Abstract
Sarcopenia is an age-associated loss of skeletal muscle mass and strength that increases the risk of disability. Calorie restriction (CR), the consumption of fewer calories while maintaining adequate nutrition, mitigates sarcopenia and many other age-related diseases. To identify potential mechanisms by which CR preserves skeletal muscle integrity during aging, we used mRNA-Seq for deep characterization of gene regulation and mRNA abundance in skeletal muscle of old mice compared with old mice subjected to CR. mRNA-Seq revealed complex CR-associated changes in expression of mRNA isoforms, many of which occur without a change in total message abundance and thus would not be detected by methods other than mRNA-Seq. Functional annotation of differentially expressed genes reveals CR-associated upregulation of pathways involved in energy metabolism and lipid biosynthesis, and downregulation of pathways mediating protein breakdown and oxidative stress, consistent with earlier microarray-based studies. CR-associated changes not noted in previous studies involved downregulation of genes controlling actin cytoskeletal structures and muscle development. These CR-associated changes reflect generally healthier muscle, consistent with CR's mitigation of sarcopenia. mRNA-Seq generates a rich picture of the changes in gene expression associated with CR, and may facilitate identification of genes that are primary mediators of CR's effects.
Keywords: deep sequencing, novel transcripts, sarcopenia, aging
sarcopenia is a progressive decline in muscle mass and function that is common in humans over 60 yr of age; compromised muscle function increases the risk of injury and causes a predisposition to disability and mortality (1, 23), making it a significant public health issue. Understanding of this age-related disease and its underlying mechanisms has the potential to suggest means of preventing or reversing the process. Calorie restriction (CR), the consumption of fewer calories while maintaining adequate nutrition, mitigates aging and extends both health and life spans of a wide variety of experimental animals (21, 28), and delays the onset and decreases the incidence and severity of a wide range of age-related diseases including cancer, cardiovascular diseases, and neurodegeneration (28). In regard to sarcopenia, CR has been shown to attenuate the age-associated loss of muscle mass and function in rodents and nonhuman primates (3, 11). CR is associated with mitigation of the age-induced decline in mitochondrial function and decreases the mitochondrial production of reactive oxygen species, which are the major determinants of oxidative damage in skeletal muscle cells (5). However, the mechanisms by which CR preserves the skeletal muscle integrity during aging remain largely unknown. Given the pleiotropic ameliorating effects of CR on age-related physiological changes, understanding how CR modulates skeletal muscle physiology during aging will facilitate discovery of pathways involved in sarcopenia.
To date, studies exploring the effects of CR on tissues gene expression have relied on microarrays. Deep sequencing, a set of technologies that produce very large amounts of sequence data from nucleic acid specimens, is rapidly replacing microarrays as the technology of choice for measuring gene expression. High-throughput sequencing of cDNA libraries (mRNA-Seq) has superior ability to capture the scale and complexity of whole transcriptomes (24). While array design relies on prior knowledge of the transcripts being interrogated, mRNA-Seq requires no design and allows discovery of new transcripts and identification of alternate splicing isoforms of known transcripts. Furthermore, microarray methods lack the dynamic range to detect and quantify low abundance transcripts, but deep sequencing can identify transcripts that are expressed at levels below the threshold of detection by microarrays (24). In addition, deep sequencing eliminates background problems that result from cross-hybridization in microarrays, thus facilitating interpretation of the signal and obviating the nonlinear data manipulation steps required by microarrays. Therefore, the application of deep sequencing to mRNA studies has the potential not only to discover novel isoforms but also to provide accurate and sensitive measurement of mRNA levels and detect expression of rare but functionally significant mRNAs.
Given the advantages of deep sequencing over microarray platforms for gene expression analysis, and the fact that deep sequencing has not yet been applied to analysis of CR's effects on gene expression, we have used mRNA-Seq to conduct a deep characterization of mRNA expression and gene regulation in skeletal muscle from old mice compared with old mice subjected to a long-term CR. We have used mRNA-Seq results to predict biological processes regulated by the CR-induced changes in the muscle. This study has revealed the great complexity of CR-induced changes in mRNA expression and evidence that CR results in a range of changes in gene expression that are broadly consistent with CR's beneficial effects.
