HIV-gag.
FIGURE 3.
Increased antigen-specific immune responses induced by TGF-β1-silenced OvC cells. A, PBMC from 3 healthy donors were sensitized with autologous DC pulsed with homogenates from OvCar3-TGF-β1 or OvCar3-control OvC cells at ratio of 10:1 in 6-well plates for 7 days. The sensitized PBMC were evaluated for immune responses against mesothelin or HE4 antigen by ELISPOT using mature DC pulsed with mesothelin or HE4 antigen as stimulators. B, PBMC from 3 healthy donors were sensitized with autologous DC pulsed with homogenates from He207-TGF-β1 or He207-control OvC cells at ratio of 10:1 in 6-well plates for 7 days. The sensitized PBMC were evaluated for immune responses against mesothelin or HE4 antigen by ELISPOT using mature DC pulsed with mesothelin or HE4 antigen as stimulators. Data represented as mean ±SD of triplicate cultures. *, p<0.001, silence vs control cell lines.
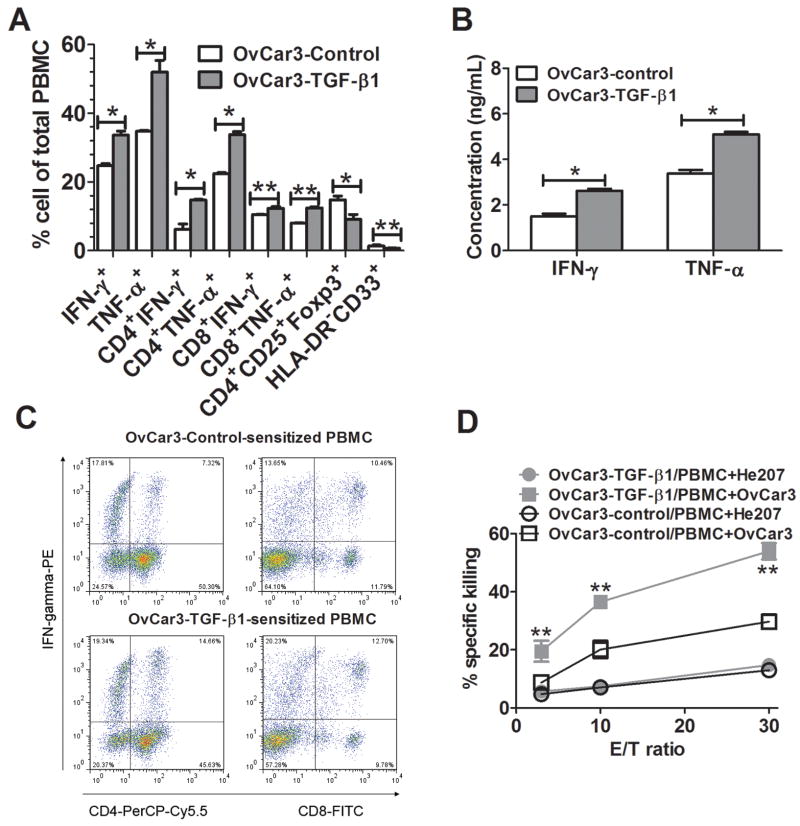
Sensitization with TGF-β1-Silenced OvC Cells Enhanced Th1/Tc1 Type Immune Responses
We next analyzed by flow cytometry populations of monocyte-depleted PBMC which had been cocultured with autologous DC that had been pulsed with homogenates from OvC cells whose TGF-β1 gene had, or had not, been silenced. There was a significantly higher percentage of IFN-γ- and TNF-α-producing CD4+ and CD8+ T when the homogenates were derived from cells with a silenced TGF-β1 gene (Fig. 4A), which was consistent with elevated concentrations of IFN-γand TNF-αin the corresponding supernatants (Fig. 4B). The increase in IFN-γ- and TNF-α-positive T cells was accompanied by fewer cells expressing markers characteristic for Treg (CD4+CD25+Foxp3+; ref 24) or myeloid derived suppressor cells (MDSC; Lin-HLA-DR-CD33+; ref 37) in the cultures, and a representative dotplot is shown in Fig. 4C. We obtained similar results with both He207 or He235 OvC cells (data not shown). Importantly, there was a significantly increased CTL activity of PBMC sensitized to homogenate from TGF-β1-silenced OvCar 3 cells as compared control-silenced OvCar3 (Fig. 4D) and the CTL activity was higher against OvCar3 than against a different OvC line, He207.
