Abstract
Background & Aims
Studies of the pressure response of the upper esophageal sphincter (UES) to simulated or spontaneous gastroesophageal reflux have shown conflicting results. These discrepancies could result from uncontrolled influence of variables such as posture, volume, and velocity of distension. We characterized in humans the effects of these variables on UES pressure response to esophageal distension.
Methods
We studied 12 healthy volunteers (average 27±5 years old, 6 male) using concurrent esophageal infusion and high-resolution manometry to determine UES, lower esophageal sphincter, and intraesophageal pressure values. Reflux events were simulated by distal esophageal injections of room-temperature air and water (5, 10, 20, and 50 ml) in individuals in 3 positions (upright, supine and semi-supine). Frequencies of various UES responses were compared using χ2 analysis. Multinomial logistical regression analysis was used to identify factors that determine the UES response.
Results
UES contraction and relaxation were the overriding responses to esophageal water and air distension, respectively, in a volume-dependent fashion (P<.001). Water-induced UES contraction and air-induced UES relaxation were the predominant responses among individuals in supine and upright positions, respectively (P<.001). The prevalence of their respective predominant response significantly decreased in the opposite position. Proximal esophageal dp/dt significantly and independently differentiated the UES response to infusion with water or air.
Conclusions
The UES response to esophageal distension is affected by combined effects of posture (spatial orientation of the esophagus), physical properties, and volume of refluxate, as well as the magnitude and rate of increase in intraesophageal pressure. The UES response to esophageal distension can be predicted using a model that incorporates these factors.
Keywords: esophagus, stomach, airway, GERD
INTRODUCTION
Gas, liquid and their mixture are the main components of gastro-esophageal reflux (GER). The consequence of their entry into pharynx in terms of airway safety and potential aspiration is quite different especially during sleep. Over the past century a number of reflexes emanating from esophagus have been identified such as secondary peristalsis2, upper esophageal sphincter (UES) contraction3, and closure of the vocal cords4, which in isolation or in combination through different mechanisms protect the airway against aspiration of gastric contents. The function of the human UES in response to esophageal distention either by a balloon or simulated GER using esophageal perfusion of air and liquid or spontaneous GER has been studied extensively for more than 50 years3–16. Although some of these studies reported a UES contraction response to esophageal distension with a balloon3, 12, 16, non-acid and acid liquid 3, 5, 13, 14 and esophageal common cavity7, these observations have been challenged by studies that either did not demonstrate a UES contraction response8, 15 or observed UES relaxation6 during simulated or spontaneous esophageal acid exposure.
Center to the complexity of UES response to reflux events is the variable physical properties of the refluxate and the influence of posture on its impact, which eludes quantification and qualification during studies of naturally occurring reflux episodes. Other factors such as technical difficulties in measuring UES pressure in some studies due to the axial movement of the sphincter, hydrostatic effects of perfused systems, and inherent differences in fidelity of recording technique may have contributed to the discrepancies observed among various studies. Some investigators have correlated the UES response to transient lower esophageal sphincter relaxation (TLESR), but have ignored the fundamental effect of intra-esophageal pressure change and its associated esophageal wall tension due to distention on UES pressure6. Others have used common cavity as a surrogate marker of GER without incorporating the effect of the physical properties of the reflux material, which have caused increased intra-esophageal pressure7. These shortcomings have resulted in confusing and at times contradictory findings.
A recent report investigating postprandial GER episodes during TLESR in humans showed that the physical properties of the refluxate and the posture of subjects correlated with the type of UES response17. The majority of GER episodes in the recumbent posture contained liquid only and were associated with UES contraction. In contrast, GER episodes in the upright posture mainly contained air component and were coupled with UES relaxation. Furthermore, it was proposed that the rate of esophageal pressure increase could have been the distinctive factor for the differing UES response to GER episodes 17. However, volume and physical properties of the refluxate could not be controlled in this study, and therefore it was difficult to determine whether the subject’s posture, the physical property of refluxate or the magnitude and the rate of esophageal pressure increase were the determinants of the UES response17.
The present study aims to test the unifying hypothesis that UES pressure response to GER is multifactorial and is influenced by physical property and volume of the distending agent, spatial orientation of the esophagus and the velocity and magnitude of its distention.
METHODS
Study Protocol
We enrolled 12 healthy young volunteers (27 ± 5 yrs, 6 females). The Institutional Review Board Committee of the Medical College of Wisconsin approved the study protocol and each subject provided both verbal and written consent before enrollment in the study. They had no history of known gastrointestinal disorders and were not taking any medications that may have affected gastrointestinal tract. Transnasal endoscopy was performed in all subjects prior to the study and showed no erosive esophagitis or other luminal abnormalities of the esophagus, gastroesophageal junction and stomach18. Subjects were comfortably studied in a powered medical examination chair with stretched legs in upright, supine and semi-supine (45 degree) postures. Following 6 hours of fasting and topical lidocaine application, a solid-state high resolution manometry catheter with 36 circumferential pressure sensors, spaced 1 cm apart (Sierra Scientific, Los Angeles, CA), was introduced trans-nasally in a fashion that at least 3 proximal sensors were located in the pharynx. Subsequently, a 3 mm outer diameter injection tube was placed through the same nosrtril in a fashion that the injection port was located 5 cm above the upper border of lower esophageal sphincter 1. Gastroesophageal reflux events were simulated by intra-esophageal injection of room air and tap water (allowed to acclimate to room temperature for 30 minutes) uniformly by a single operator (AB) using a 60 mL syringe. Exact timing and type of the injection were documented by the second investigator (JL) using event marker feature of the Manoscan® system. Rate of perfusion was evaluated in vitro by a series of trials recorded on Narco Bio-Systems MMS 200 physiologic recorder showing a rate of 50 mL/s for air and 10 mL/s for water injections. We tested three trials of 5, 10, 20 and 50 mL volumes of each substance in a random order resulting in 72 events for analysis in each subject. Each perfusion was only performed when the UES, esophagus and LES were at baseline for at least three tidal volume respiratory cycles and 20s after a preceding peristaltic event. After each perfusion, manometry was carefully monitored for 20s and then subjects were cued to swallow and clear esophageal contents. Esophageal clearance was confirmed by an effective peristaltic wave and return of intra-esophageal pressure to baseline. All volunteers tolerated the procedure except one who reported minor nausea during 50 mL of liquid injection in the supine position who was able to finish the protocol in another session.