MATERIALS AND METHODS
Mice and Diets
One-month-old male mice of the long-lived B6C3F1 strain were purchased from Harlan (Indianapolis, IN). One week after arrival, mice were individually housed and randomly assigned to one of two groups, control or CR. Control mice were fed 93 kcal/wk of a defined control diet (AIN-93M, diet no. F05312, BIO-SERV). CR mice were fed 52.2 kcal/wk of a defined CR diet (AIN-93M 40% Restricted, diet no. F05314, BIO-SERV). The CR mice consumed ∼40% fewer calories than the control group. The CR diet was enriched so that the CR mice consumed approximately the same amount of protein, vitamins, and minerals per gram of body weight as the control mice. All mice had free access to water. Mice were maintained at 20–24°C and 50–60% humidity with lights on from 0600 to 1800. Sentinel mice were kept in the same room as the experimental mice, and serum samples were screened every 6 mo for titers against 11 common pathogens. No positive titers were found during these studies. After 25 mo of CR treatment, mice were euthanized, and muscles that occupy the thigh (mainly quadriceps and adductor muscles) were rapidly excised and flash-frozen in liquid nitrogen. A group of control mice were euthanized at 7 mo of age and used as a young group in quantitative real-time polymerase chain reaction (qPCR) studies. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Riverside.
mRNA-Seq Sample Preparation
Muscles that occupy the thigh (mainly quadriceps and adductor muscles) were excised, flash-frozen in liquid nitrogen, and ground to a fine powder under liquid nitrogen. We used muscles from one control and one CR mouse to generate 76-bp paired-end mRNA-Seq data. Total RNA was extracted from muscle tissue with RNeasy Mini (QIAGEN) including the optional on-column DNase digestion step. RNA integrity was confirmed using the Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA) according to the manufacturer's instructions. Poly(A)+ mRNA was purified from 10 μg of total RNA, and fragmented (∼200 nt) prior to cDNA synthesis with random priming. The cDNA was converted into paired-end libraries using the Illumina mRNA-Seq Sample Prep Kit according to the manufacturer's protocol. The library preparation steps included end-repair, A-tailing, ligation of paired-end adapters, size selection and preamplification. Paired-end sequencing of the libraries was carried out using the Illumina GAII according to the manufacturer's instructions, using 76 cycles. Image deconvolution and quality values calculation were performed using the modules of the Illumina pipeline.
Tophat/Cufflinks Analysis of Sequencing Reads
Paired-end sequencing reads were aligned to the mouse reference genome (NCBI37/mm9) with Tophat v1.2.0 (29) using the default parameters with the exception of allowing up to 10 alignments to the reference for a given read, instead of the default value of 40. The data aligned by Tophat were processed by Cufflinks (30) to assemble transcripts and to measure their relative abundances in FPKM units (fragments per kilobase of exon per million fragments mapped). Assembled transcripts from control and CR muscle samples were compared with the RefSeq refFlat annotated transcriptome downloaded from the UCSC genome browser and tested for differential expression using the Cuffcompare and Cuffdiff utilities included in the Cufflinks package. Cuffdiff was run with FPKM upper-quartile normalization and a false discovery rate (FDR) threshold of 5%. Cufflinks calculates differential expression at the transcript, primary transcript, and whole gene levels. It also measures differential splicing and promoter usage and discovers novel isoforms of annotated genes. Analysis results were submitted to the National Center for Biotechnology Information's (NCBI's) Gene Expression Omnibus (GEO) database (GSE33863).
qPCR
Total RNA was extracted from thigh muscles of young control, old control, and old CR mice (n = 4 for each group) with RNeasy Mini (QIAGEN) from frozen skeletal muscles, and was used to generate cDNA with the High Capacity cDNA RT Kit (Applied Biosystems) or the QuantiTect Reverse Transcription Kit (Qiagen). The cDNA generated with the High Capacity cDNA RT Kit was used to quantify mRNA levels using primers specific for fasn, igfn1, and gapdh. Primers were designed using Primer3Plus (31), and the sequences are listed in Supplemental Table S11.1 All PCR primer sites span introns. PCR assays were performed in quadruplicate on an ABI 7900HT Real-Time PCR System using the default settings according to the manufacturer's instructions and using 2X Power SYBR Green PCR Master Mix (Applied Biosystems), with no-template and reverse transcriptase reactions as negative controls. Expression levels were determined using the standard curve obtained for each primer pair. The cDNA generated with the QuantiTect Reverse Transcription Kit was used to quantify mRNA levels using Qiagen QuantiTect Primer Assays specific for Acly, Cdkn1a, Dupd1, Fbxo32, Gadd45a, Myf6, Myl2, Ppp1r1a, Scd3, Tnnc1, Tnni1, Trim63, and Gapdh (Supplemental Table S11). The real-time PCR reactions were performed in triplicate with QuantiTect SYBR Green PCR Kit (Qiagen) on the CFX96 Real-time PCR system (Bio-Rad). The delta-delta comparative threshold method was used to determine the fold change between control and CR groups. All qPCR reactions were analyzed on agarose gels to ensure that products were of the predicted size, and dissociation curves of the PCR products were checked to confirm that a single product was amplified.