FIGURE 4.
Enhanced Th1/Tc1 immune response induced by TGF-β1-silenced OvC cells. A, PBMC from 3 healthy donors were sensitized with autologous DC pulsed with homogenates from OvCar3-TGF-β1 or OvCar3-control OvC cells at ratio of 10:1 in 6-well plates for 7 days. The sensitized PBMC were evaluated for IFN-γ- and TNF-α-producing CD4+ or CD8+, CD4+CD25+Foxp3+ and HLA-DR-CD33+ cells. B, The supernatants from A were assayed for IFN-γ and TNF-α production by ELISA. C, A representative dotplot showing IFN-γ-producing CD4+ or CD8+ T cells in sensitized PBMC. D, Sensitized PBMC were evaluated for tumor cell-specific killing activity in 51Cr release assays using the sensitizing OvCar3 cells or cells from a different OvC, He207, as targets. Data were expressed as mean ± SD of triplicate cultures. *, p<0.001, **, p<0.05, silence vs control cell lines.
TGF-β1-Silenced Autologous OvC Cells Induce an Increased Immune Response by PBMC from a Patient to Her Own OvC Cells and to Mesothelin
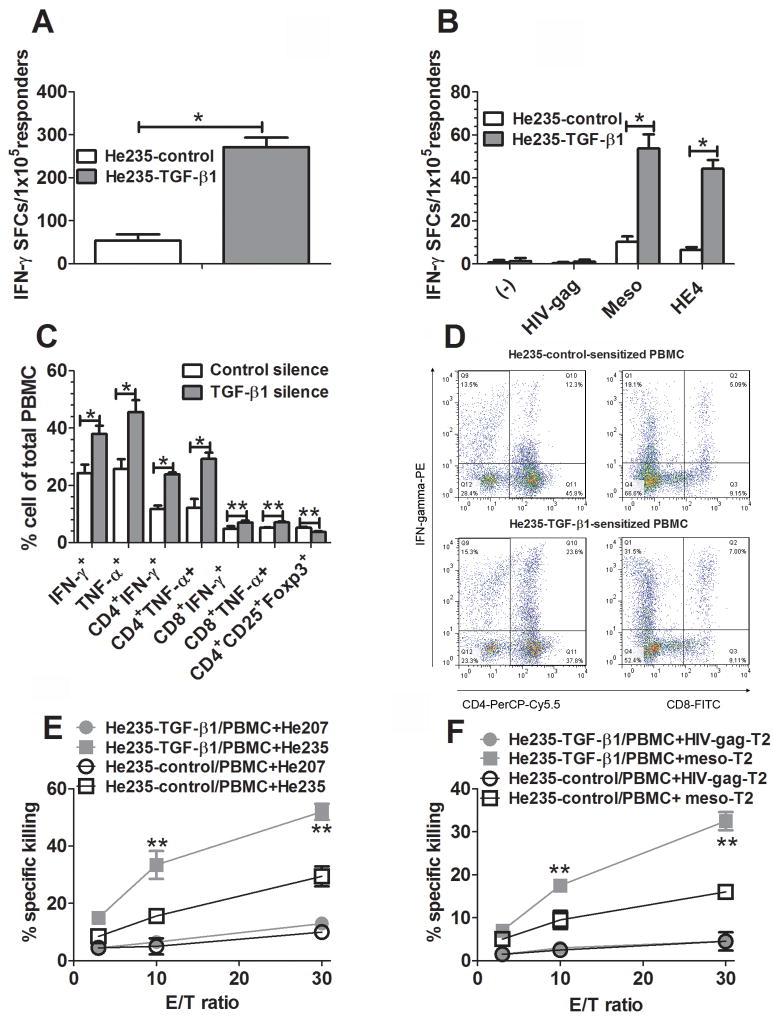
To further explore the clinical relevance of sensitization with TGF-β1-silenced OvC cells, we performed experiments similar to those described above sensitizing PBMC from a patient (#235) with OvC ovarian cancer against antigens expressed by her autologous tumor cells. DC isolated from the patient's DC were pulsed with homogenate from her autologous OvC whose TGF-β1 gene had either been silenced or left intact as a control. As shown in Fig. 5A and B, silencing of the TGF-β1 gene induced a significantly elevated immune response of DC pulsed with the corresponding homogenate to both tumor cells and mesothelin, as indicated by significantly increased ELIspots; it is notworthy that there was no increased response to HE4 which is not expressed by HE235 cells. Immunological phenotyping showed that PBMC sensitized with homogenates from cells with a silenced TGF-β1 gene contained many more IFN-γ or TNF-α-producing CD4+ and CD8+ T cells and fewer cells with markers characteristic of Treg (Fig. 5C); a representative dotplot is shown in Fig. 5D. Furthermore, PBMC sensitized against homogenates from He235 cells with a silenced TGF-β1 gene exhibited an increased CTL response to both autologous He235 OvC cells (Fig. 5 E) and to T2 cells that had been pulsed with a mesothelin-derived HLA-A2-restricted peptide (Fig. 5F).