Pressure Data Analysis
All pressure measurements were recorded at a 35HZ frequency and measured in reference to the atmospheric pressure (except LES that was measured in reference to gastric pressure). UES and LES pressures were measured utilizing the e-sleeve function of the Manoview ® software. All UES, LES and esophageal baseline pressures were measured as peaks and troughs over 3 tidal volume respiratory cycles at stable resting conditions when no pharyngeal, gastric, esophageal, UES or LES events were present. Type, frequency, amplitude, onset, and duration of the UES response along with the LES and esophageal body response were recorded. All responses were determined in a 10 second window after the injection. Percent UES relaxation and percent UES contraction were calculated as a fraction of the maximum possible relaxation (baseline UES pressure-proximal esophageal pressure) and as a fraction of the maximum UES contraction pressure, respectively. UES relaxation was considered complete (100%) if an audible belch was documented or the nadir UES pressure equalized to the proximal esophageal pressure. Due to considerable variability of the resting UES pressure even at rest we decided to set a conservative threshold (10 mmHg) for determining the UES response6, 17. UES response was categorized as an ordinal variable: contraction (UES pressure exceeded the peak baseline pressure by more than 10 mmHg); relaxation (UES pressure dropped more than 10 mmHg below baseline trough pressure); or no response. It has been observed that often prior to and almost always after a swallow related UES relaxation (as UES participates in pharyngoesophageal peristaltic wave) there is an increase in UES tone that has been linked to the preceding relaxation rather than an independent event19. We considered UES contraction as a response only if it was not preceded by a UES relaxation response and not followed by UES relaxation within 3 seconds.
Distal and proximal esophageal pressures were measured as an average pressure between two adjacent pressure sensors located 3–5cm above the LES and 3–5cm below the UES respectively 17, and avoided sensors that were affected by cardiovascular pressure markings. The extent of esophageal intraluminal pressure increase was recorded as an ordinal variable: 1) if proximal and distal esophageal pressure increased more than 3 mmHg above baseline during perfusion; 2) if esophageal pressure increased more than 3 mmHg above baseline during perfusion only in distal segment; and 3) if esophageal pressure did not increase more than 3 mmHg in either segment. Esophageal pressure and peak rate of pressure increase (dp/dt) within a 0.1 s window was measured during the perfusion in the proximal and distal esophageal segments as continuous variables.
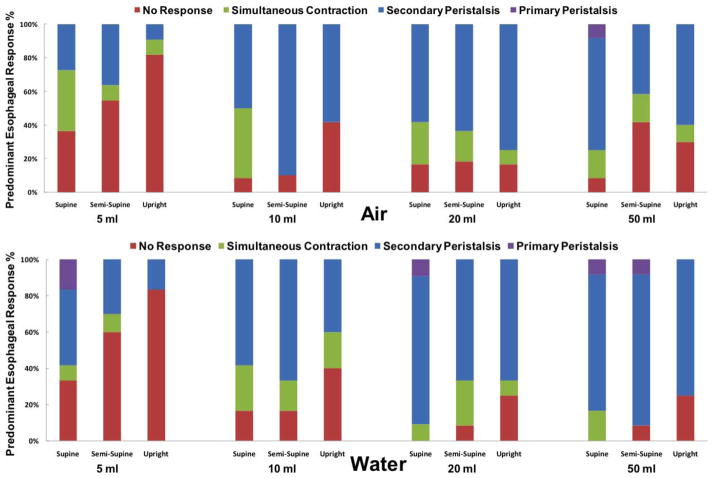
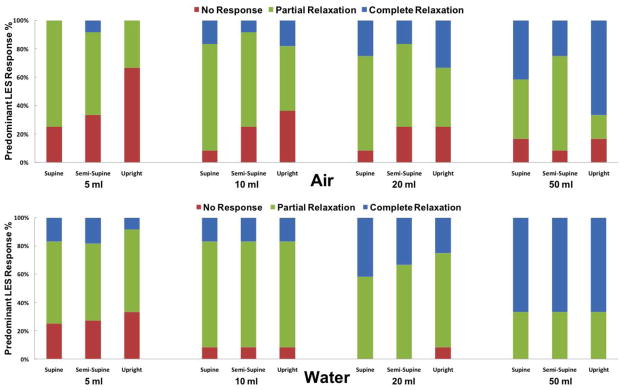
Esophageal clearing response to distal esophageal perfusion could be classified into four categories: (1) tertiary or simultaneous esophageal contraction (>20 mmHg); (2) secondary peristalsis (>20 mmHg); 20 primary peristalsis (>20 mmHg); and, (4) no response. LES response to distal esophageal perfusion was classified into three types: (1) complete relaxation defined as equalization of the LES pressure to gastric pressure; (2) partial relaxation defined as a pressure drop > 5 mmHg; and, 20 no response. The predominant UES, LES and esophageal response was considered mode of three trials and recorded as an ordinal variable.
Statistical Methods
Data are presented as mean ± standard deviation unless otherwise specified. Data was analyzed using SAS analysis software. Odds ratios (OR) of each covariates within the outcome category were determined to estimate their effect on the probability. Multinomial logistical regression analysis was used to model the three UES outcomes as ordinal variable (contraction, no response and relaxation) and determine the contribution of posture, volume, extent of esophageal pressure increase, and rate of esophageal pressurization to UES response. Pseudo R-square was used to assess the quality of the fit by the model and is analogous to the R-square in linear regression, which explains how much of the variation is explained by the model. It is a measure of how much of the log likelihood for the null parameter model compared to the full parameter model that can be explained by the multinomial logistic regression model just constructed. Generalized linear model testing was used to model the UES pressure amplitude response as continuous variable using posture, volume of perfusate, and extent and rate of esophageal pressurization as predictors.