Western Blot Analysis
Total protein was extracted from skeletal muscle from old control and old CR mice (n = 3 for each group). Approximately 60 mg of frozen skeletal muscle tissue were sonicated in 350 μl of SDS buffer (50 mM Tris·HCl pH 6.8, 2% SDS, and 10% glycerol) for 20 s at output 7 (Sonifier Analog Cell Disruptor - model S-450A), and centrifuged at 16,000 g for 20 min. Supernatant was collected, and protein concentrations were determined by Pierce BCA Protein Assay Kit (Thermo Scientific). Proteins were separated by SDS-PAGE and transferred to PVDF membrane. We used antibodies against fasn and pan-actin from Cell Signaling and igfn1 from Santa Cruz Biotechnology. Pan-Actin antibody detects endogenous levels of total actin (all isoforms). Western blots were developed with Amersham ECL Advance chemiluminescence, using standard procedures.
RT-PCR
We used RT-PCR to validate the expression and splicing of novel exons. Total RNA extracted with RNeasy Mini (QIAGEN) from frozen mouse liver and muscle was used to generate cDNAs with the High Capacity cDNA RT Kit per manufacturer's instructions (Applied Biosystems). The cDNAs were used as templates for PCR amplification with HotStarTaq DNA Polymerase (QIAGEN) using the cycling program suggested by the manufacturer. The primers (Supplemental Table S11) were designed to cross the novel splice junctions predicted by Cufflinks. The same sets of primers were used with mouse genomic DNA as control. PCR products were analyzed on 2% agarose gels and fragment size was determined by comparison to a 100 bp DNA Ladder.
Functional Annotation of the Differentially Expressed Genes
To characterize biological processes affected by CR-induced mRNA expression changes, we used Gene Ontology (GO) and the functional annotation clustering feature of DAVID (15) to functionally annotate genes that were differentially expressed in the CR mouse muscle (genes listed in Supplemental Table S1) and genes that displayed isoform switching in the CR muscle without a change in total mRNA abundance (genes listed in Supplemental Table S9). We also used the clustering analysis to analyze the significance of KEGG pathways in our data. The functional annotation clustering tool measures the similarities among GO terms based on the extent of their associated genes and assembles the similar and redundant GO terms into annotation clusters. Each GO term in a cluster is assigned a Fisher Exact P value representing the degree of enrichment of the GO term in the input gene list. Each cluster is assigned an enrichment score to rank its biological significance. This enrichment score is derived from the geometric mean (in −log scale) of member's P values. Thus, a biologically significant cluster (high enrichment score) is generated only when most of its GO term members have significant enrichment values (low Fisher Exact P values). The resulting clusters were further curated to keep only GO terms with P values < 0.05.
Microarray Analysis of Mouse Atrophied Skeletal Muscle
MIAME-compliant raw data from Agilent whole genome arrays of skeletal muscle from control and mice with hind-limb suspension (22) were obtained from the GEO repository GSE9802. The arrays were analyzed with Bioconductor limma package to identify genes differentially expressed in atrophied muscle (27). Limma uses linear models to analyze microarray experiments. Briefly, the intensity data were imported with implementing the “normexp” method with an offset of 1 to correct the background and a “quantile” normalization method to normalize between arrays. Replicate spots were averaged with the “avereps” function. Differentially expressed genes were obtained by fitting a linear model to the normalized data followed by computing empirical Bayes statistics. The P values were adjusted for multiple testing using Benjamini and Hochberg's method to control the FDR. Genes with FDR <0.05 were selected as differentially expressed. The differentially expressed genes were separated into two lists of upregulated and downregulated genes, which were then compared with the genes downregulated and upregulated by CR using a four-way Venn diagram.