FIGURE 5.
Increased sensitization of PBMC by DC pulsed with homogenates of autologous TGF-β1-silenced OvC cells. PBMC from a patient with advanced ovarian cancer were sensitized with her DC pulsed with homogenates from autologous He235-TGF-β1 or He235-control cells at ratio of 10:1 in 6-well plates for 7 days. A, Sensitized PBMC were evaluated for tumor cell-specific immune response by ELIspot using MMC-treated WT He235 cells as stimulators. B, Sensitized autologous PBMC were evaluated by ELISPOT using DC pulsed with mesothelin or HE4 antigen as stimulators. C, Sensitized PBMC were evaluated for IFN-γ- and TNF-α-producing CD4+ or CD8+, CD4+CD25+Foxp3+ and HLA-DR-CD33+ cells. D, A representative dotplot showing IFN-γ-producing CD4+ or CD8+ T cells in sensitized PBMC. E, Sensitized PBMC were evaluated for tumor cell-specific killing activity in CytoTox 96®non-radioactive cytotoxicity assays using autologous He235 cells or heterologous He207 cells as targets. F, Sensitized PBMC were evaluated for antigen-specific CTL activity in CytoTox 96®non-radioactive cytotoxicity assays using HLA-A2-restricted mesothelin peptide-pulsed T2 cells as targets. Data were expressed as mean ± SD of triplicate wells. *, p<0.001, **, p<0.05, silence vs control cell lines.
Discussion
Ovarian carcinomas are attractive targets for therapeutic cancer vaccination both because of a great clinical need for patients with advanced disease and because conventional therapy can effectively reduce the tumor load, providing a situation similar to that when several cancer vaccines can cure tumors in preclinical models. Vaccination with engineered tumor cells or DC that have been ‘pulsed’7, 37-39 or transfected8 with tumor material is likely to engage a much larger part of the immunological repertoire and thereby be more efficacious than a vaccine that is specific for one or a few epitopes. However, the ability of many tumors to make immunosuppressive molecules, among which TGF-β is most powerful,20,24 may dramatically decrease their efficacy as source of a vaccine.
Previous studies have demonstrated that silencing of the TGF-β gene in tumors of mouse, rat or human origin increases their immunogenicity.31-36 In this study, we confirmed published data demonstrating that cultured cells from human OvC cells make TGF-β114,15and demonstrated that this production can be prevented by silencing the TGF-β1 gene via lentivirus-mediated shRNA interference. We then showed that PBMC from 3 healthy adult volunteers, according to ELIspot assays, were more effective when sensitized during 7 day cocultivation with MMC-treated OvC cells whose TGF-β1 gene had been silenced. Likewise, silencing of the TGF-β1 gene increased the immunogenicity of homogenates from OvC cells used to pulse DC for sensitization of autologous lymphocytes. There was an increased immune response to known OvC antigens, mesothelin and HE4, consistent with the expression of these antigens on the sensitizing OvC cells,28,29 implying that the immune responses detected with PBMC from healthy donors were not just directed to alloantigens expressed by the OvC but not by the PBMC. While previous studies demonstrated cell-mediated immunity to mesothelin,37 the demonstration of a Th1 type immune response to HE4 is novel.