RESULTS
We recorded and analyzed a total of 864 events. These constituted three repetitions of 288 test conditions generated by interaction of the volume, posture and physical properties of the distending agent namely air and liquid. There were three types of UES responses: contraction, relaxation, or no response. Prevalence of each type of response was influenced by test conditions however, UES demonstrated a predominant response to esophageal infusion defined as development of a similar response to at least two of the three repetitions in nearly all test conditions (283/288). The five test conditions without predominant response occurred during 5 mL infusion of water in upright and semi-supine posture.
Qualitative Analysis of UES Response to Esophageal Distention Induced by Distal Esophageal Perfusion
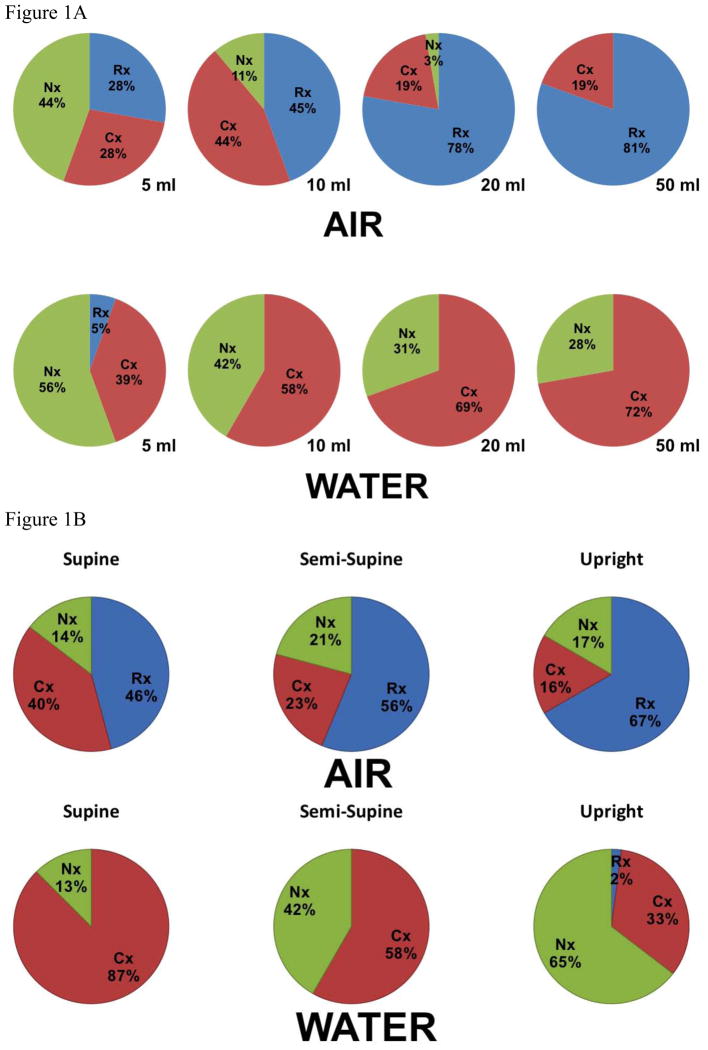
The UES pressure response was significantly dependent on the physical properties of the distending agent. Air insufflations predominantly induced a UES relaxation response, while liquid perfusion primarily evoked a UES contraction response (p<0.001, figure 1a). Frequency of the UES response to both air and water was dose dependent. In that, frequency of response induced by the increasing volume of infusate increased progressively and reached a plateau at larger volumes of 20–50 mL. Increasing volume from 5mL to 50mL resulted in fewer “no response” events of either distending agent (p<0.001). The likelihood of a UES relaxation response to 20 mL and 50 mL of air was significantly higher than 5 and 10 mL air infusion (OR=7.2, p<0.001). In contrast, the likelihood of a UES contraction response to 20 and 50 mL of water infusion was considerably higher than 5 and 10 mL (OR=5.93, p<0.001). There were no statistical differences between UES responses to 20 and 50 mL infusion for both air and liquid.
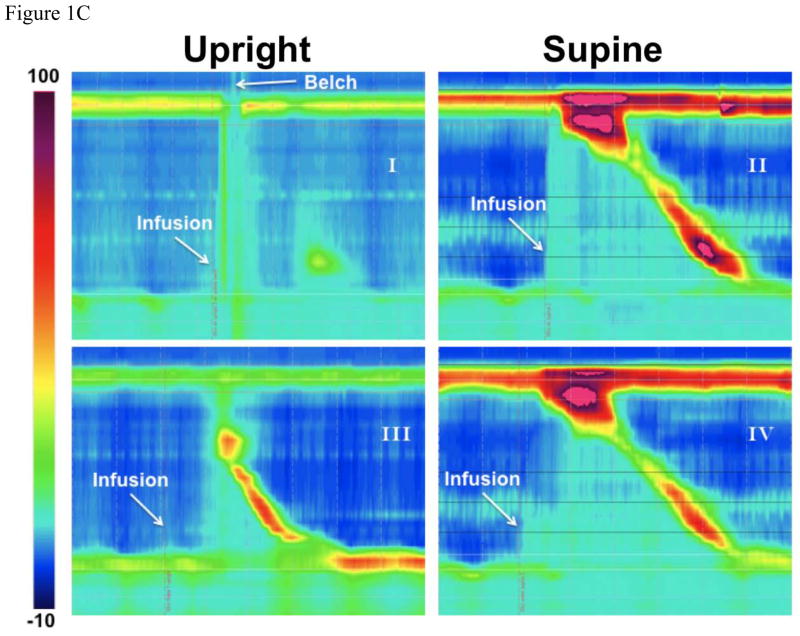
Figure 1. Qualitative characterization of UES response to distal esophageal perfusion.
a) Volume effect: frequency of predominant UES response to air and liquid distention across different volumes shown as a percentage of total (n=12, 283 trials) b) Posture effect: frequency of predominant UES response across various postures shown as a percentage of total (n=12, 283 trials) c) Panel I and II demonstrate representative examples of distal esophageal 50 mL air insufflations in upright and supine posture respectively. Panel I displays complete UES relaxation associated with a belch reflex (gastric pressure increase and pharyngeal air escape) and minimal esophageal or LES response. Panel II displays an immediate UES contractile response, complete LES relaxation and a secondary peristaltic wave. Panel III and IV demonstrate representative examples of distal esophageal 50 mL water infusions in supine and upright posture respectively. Panel III shows secondary peristalsis and complete LES relaxation without any discernable UES response. Panel IV displays a coordinated UES contractile response, secondary peristalsis and complete LES relaxation.