RESULTS
Overview of mRNA-Seq Analysis
mRNA-Seq measures transcript abundance, determines differential gene expression between experimental conditions, and identifies the type of regulation (transcriptional or posttranscriptional) governing the changes in gene expression (30). To investigate the effects of CR on gene expression and regulation in skeletal muscle, we used mRNA-Seq to analyze the transcriptome of skeletal muscle from 26 mo old control and CR mice. We isolated poly(A)+ RNA from each muscle sample, fragmented it to ∼200 nucleotides, made randomly primed cDNA, and constructed Illumina sequencing libraries. The libraries were sequenced to obtain 76 bases of sequence at each end of each fragment; longer reads and paired-end reading facilitate the identification of splice sites and isoforms. We mapped the sequencing reads to the mouse genome (NCBI37/mm9) using Tophat, which aligns reads across splice junctions without relying on known gene annotations. This approach allows identification of alternate transcription and splicing events that are not described by preexisting gene models. We obtained 23,269,244 and 19,191,332 properly paired reads for control and CR samples, respectively. The aligned fragments were assembled into transcripts by Cufflinks, which also estimates transcript abundances in FPKM. The assembled transcripts from control and CR muscle samples were compared with the RefSeq refFlat annotated transcriptome from the UCSC genome browser and tested for differential expression using the Cuffcompare and Cuffdiff utilities included in the Cufflinks package. Transcript assembly with Tophat in the absence of an annotated transcriptome reference, followed by re-estimation of the abundance of transcripts by Cuffdiff in the presence of an annotated transcriptome reference, classifies the assembled transcripts into known transcripts or novel isoforms of annotated genes. Cuffdiff also measures differential splicing and differential promoter usage.
Genes Differentially Expressed in the Aged Control and Aged CR Mouse Muscle
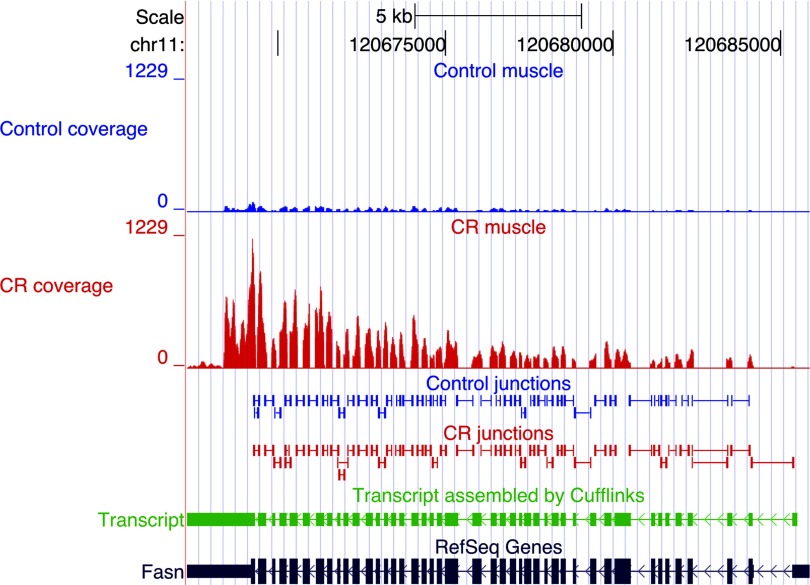
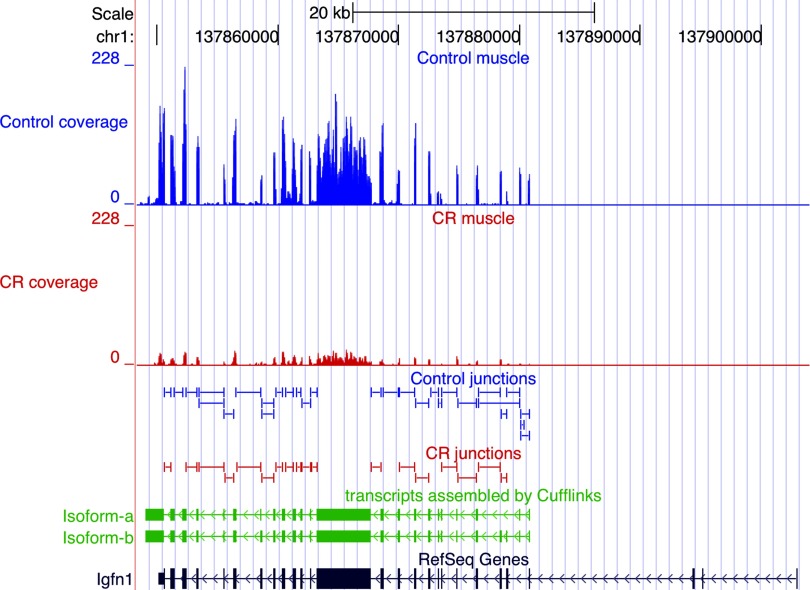
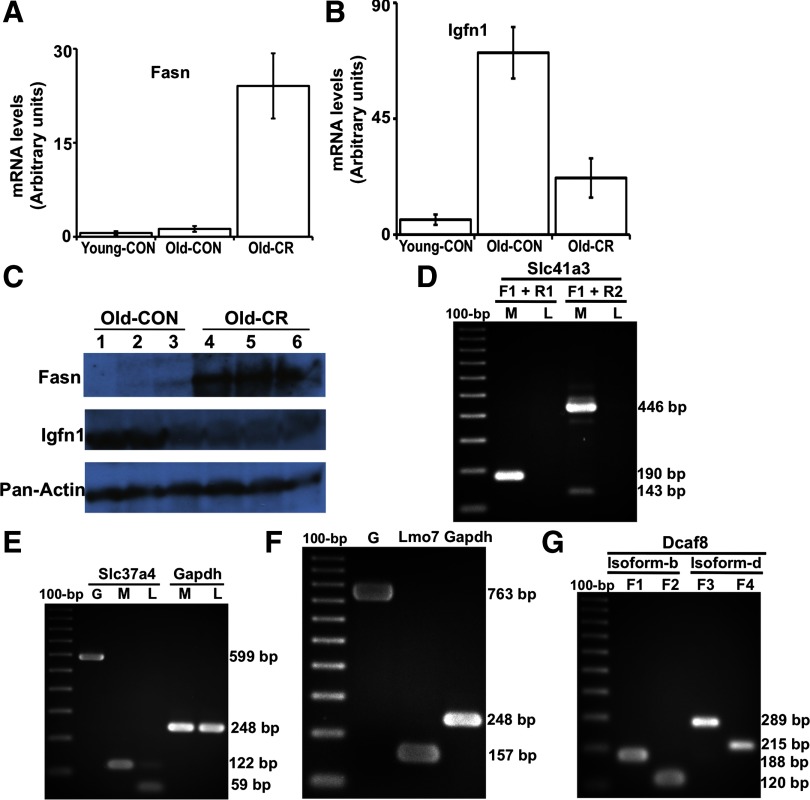