Finally, we sensitized PBMC-derived lymphocytes from a patient, #235, who has stage IV serous OvC by pulsing her DC with a homogenate from autologous He235 OvC cells whose TGF-β1-gene had either been silenced or remained intact. There was a significantly more potent ELIspot response when the DC were pulsed with a homogenate from He235 cells whose TGF-β1 gene had been silenced, and there was increased CTL activity, both against autologous He235 OvC cells and against mesothelin which is expressed by the He235 OvC but not against HE4 which is not expressed.
According to flow cytometry, sensitization against cells with a silenced TGF-β1 gene increased the frequency of CD4+ and CD8+ lymphocytes expressing IFN-γ and TNF-α while decreased the frequency of CD4+CD25+Foxp3+ cells,21 and it also decreased the frequency of cells with markers characteristic of MDSC.38 Importantly, there was an increase of antigen-specific CTL activity when the PBMC had been sensitized by homogenates from TGF-β1-silenced OvC cells. We conclude that sensitization against intact or homogenized tumor cells whose TGF-β1 gene is silenced more effectively induces a Th1/Tc1 type immune response as may be expected based on the known effects of TGF-β on the immune response, as reviewed in the Introduction.
Importantly, we could recapitulate these results by culturing OvCar3 WT cells in the presence of a TGF-β1-specific neutralizing mAb for 2 weeks before they were used to prepare homogenate for pulsing DC. However, addition of anti-TGF-β1 mAb during the in vitro sensitization of PBMC to DC pulsed with OvCar 3 WT cells did not improve the immune response as measured by the ELIspot assay, indicating that the increased immunogenicity of OvC cells whose TGF-β1 gene is silenced is not due to the removal of soluble TGF-β1. Our preliminary results indicate that silencing of TGF-β1 promotes the expression of mesothelin and HE4 antigens by OvC cells (supplementary Figure 1), and we hypothesize that this may explain their increased immunogenicity.
Our findings are noteworthy in view of the substantial number of studies performed by vaccinating with DC that have been pulsed with tumor homogenates both in animal models7,39 and in human patients,40,41 and they have implications for the development of therapeutic vaccines using whole tumor cells or DC which have been pulsed with homogenates from tumor cells or transfected with RNA or DNA prepared from the tumor cells. While pooled material from several cultured lines of the same tumor contain many shared tumor antigens, ideally, a vaccine should also engage an immune response that is directed to putative antigens that are unique for a patient's own tumor as a result of mutations and other genetic events. While vaccines using tumor peptides or genes encoding a limited number tumor epitopes are not contaminated with immunosuppressive material, their efficacy is hampered by the fact that they engage a much smaller part of the immunological repertoire than a cell-based vaccine.
We speculate that silencing of some of the genes that encode other immunosuppressive molecules, including other members of the TGF-β family, as well as B7-H1, IL-10, indoleamine 2,3-dioxygenase (IDO), prostaglandin E2, nitric oxide etc in the tumor cells used as source of a vaccine may also improve its therapeutic efficacy.42-46 The efficacy of vaccination may be further improved by engineering the tumor cells to engage immunostimulatory molecules such as GM-CSF, CD40, CD80, CD83, CD137 etc.4-6, 47-49 However, to achieve therapeutic efficacy against tumors which are larger than a few mm in diameter, the powerful immunosuppressive mechanisms at tumor sites need to be overcome.
Supplementary Material
Silencing of the TGF-β1 gene promotes the expression mesothelin and HE4 in OvC cells. A. OvCar3 and He207 OvC cells with control or silenced TGF-β1 were fixed and permeabilized with the Cytofix/Cytoperm reagent and intracelluarly stained with anti-mesothelin antibody (569) followed by FITC conjugated secondary anti-mouse antibody. The intracellular expression of mesothelin was analyzed by flow cytometry. The number in the histogram denotes the geometric mean of fluorescence intensity. Dash line, control stain; black line, OvCar3-control cells; red line, OvCar3-TGF-β1 cells. B. The supernatants were harvested from the OvCar3 and He207 OvC cells with control or silenced TGF-β1 for assaying the HE4 secretion by ELISA kit from FDI. Data were expressed as mean ±SD of triplicate wells (pM/1 million cell/24 h).