The predominant UES response to air and liquid infusions across different postures demonstrated additional independent effect on UES response (p<0.001, figure 1b). The likelihood of UES contraction induced by liquid perfusion increased significantly from the upright to the semi-supine posture (OR=2.7, p<0.005) and to the supine posture (OR=8.7, p<0.001). Air insufflations in the upright posture were more likely to result in UES relaxation (OR=4.5, p<0.001) while air-induced UES contraction was more likely to occur in supine posture (OR=3.6, p<0.005). There was no significant interaction between volume of perfusate and posture (p = 0.13). Increase in proximal esophageal pressure significantly increased the likelihood of air-induced relaxation and decreased the chance of no response (OR=1.1 for each one mmHg increase, p<0.05). On the other hand increase in proximal esophageal pressure during water infusion significantly increased the likelihood of UES contractile response (OR=1.2 for each one mmHg increase, p<0.001). The effect of esophageal pressure was partially dependent on the volume of infusate (p<0.01). Distal esophageal pressure had little effect independent from proximal esophageal pressure increase.
Examples of typical UES responses to air and water infusions are shown in figure 1c. Further details of the UES response and percentage of each type of UES response are shown in supplementary figure-1.
Quantification of UES Pressure Response to Esophageal Distention induced by Distal Esophageal Perfusion
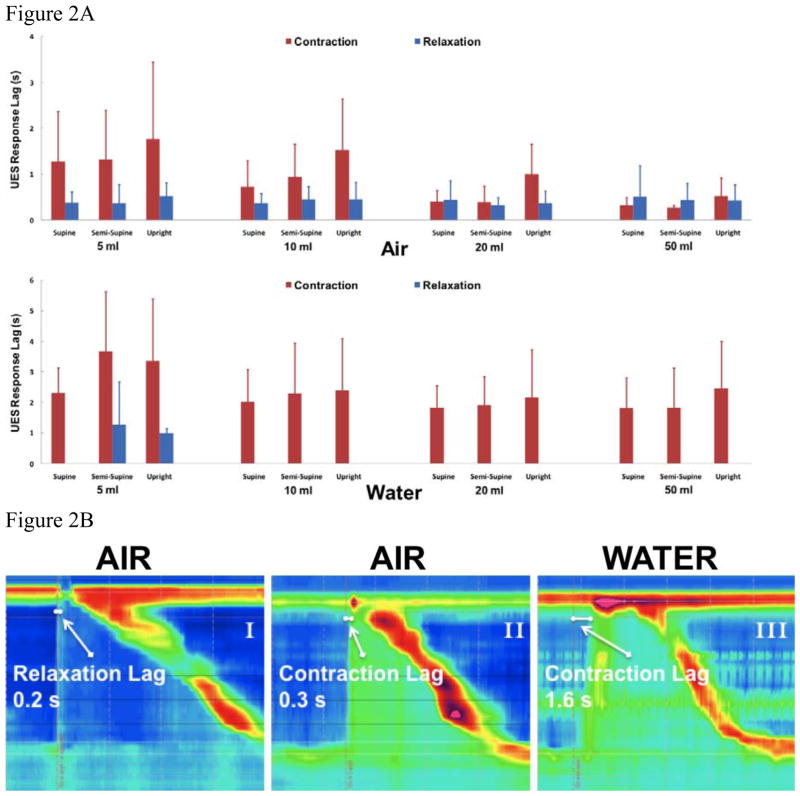
UES relaxed 64 ± 11 % in response to 5–10 mL and 83 ± 8 % in response to 20–50 mL of air insufflation (as a percentage of its baseline pressure), and contracted 32 ± 6 mmHg in response to 5–10 mL and 44 ± 11 mmHg in response to 20–50 mL of water infusion. The magnitude of UES relaxation increased significantly with increasing air volume from 5 mL to 50 mL (18±9 %, p=0.004). Timing characteristics of UES response are shown in figure 2a. UES relaxation or contraction response was universally elicited in less than 1 s (0.4 ± 0.1s) after air insufflation. In contrast the majority of UES contraction responses to water infusion were seen later than 2s (2.3 ± 0.6s) after water infusion. The UES contractile response lag for air insufflation was significantly shorter than that of water infusion (1.4 ± 0.2 s, p<0.001). In addition, the UES contraction lag time was significantly shorter in the supine posture compared to the upright position for both air and water infusion (−0.58s, p=0.03 for air; −0.65s, p=0.01 for water), and for higher volumes (20–50 mL) compared to lower volumes (5–10 mL) (−0.88s, p<0.001 for air; −0.95s, p=0.02 for water). Lag time for air induced UES relaxation response was not affected by volume or posture. Duration of UES relaxation (1.4 ± 0.3 s) was shorter compared to the duration of UES contraction response (7.7 ± 1.8 s) with no difference across volumes or postures for either response. Examples of UES response lag time under different test conditions are displayed in figure 2b. Further characteristics of the UES response including pressure amplitude and response duration are presented in supplementary figure 1.
Figure 2. Timing of UES response across all study conditions during all 864 events (n=12).
a) UES response lag after esophageal distention b) Distinctive UES response lags in response to air and water infusion. Panels I & II display a very short UES relaxation and contraction lag with air insufflation. Panel III demonstrates a more delayed UES contraction response with water perfusion.