Cufflinks/Cuffdiff measured the differential expression at the transcript, primary transcript, and whole gene levels; expression levels of primary transcripts and genes were computed by summing the FPKMs of all transcripts derived from a primary transcript or within a gene. Selection criteria implemented in determining the significance of differential expression of whole gene, primary transcript or isoform were: 1) expression level >10 FPKM in at least one of the states (control or CR), 2) FDR <5%, 3) fold change ≥1.5. The 10 FPKM threshold was arbitrarily chosen as a very conservative upper bound on possible noise reads. Genes were annotated by comparing transcripts assembled by Cufflinks to the mouse RefSeq refFlat reference annotation. At the whole gene level, CR significantly changed the expression of 435 annotated genes (Supplemental Table S1); 171 genes were upregulated, and 264 genes were downregulated in the muscle from CR mice. An example of a gene induced by CR is fatty acid synthase (fasn) (Fig. 1); an example of a gene suppressed by CR is immunoglobulin-like and fibronectin type III domain containing 1 (igfn1) (Fig. 2). We verified the CR-induced changes in the fasn and igfn1 mRNAs with qPCR in the same muscle samples used for the Illumina sequencing, and in additional muscle samples from aged control and aged CR mice, as well as young control mice. qPCR indicates that fasn expression did not change with age but was induced by CR (Fig. 3A); expression of igfn1 increased with age, but CR significantly suppressed the age-associated increase (Fig. 3B). Igfn1 is specifically expressed in skeletal muscle and has been reported to be highly upregulated in atrophied muscle (20). The increased expression of igfn1 with age and its suppression by CR suggest that CR may oppose age-associated sarcopenia. Western blotting showed CR-induced changes at the protein level consistent with changes in levels of fasn and igfn1 mRNA in the old control and CR mice (Fig. 3C).
Fig. 1.
mRNA-Seq analysis of fatty acid synthase (fasn) gene expression. Shown here is a UCSC browser view illustrating the fasn gene with sequence coverage depth (quantity of reads, y-axis) at the top [blue, control; red, calorie restriction (CR)]. The peaks mark expressed exons and are consistent with RefSeq annotation (dark blue at bottom). Differential expression of the fasn gene is indicated by the considerably fewer reads mapping to the exons in the control than in the CR sample. Splice junctions identified by Tophat from control and CR datasets are shown below the sequence alignments; all known splice junctions were detected. The fasn transcript assembled by Cufflinks independently of any gene annotation is shown in green; it coincides precisely with the RefSeq annotation.