Acknowledgments
Our work was supported by a grant from Marsha Rivkin Center for Ovarian Cancer Research and by grant RO1-112073, from NIH. We thank Dr. N. Kiviat, Dr Y. Guo and Mrs K. Agnew for support and Dr D. Gretch for use of his facilty to conduct CTL experiments with 51Cr labeled target cells.
Grant support: This work was supported by RO1 CA134487 from National Institutes of Health and by a grant from Marsha Rivkin Center for Ovarian Cancer Research.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Bielas JH, Loeb KR, Rubin BP, et al. Loeb La. Human cancers express a mutator phenotype. Proc Natl Acad Sci U S A. 2006;103:18238–18242. doi: 10.1073/pnas.0607057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morton DL, Barth A. Vaccine therapy for malignant melanoma. CA Cancer J Clin. 1996;46:225–244. doi: 10.3322/canjclin.46.4.225. [DOI] [PubMed] [Google Scholar]
- 3.Harada M, Li YF, El-Gamil M, et al. Melanoma-Reactive CD8+ T cells recognize a novel tumor antigen expressed in a wide variety of tumor types. J Immunother. 2001;24:323–333. doi: 10.1097/00002371-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patiens. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardoll DM. Therapeutic vaccination for cancer. Clin Immunol. 2000;95:S44–62. doi: 10.1006/clim.1999.4819. [DOI] [PubMed] [Google Scholar]
- 6.Ye Z, Hellstrom I, Hayden-Ledbetter M, et al. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 7.Fields RC, Shimizu J, Mule' JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson RB, Kasid A, Huff KK, et al. Activation of growth factor secretion in tumornigenic states of breast cancer induced by 17 beta-estradiol or v-Ha-ras oncogene. Proc Natl Acad Sci U S A. 1987;84:837–841. doi: 10.1073/pnas.84.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu SL, Zhang WC, Akiyama Y, et al. Genomic structure of the transforming growth factor beta type II receptor gene and its mutation in hereditary nonpolyposis colorectal cancers. Cancer Res. 1996;56:4595–4598. [PubMed] [Google Scholar]
- 11.Park K, Kim SJ, Bang YJ, et al. Genetic changes in the transforming growth factor beta (TGF-beta) type II receptor gene in human gastric cancer cells: correlation with sensitivity to growth inhibition by TGF-beta. Proc Natl Acad Sci U S A. 1994;91:8772–8776. doi: 10.1073/pnas.91.19.8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumoto S, Artita N, Ohnishi T, et al. Microsatellite instability and mutated type II transforming growth factor-beta receptor gene in gliomas. Cancer Lett. 1997;112:251–256. doi: 10.1016/s0304-3835(96)04583-1. [DOI] [PubMed] [Google Scholar]
- 13.Derynck R, Goeddel DV, Ullrich A, et al. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal groth factor receptor by human tumors. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- 14.Bartlett JM, Langdon SP, Scott WN, et al. Transforming roth factor-beta isoform expression in human ovarian tumours. Eur J Cancer. 1997;33:2397–2403. doi: 10.1016/s0959-8049(97)00304-3. [DOI] [PubMed] [Google Scholar]
- 15.Gordinier ME, Zhang HZ, Patenia R, et al. Quantitative analysis of transforming growth factor beta 1 and 2 in ovarian carcinoma. Clin Cancer Res. 1999;5:2498–2505. [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JC, Lee KM, Kim DW, et al. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 19.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobie JJ, Wu RS, Kurt RA, et al. Transforming growth factor β inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 21.Flavell RA, Sanjabi S, Wrzesinski SH, et al. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larmonier N, Marron M, Zeng Y, et al. Tumor-derived CD4(+)CD25(+)regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, et al. CD4−8− dendritic cells prime CD4+ T regulatory 1 cells to suppress anti-tumor immunity. J Immunol. 2005;175:2931–2937. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 24.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 25.Wrzesinsky SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Jaffar J, Zhou J, et al. Inhibition of TGFbeta 1 makes nonimmunogenic tumor cells effective for therapeutic vaccination. J Immunother. 2009;32:232–239. doi: 10.1097/CJI.0b013e318197ac86. [DOI] [PubMed] [Google Scholar]
- 27.Tsang MLS, Weatherbee JA, Dietz M, et al. TGF-beta specifically inhibits the IL-4 dependent proliferation of multifactor-dependent murine T-helper and human hematopoietic cell lines. Lymphokine Res. 1990;9:607–609. [Google Scholar]