Intra-esophageal Pressure Change during Distal Esophageal Perfusion
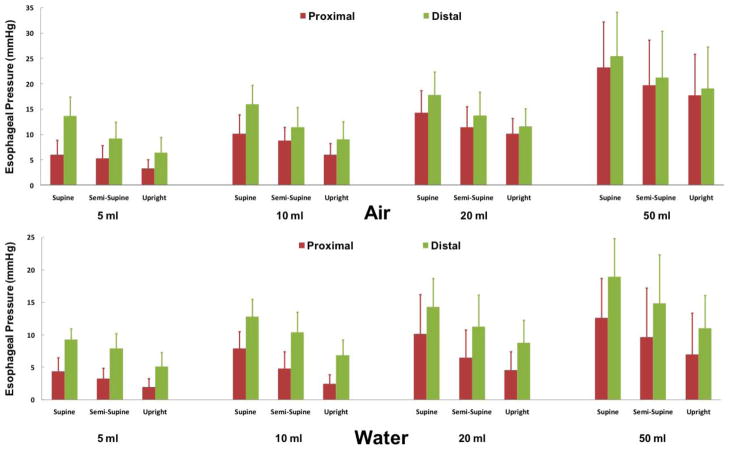
The proximal extent of the intra-luminal pressure increase along the length of the esophagus due to distal perfusion was different between air and water. All air insufflation trials resulted in a discernable pressure increase in the distal esophagus. Majority of the pressure increases associated with air volumes of more than 10mL (95%) produced an esophageal pressure rise in both distal and proximal segments (figure 3). In contrast, some 5 mL water infusions (26%) did not result in a discernable esophageal pressure rise especially in upright posture. Interestingly a significant proportion of water infusions (52%), even at 50mL volume (31%), did not result in proximal esophageal pressure increase. Measurement of esophageal pressure in the distal and proximal esophagus during water infusions and air insufflations demonstrated a significant effect of posture and volume (p<0.001). In that both supine posture and larger volumes were associated with higher pressure increases (figure 3).
Figure 3. Esophageal pressure during distal esophageal perfusion.
Amplitude of esophageal pressure in proximal and distal segments across all study conditions during all 864 events (n=12)
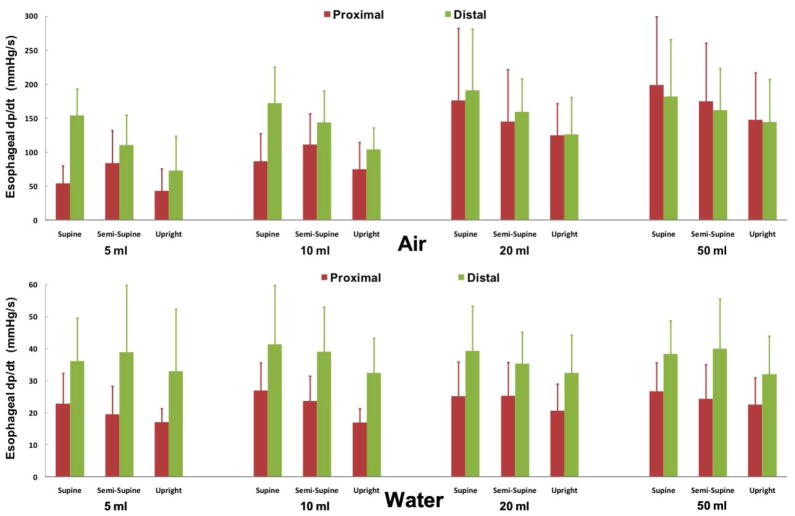
The rate of esophageal pressure increase (dp/dt) associated with air insufflations (143 ± 35 mmHg/s) were significantly faster than that of water infusions (37 ± 3 mmHg/s), which were largely indistinguishable from baseline luminal pressure fluctuations (figure 4). Both distal and proximal esophageal air-induced dp/dt significantly increased with increasing volume (p<0.001) and in supine compared to upright posture (p<0.02). In contrast, only proximal esophageal water-induced dp/dt significantly increased with increasing volume (p< 0.05) and supine posture (p=0.001). Rate of proximal esophageal pressure increase (dp/dt) for larger volumes of air infusion (20mL and 50mL) associated with UES relaxation was significantly higher than those associated with UES contraction (p<0.01, supplementary figure 2).
Figure 4. Rate of esophageal pressure increase (dp/dt) during distal esophageal perfusion.
Average dp/dt across all study conditions during all 864 events (n=12)
Esophageal and LES Pressure Response to Esophageal Distention induced by Distal Esophageal Perfusion
Esophageal body response if present could be categorized into simultaneous (tertiary) contractions, primary and secondary peristalsis. Esophageal response to esophageal distention was volume and posture dependent. Likelihood of an esophageal response increased with changing position from upright to supine posture and increasing volume of the infusate (figure 5). 58% of 5mL infusions of either substance (39/67) did not elicit a predominant esophageal response but for volumes larger than 10 mL, secondary peristalsis was the predominant esophageal response in majority of instances (65%=136/208, figure 5) irrespective of posture or the distending agent. Esophagus either remained silent or generated simultaneous contractions in the remaining substantive minority of the infusions (>30%). Primary peristalsis response was exceedingly rare constituting only 2% of episodes. Esophageal peristalsis when present was elicited within 3.9 ± 1.6 s after air insufflation and 5.4 ± 1.7s after water infusion. During water infusions, a pattern of more frequent peristaltic response with shorter lag time related to increasing volume was apparent (supplementary figure 3).
Figure 5. Esophageal response to distal esophageal perfusion (n=12).
Frequency of predominant esophageal response to air and liquid distention across different postures shown as a percentage of total (275/288 trials)
Predominant LES response to esophageal distention of either substance if present was strictly relaxation (no contraction observed) and likelihood of relaxation increased with increasing volume of the infusate. In overwhelming majority of instances, LES pressure response to distal esophageal distention of air or water was relaxation (83%=237/286, figure 6). LES did not exhibit any pressure change during the remaining infusions (17%). LES relaxations occurred 1.8 + 0.5 s after air insufflation and 2.2 + 0.2 s after water infusion. The onset of LES relaxation for the majority of trials could be correlated to either a UES response or to an esophageal contractile event. For water perfusions, increase in the volume of infusate was associated with an increase in the frequency of complete LES relaxation. Lag time in LES response to esophageal air insufflation diminished precipitously as the volume of infusate increased (2.2s for 5mL vs 0.9s for 50mL). Lag time in LES response to distal esophageal perfusion of water was relatively stable across various postures and volumes (supplementary figure 4).