Fig. 2.
mRNA-Seq analysis: CR decreases igfn1 expression. Shown is a UCSC browser view illustrating the igfn1 (Immunoglobulin-like and fibronectin type III domain containing 1) gene with sequence coverage depth (quantity of reads, y-axis) at the top (blue, control; red, CR). The peaks mark expressed exons and are consistent with RefSeq annotation (dark blue at bottom). Decreased expression of igfn1 in CR muscle is indicated by the considerably fewer reads mapping to the exons in CR compared with control. Splice junctions identified by Cufflinks from control and CR datasets are shown below the sequence alignments. The igfn1 transcripts assembled by Cufflinks independently of any gene annotation are shown in green (isoform-a and isoform-b); the isoforms are the products of alternative splicing and both have alternative last exons.
Fig. 3.
qPCR and Western blotting: validating the differential expression of fasn and igfn1 and agarose gels of RT-PCR products verifying the expression and splicing of novel exons. A: qPCR validation of the differential expression of fasn. The change in gene expression identified by mRNA-Seq were confirmed with qPCR by comparing mRNA levels from old control and old CR samples. We also added a young control group to measure the age effects on fasn muscle expression (n = 4 for each of the 3 groups). CR induced fasn expression, but age did not affect it. mRNA levels were normalized to GAPDH control. B: qPCR validation of the differential expression of igfn1. The change in gene expression identified by mRNA-Seq were confirmed with qPCR by comparing mRNA levels from old control and old CR samples. Age increased igfn1 expression, but CR suppressed the age-associated increase. C: Western blots showing the level of fasn, igfn1, and pan-actin in protein samples extracted from the skeletal muscle of old control and old CR mice (n = 3 for each group). D: expression and splicing of the novel exon in the Slc41a3 gene locus shown in Fig. 4. The forward primer specific to exon 1 (F1) and reverse primer specific to exon 2 (R1) were designed to amplify a 190 bp product from isoform-a which includes the second exon. The forward primer specific to exon 1 (F1) was also used with a primer specific to exon 3 (R2) to amplify a 446 bp product that further validated isoform-a, and a 143 bp product that validates isoform-b, which lacks the second exon. The lanes are labeled as M (muscle cDNA) and L (liver cDNA). Neither isoform is expressed in the liver. E: expression and splicing of the novel exon in the Slc37a4 gene locus shown in Fig. 5. The primers amplify a 599 bp product from genomic DNA (lane G), a 122 bp PCR product from muscle cDNA (lane M) when the novel exon is expressed and included in the transcript, and a 59 bp PCR when the novel exon is excluded. Both isoforms were expressed in the liver (lane L), but only the transcript that included the novel exon was expressed in muscle. A pair of primers to amplify cDNA of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as a control. F: expression and splicing of the novel leader exon in the Lmo7 gene locus shown in Fig. 6. The primers amplify a 763 bp product from genomic DNA (lane G) and a 157 bp PCR from muscle cDNA, verifying the expression and splice junction of the novel exon. G: expression of the leader exons of the novel isoforms b and d in the Dcaf8 gene locus shown in Fig. 9. A forward primer (F1) that maps to an intronic region of the RefSeq canonical Dcaf8 is designed to be specific to exon 1 of the novel isoform-b. The primer F1 amplifies a 188 bp product from muscle cDNA when used with a reverse primer specific to exon 2 (R1), verifying the expression of the novel leader exon of isoform-b. A second forward primer (F2), downstream of F1 amplifies a shorter PCR product (120 bp) when used with the same reverse primer R1. Similarly, expression of the leader exon of the novel isoform-d was verified by a forward primer (F3) that maps to an intronic region of the RefSeq canonical Dcaf8, designed to be specific to exon 1 of isoform-d. The primer F3 amplifies a 289 bp product from muscle cDNA when used with a reverse primer (R2) specific to exon 2 of isoform-d, verifying the expression of the novel leader exon of isoform-d. A second forward primer (F4), downstream of F3 amplifies a shorter PCR product (215 bp) when used with the same reverse primer R2. Primer sequences are shown in Supplemental Table S11.
Novel mRNA Isoforms Detected in the Skeletal Muscle of Control and CR Mice
Since mRNA-Seq can discover novel exons and their associated splice junctions (30), we analyzed our data to identify CR-associated differential expression of alternative transcript isoforms. We considered only those novel transcripts whose expression was significantly altered by CR and identified 871 novel isoforms of known transcripts annotated in RefSeq refFlat (Supplemental Dataset). The novel isoforms resulted from various events such as alternative promoter usage, alternative splicing, and alternative cleavage and polyadenylation.