- 28.Scholler N, Fu N, Yang Y, et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci U S A. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellström I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 30.Hellstrom I, Ledbetter JA, Scholler N, et al. CD3-mediated activation of tumor-reactive lymphocytes from patients with advanced cancer. Proc Natl Acad Sci U S A. 2001;98:6783–6788. doi: 10.1073/pnas.021557498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 32.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11:6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spearman M, Taylor WR, Greenberg AH, et al. Antisense oligodeoxyribonucleotide inhibition of TGF-beta 1 gene expression and alterations in the growth and malignant properties of mouse fibrosarcoma cells. Gene. 1994;149:25–29. doi: 10.1016/0378-1119(94)90408-1. [DOI] [PubMed] [Google Scholar]
- 34.Fakhrai H, Dorigo O, Lin H, et al. Eradication of established intracranial rat gliomas by transforming growth factor beta anisense therapy. Proc Natl Acad Sci U S A. 1996;93:2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fakhrai H, Mantil JC, Liu L, et al. Phase I clinical trial of a TGF-beta antisense-modified tumor vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052–1060. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 36.Schlingensiepen KH, Fischer-Blass B, Schmaus S, et al. Antisense therapeutics for tumor treatment: the TGF-beta2 inhibitor AP 12009 in clinical development against malignant tumors. Cancer Res. 2008;177:137–150. doi: 10.1007/978-3-540-71279-4_16. [DOI] [PubMed] [Google Scholar]
- 37.Johnston FM, Tan MC, Tan BR, Jr, et al. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin Cancer Res. 2009;15:6511–6518. doi: 10.1158/1078-0432.CCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert LA, Gibson GR, Maloney M, et al. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–646. [PubMed] [Google Scholar]
- 40.Chang AE, Redman BG, Whitfield JR, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 41.Fadul CE, Fisher JL, Hampton TH, et al. Immune response in patients with newly diagnosed glioblastoma multiforme rreated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother. 2011;34:382–389. doi: 10.1097/CJI.0b013e318215e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 43.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Rask K, Zhu Y, Wang W, et al. Ovarian epithelial cancer: a role for PGE2-synthesis and signalling in malignant transformation and progression. Mol Cancer. 2006;5:62. doi: 10.1186/1476-4598-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliver RT, Nouri AM, Crosby D, et al. Biological significance of beta hCG, HLA and other membrane antigen expression on bladder tumours and their relationship to tumor infiltrating lymphocytes (TIL) J Immunogenet. 1989;16:381–390. doi: 10.1111/j.1744-313x.1989.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 48.Yang SC, Yang Y, Raycraft J, et al. Melanoma cells transfected to express CD83 induce anti-tumor immunity that can be increased by also engaging CD137. Proc Natl Acad Sci U S A. 2004;101:4990–4995. doi: 10.1073/pnas.0400880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivares J, Kumar P, Yu Y, et al. Phase I trial of TGF-{beta}2 antisense GM-CSF gene-modified autologous tumor cell (TAG) vaccine. Clin Cancer Res. 2011;17:183–192. doi: 10.1158/1078-0432.CCR-10-2195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Silencing of the TGF-β1 gene promotes the expression mesothelin and HE4 in OvC cells. A. OvCar3 and He207 OvC cells with control or silenced TGF-β1 were fixed and permeabilized with the Cytofix/Cytoperm reagent and intracelluarly stained with anti-mesothelin antibody (569) followed by FITC conjugated secondary anti-mouse antibody. The intracellular expression of mesothelin was analyzed by flow cytometry. The number in the histogram denotes the geometric mean of fluorescence intensity. Dash line, control stain; black line, OvCar3-control cells; red line, OvCar3-TGF-β1 cells. B. The supernatants were harvested from the OvCar3 and He207 OvC cells with control or silenced TGF-β1 for assaying the HE4 secretion by ELISA kit from FDI. Data were expressed as mean ±SD of triplicate wells (pM/1 million cell/24 h).