Figure 6. LES response to distal esophageal perfusion (n=12).
Frequency of predominant LES response to air and liquid distention across different postures shown as a percentage of total (286/288 trials)
A Model for Predicting UES Response to Simulated Gastroesophageal Reflux Events
In the combined model of UES response to air and water, variables had different level of influence. Posture had a pseudo-R2 of 4.5% (p<0.001), volume pseudo-R2 of 7.4% (p<0.001), proximal dp/dt pseudo-R2 =23.6% (p<0.0001), proximal esophageal pressure pseudo-R2 =1.3% (p<0.01), and the nature of distending substance (air versus water) added a pseudo-R2 5.5% (p<0.001) above and beyond the effect of other variants to the model. The total pseudo-R2 of for all 5 variants of combined predictive model was 39.8%. When modeling the UES response within air insufflations only we found that volume had the strongest effect (pseudo-R2 =19.6%, p<0.0001), posture had a pseudo-R2 of 3.5% (p<0.001), proximal dp/dt 5.3% (p<0.001) and proximal esophageal pressure added a small 0.8% (p<0.05) to the model. The total pseudo-R2 for modeling UES response to air distention was 29.2%.
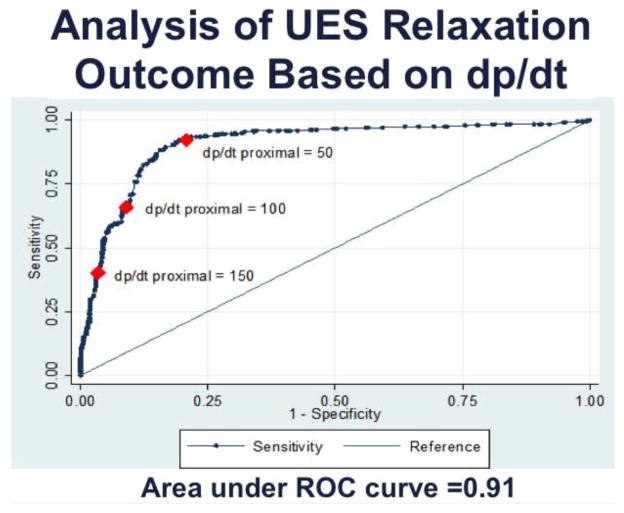
In the model of UES pressure response to water distention, posture had the strongest effect with pseudo-R2 of 10.6% (p<0.001), followed by volume 8.3% (p<0.001), proximal dp/dt pseudo-R2 of 2.3% (p<0.01) and proximal esophageal pressure of 2.7% (p<0.01). The total pseudo-R2 for modeling the UES response to water distention was 23.9%. As seen one of the major factors in the combined model to predict UES response was proximal esophageal dp/dt. This factor for the most part differentiated air from water infusions and within each group remained significant. Graphical plot of UES relaxation response as a binary outcome against dp/dt of all events showed a receiver operating characteristic (ROC) curve with area under the curve (AUC) of 0.91 for dp/dt (figure 7). Discriminating threshold of dp/dt=82 had the highest accuracy of 86.1% in classification of UES response, and as expected higher dp/dt had higher specificity and lower dp/dt had higher sensitivity in predicting the UES relaxation response.
Figure 7. Effect of dp/dt on UES response.
Receiver operating characteristic (ROC) curve of UES relaxation as a binary outcome (yes or no) plotted against dp/dt of esophageal distention for all events (n=12, 864 events). ROC area under the curve is 0.91 and best classifier is dp/dt=82 mmHg/s with accuracy of 86.1%.
Distal dp/dt and distal esophageal pressure did not add significantly to the ability of the model to predict which of the outcomes will occur in combined or separate models. More complex models involving interaction terms were also examined and only had an insignificant effect.
DISCUSSION
Esophagus is a richly refelxogenic organ capable of initiating a number of distention induced reflexes that involves the glottis, UES, LES and esophagus itself 21–24. Esophageal distention in normal individuals initiates a well-orchestrated response between the UES, body of the esophagus and LES to protect the upper airways from deleterious contact of gastric content while allowing safe ventilation of gastric and esophageal gas. Esophageal and UES pressure response to esophageal distention has been described as early as 33 weeks of gestational age25. Aging on the other hand selectively affects the esophageal and UES response to esophageal distention. In that, while UES contractile response remain intact to balloon distention, UES relaxation response to air distention increases significantly at the expense of decreased secondary peristalsis26.
In this study we quantified and compared in detail the UES response to air and liquid of various volumes in different postures. Study findings indicate that UES response to esophageal distention is multifactorial including spatial orientation of the esophagus, rate and magnitude of proximal intra-esophageal pressure increase, physical property and volume of the refluxate. In that: 1) UES contraction and relaxation were the overriding responses to esophageal water and air distension respectively in a volume dependent fashion. 2) Water induced UES contraction and air induced UES relaxation were the more prevalent responses in supine and upright postures respectively and frequency of their predominant response significantly decreased in the opposite posture. 3) Proximal esophageal dp/dt independently predicted the UES response to water and air infusion.
The effect of posture on UES response to liquid distention simulating GER events favors airway protection. UES response shifts to contraction as subject goes from upright to supine posture, which is presumably the preferred position for sleep. It should be noted however, that orientation of esophagus and composition of infusate interact with each other as air always moves upward resulting in proximal esophageal distention while liquid gravitates downward and requires larger volume to reach proximal esophagus. Of clinical importance is the fact that UES contractile response to liquid infusion is absent in more than a quarter of instances of large volume infusions in semi-supine posture. This finding may have ramifications in airway protection especially during sleep in which the UES resting pressure is exceedingly low27. This issue even becomes more relevant for patients with GERD. In fact recently we have shown that in GERD patients with regurgitation UES response to liquid esophageal distention is altered28 and their esophago-UES contractile reflex is not as robust as healthy volunteers. This deficit may predispose these patients to pharyngeal reflux and its consequences such as aspiration pneumonia or other supraesophageal complications.