The 871 novel transcripts (Supplemental Dataset) that changed expression with CR were further analyzed to identify some of the most common types of alternative events that give rise to new isoforms. To ensure that they are novel, transcripts were checked against the combined mainstream transcript databases of RefSeq, UCSC, Ensembl, and VEGA annotations, using the BEDTools suite (25). This analysis revealed that the majority of the novel transcripts (854 of the 871) had a 5′ exon and/or 3′ exon that did not overlap with any annotated 5′ untranslated region (UTR) or 3′ UTR in the combined transcript databases. The 5′ exons of the novel isoforms were further selected to be at least 200 bp away from any known 5′ UTR. Thus, this category of novel transcript includes transcripts with alternative leader exons that arose from alternative promoter usage, transcripts with alternative last exons that arose from alternative cleavage and polyadenylation, or both types of alternative events occurring in the same transcript. Other types of observed alternative transcripts included novel exons that were absent from annotated transcripts, or known exons that were excluded in novel transcripts. Other transcripts, not included in the categories described so far, were characterized as novel because they were the products of alternative 5′ splice site and/or alternative 3′ splice site events. It should be noted that novel transcripts with an alternative leader exon and/or alternative last exon, and novel transcripts with excluded exons, can also harbor alternative 5′ and 3′ splice site events.
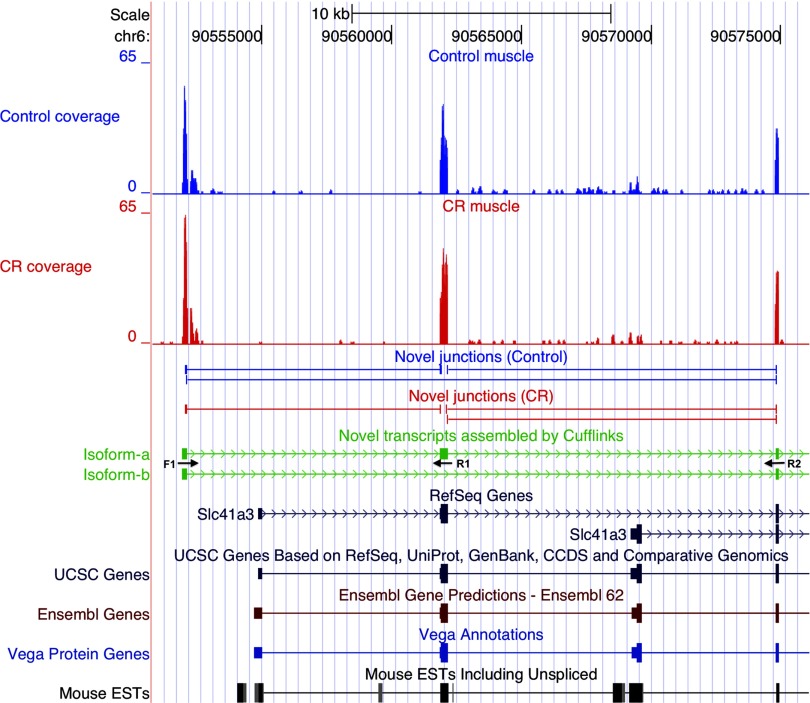
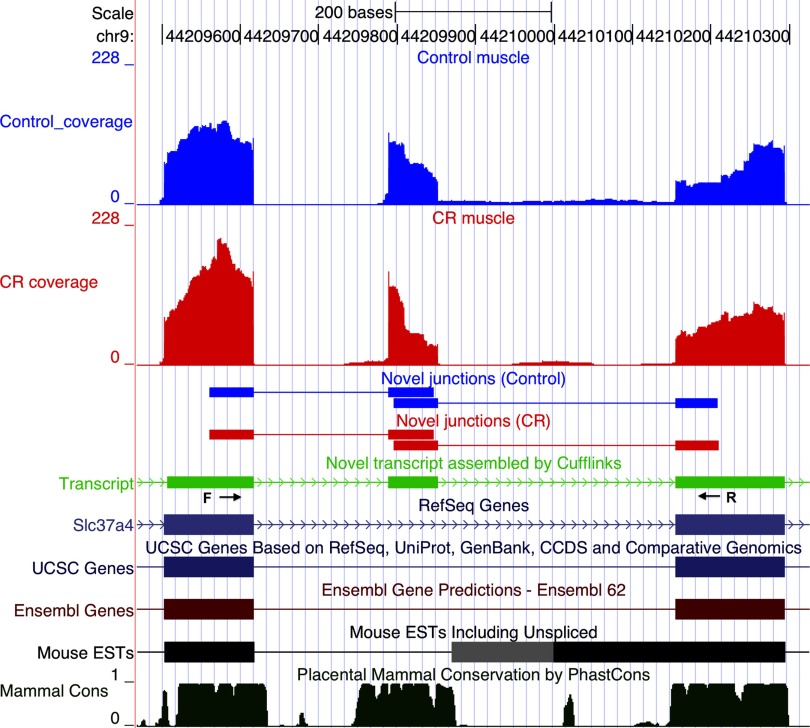
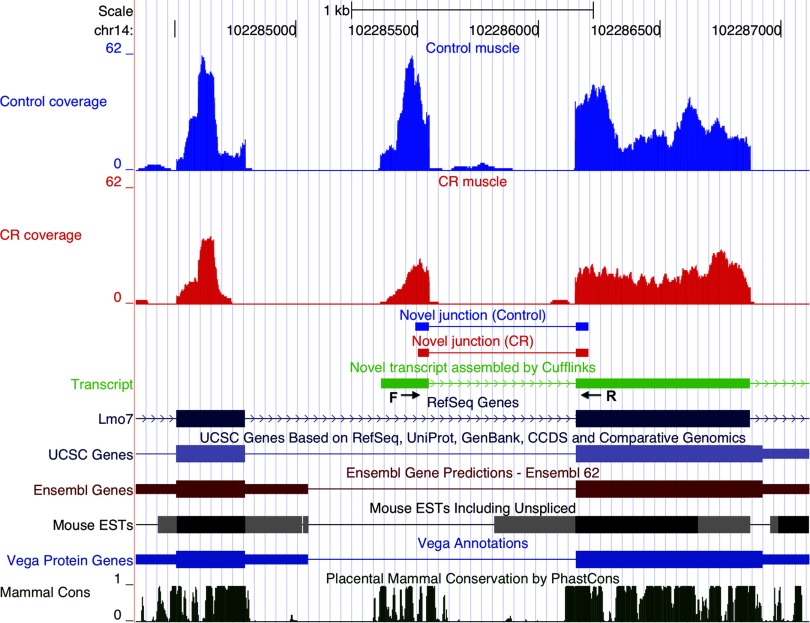
Examples of alternate transcript events are illustrated in Figs. 4–6. Slc41a3 has two primary transcripts annotated in RefSeq. A novel third primary transcript with two isoforms (isoform-a and isoform-b, Fig. 4) was identified by Cufflinks; both isoforms were initiated at a novel transcription start site. The second exon was included in isoform-a