Although the majority of in-vivo reflux events are under normal conditions acidic our original design of the study didn’t address the effect of acidity of refluxate on UES response.
To address this issue, we studied seven additional healthy volunteers (post-hoc) using infusion of 0.1 normal hydrochloric acid (5, 10 and 20 mL) following the same protocol and analysis technique described in method section. We found that frequency of UES response induced by esophageal acid infusion compared to those of water infusion did not show any statistical differences (in terms of volume of the infusate and spatial orientation of the esophagus). Acid infusion similar to water infusion induced a volume dependent increase in frequency of UES contractile response and did not result in any UES relaxation response. Acid infusion typically provoked no UES response in upright position, but as subject moved to supine posture UES response mainly shifted to contraction, comparable to responses seen with water infusion (Supplementary figure 5). Therefore, we conclude that acidity of the infusate does not affect the UES response.
Considering the rich vagal innervation of the esophageal mucosal, submucosal and muscular layers with different response characteristics and capabilities in mediating UES response to esophageal distention, the observed variability of UES pressure change due to esophageal infusion of various distending agents in different positions is not surprising. From our airway safety perspective it seems fundamental to UES function to prevent reflux of fluid into the pharynx during GER events. This requires a response selection capability by the esophagus while dealing with different physical properties of the refluxate and various postures.
A number of neural characteristics of the esophageal innervation have been described that can explain the various responses observed in the present and previous studies. Among them are the ability of the esophageal receptors to encode rate of pressure change such as those observed with liquid and air distention29–31. Presence of rapidly adapting mucosal and slowly adapting deep muscular receptors23, 29, 32 relaying fine sensation or luminal chemicals and distention or contraction respectively has been reported. A new subgroup of afferent fibers responsive to both mucosal fine stroke and muscle tension have also been identified in the esophagus, which could be located in the muscularis mucosa exhibiting both properties33. Recent studies using mucosal anesthesia in humans34 and cats29 as well as mucosal stripping in cats29 suggests mediation of UES relaxation by esophageal distention superficially located mucosal receptors, and UES contraction response surviving mucosal anesthesia and mucosal stripping to the muscular receptors34.
Differential UES response to proximal and distal esophageal stimulations by air injection has been reported previously in humans 13, 16. These experiments have shown that UES relaxation response was more common after proximal (106/121 trials) than distal (19/112 trials) esophageal air insufflations in a human model of contracted UES induced by mid-esophageal balloon distention which also served to separate the esophagus into proximal and distal compartments 16. On the other hand, earlier studies have shown that the human UES contractile response to slow intra-esophageal fluid infusion is augmented by acidity and proximity of the liquid to the UES13. These later studies used a single side hole for recording UES pressure response, which could suffer some limitations due to potential axial movement of the UES.
Preponderance of evidence derived from basic and clinical studies so far, suggests the existence of a complex reflex circuitry determining the UES pressure response to esophageal distention depending on the type and number of activated mucosal, submucosal and muscular receptors and their relative concentration and location in relationship to UES. Based on the findings of the present and previous studies not only the physical properties of the GER play a role in activation of these reflex circuitries, but also the spatial orientation of the esophagus through influencing the distention and affecting the proximal extension of the refluxate significantly affect the UES response. In summary, UES pressure response to esophageal distention induced by reflux of gastric content is multifactorial. These factors include spatial orientation of the esophagus, rate and magnitude of proximal intra-esophageal pressure increase, physical property and volume of the refluxate. Variations in the combination of these factors result in variation in the UES pressure response to reflux events. As such generalization of UES response under a given set of circumstances representing only a limited number of factors is best avoided.
Supplementary Material
Acknowledgments
Supported in part by NIH Grant 5R01DK025731-29, 2T32DK061923-06, 5 P01 DK068051-05 and 1UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, NIH.
Abbreviations
- UES
Upper esophageal sphincter
- Lower esophageal sphincter 1
GER, Gastroesophageal reflux
- TLESR
Transient lower esophageal sphincter relaxation
Footnotes
The authors have no conflict of interest to disclose.
Dr. Babaei had a major role in all aspects of this manuscript including study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content and statistical analysis. Dr. Rahimi Naini contributed to the analysis and interpretation of data along with statistical analysis and technical support. Omar Katib contributed to the analysis of data. Justin Lee contributed to data acquisition and analysis. Raymond Hoffman and Ke Yan performed the statistical analysis. Dr. Shaker had a major role in study concept and design, drafting of the manuscript, funding, supervision and critical revision of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leslie P, Drinnan MJ, Ford GA, Wilson JA. Swallow respiratory patterns and aging: presbyphagia or dysphagia? J Gerontol A Biol Sci Med Sci. 2005;60:391–5. doi: 10.1093/gerona/60.3.391. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer SJ. On the causes of the orderly progress of the peristaltic movements in the esophagus. Americal Journal of Physiology. 1899;2:266. [Google Scholar]
- 3.Creamer B, Schlegel J. Motor responses of the esophagus to distention. J Appl Physiol. 1957;10:498–504. doi: 10.1152/jappl.1957.10.3.498. [DOI] [PubMed] [Google Scholar]
- 4.Shaker R, Ren J, Kern M, Dodds WJ, Hogan WJ, Li Q. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol. 1992;262:G621–8. doi: 10.1152/ajpgi.1992.262.4.G621. [DOI] [PubMed] [Google Scholar]
- 5.Andreollo NA, Thompson DG, Kendall GP, McIntyre AS, Earlam RJ. Motor responses of the upper esophageal sphincter and body to intraluminal acid. Braz J Med Biol Res. 1989;22:51–60. [PubMed] [Google Scholar]
- 6.Pandolfino JE, Ghosh SK, Zhang Q, Han A, Kahrilas PJ. Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); it is mainly about microburps. Neurogastroenterol Motil. 2007;19:203–10. doi: 10.1111/j.1365-2982.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 7.Torrico S, Kern M, Aslam M, Narayanan S, Kannappan A, Ren J, Sui Z, Hofmann C, Shaker R. Upper esophageal sphincter function during gastroesophageal reflux events revisited. Am J Physiol Gastrointest Liver Physiol. 2000;279:G262–7. doi: 10.1152/ajpgi.2000.279.2.G262. [DOI] [PubMed] [Google Scholar]
- 8.Vakil NB, Kahrilas PJ, Dodds WJ, Vanagunas A. Absence of an upper esophageal sphincter response to acid reflux. Am J Gastroenterol. 1989;84:606–10. [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Dodds WJ, Dent J, Wyman JB, Hogan WJ, Arndorfer RC. Upper esophageal sphincter function during belching. Gastroenterology. 1986;91:133–40. doi: 10.1016/0016-5085(86)90449-x. [DOI] [PubMed] [Google Scholar]
- 10.Sabanathan S, Bryan AJ. The upper oesophageal sphincter and its response to gastrooesophageal reflux. J R Coll Surg Edinb. 1985;30:167–72. [PubMed] [Google Scholar]
- 11.Winship DH. Upper esophageal sphincter: does it care about reflux? Gastroenterology. 1983;85:470–2. [PubMed] [Google Scholar]
- 12.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distention in man. Gastroenterology. 1977;72:1292–8. [PubMed] [Google Scholar]
- 13.Gerhardt DC, Shuck TJ, Bordeaux RA, Winship DH. Human upper esophageal sphincter. Response to volume, osmotic, and acid stimuli. Gastroenterology. 1978;75:268–74. [PubMed] [Google Scholar]
- 14.Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol. 1978;13:821–6. doi: 10.3109/00365527809182197. [DOI] [PubMed] [Google Scholar]
- 15.Kahrilas PJ, Dodds WJ, Dent J, Haeberle B, Hogan WJ, Arndorfer RC. Effect of sleep, spontaneous gastroesophageal reflux, and a meal on upper esophageal sphincter pressure in normal human volunteers. Gastroenterology. 1987;92:466–71. doi: 10.1016/0016-5085(87)90143-0. [DOI] [PubMed] [Google Scholar]
- 16.Aslam M, Kern M, Shaker R. Modulation of oesophago-UOS contractile reflex: effect of proximal and distal esophageal distention and swallowing. Neurogastroenterol Motil. 2003;15:323–9. doi: 10.1046/j.1365-2982.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 17.Babaei A, Bhargava V, Mittal RK. Upper esophageal sphincter during transient lower esophageal sphincter relaxation: effects of reflux content and posture. Am J Physiol Gastrointest Liver Physiol. 2010;298:G601–7. doi: 10.1152/ajpgi.00486.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaker R, Saeian K. Unsedated transnasal laryngo-esophagogastroduodenoscopy: an alternative to conventional endoscopy. Am J Med. 2001;111(Suppl 8A):153S–156S. doi: 10.1016/s0002-9343(01)00852-x. [DOI] [PubMed] [Google Scholar]
- 19.Code C, Schlegel J. Handbook of Physiology. Vol. 4. Washington, DC: American Physiological Society; 1968. Motor action of the esophagus and its sphincters; pp. 1821–1839. [Google Scholar]
- 20.Chen PH, Golub JS, Hapner ER, Johns MM., 3rd Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia. 2009;24:1–6. doi: 10.1007/s00455-008-9156-1. [DOI] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol. 2007;102:2286–93. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadcherla SR, Shaker R. Esophageal and upper esophageal sphincter motor function in babies. Am J Med. 2001;111(Suppl 8A):64S–68S. doi: 10.1016/s0002-9343(01)00848-8. [DOI] [PubMed] [Google Scholar]
- 23.Lang IM, Shaker R. Anatomy and physiology of the upper esophageal sphincter. Am J Med. 1997;103:50S–55S. doi: 10.1016/s0002-9343(97)00323-9. [DOI] [PubMed] [Google Scholar]
- 24.Shaker R, Dodds WJ, Ren J, Hogan WJ, Arndorfer RC. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992;102:857–61. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 25.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr. 2003;143:31–8. doi: 10.1016/S0022-3476(03)00242-7. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Shaker R, Kusano M, Podvrsan B, Metwally N, Dua KS, Sui Z. Effect of aging on the secondary esophageal peristalsis: presbyesophagus revisited. Am J Physiol. 1995;268:G772–9. doi: 10.1152/ajpgi.1995.268.5.G772. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Bajaj S, Dua KS, Jaradeh S, Rittmann T, Hofmann C, Shaker R. Influence of sleep stages on esophago-upper esophageal sphincter contractile reflex and secondary esophageal peristalsis. Gastroenterology. 2006;130:17–25. doi: 10.1053/j.gastro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi Naini S, Dua K, Massey B, Hogan H, Shaker R. Defective Esophago-UES Contractile Reflex in Patients with Complaints of Regurgitation and Supraesophageal Symptoms. Gastroenterology. 2011;140(Supplement):S-44. [Google Scholar]
- 29.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1246–63. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 30.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. The Journal of physiology. 1998;512 (Pt 3):907–16. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. Journal of neurophysiology. 1989;61:1001–10. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 32.Freiman JM, El-Sharkawy TY, Diamant NE. Effect of bilateral vagosympathetic nerve blockade on response of the dog upper esophageal sphincter (UES) to intraesophageal distention and acid. Gastroenterology. 1981;81:78–84. [PubMed] [Google Scholar]
- 33.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512 (Pt 3):907–16. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczesniak MM, Fuentealba SE, Burnett A, Cook IJ. Differential relaxation and contractile responses of the human upper esophageal sphincter mediated by interplay of mucosal and deep mechanoreceptor activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G982–8. doi: 10.1152/ajpgi.00496.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.