and excluded from isoform-b. The novel alternative transcription initiation site is supported by the presence of novel downstream junctions and the lack of upstream junctions in both control and CR samples. RT-PCR amplified an appropriately sized 190 bp product from muscle cDNA that validated isoform-a, which includes the second exon (Fig. 3D). When a primer specific to exon 3 (Fig. 4) was used, PCR yielded a 446 bp product that further validated isoform-a and an additional 143 bp product that validated isoform-b, which excludes the second exon. No amplification products were obtained when the same primers were used with liver cDNA. In another gene, Slc37a4, Cufflinks identified an exon not present in the annotated transcript databases but conserved in mammals (Fig. 5). Upstream and downstream novel junctions that give further support to the new exon were also detected. RT-PCR validated the expression of the novel exon by amplifying an appropriately sized product from muscle and liver cDNAs; this is the sole PCR product in muscle, but only a minor product in liver where the RefSeq annotated isoform is dominant (Fig. 3E). Another example is an alternative leader exon isoform of Lmo7 mRNA (Fig. 6). The novel first exon is conserved in mammals, is not annotated in the mainstream UCSC transcripts databases, and is supported by the presence of downstream novel junctions and the lack of upstream junctions in both control and CR samples. Expression and splicing of the novel exon were confirmed by an appropriately sized PCR product (Fig. 3F).
Fig. 4.
Novel isoforms of the Slc41a3 gene mRNA. Screenshots from the UCSC genome browser, displaying mRNA-Seq reads from control and CR muscle samples mapping to annotated and novel exons in the Slc41a3 gene locus. The reads aligned to the Slc41a3 locus are shown at top (control, blue; CR, red). Slc41a3 transcripts annotated in the mainstream transcript databases are shown in the lower portion of the figure. mRNA-Seq detected two novel isoforms (isoform-a and isoform-b, in green) and their associated novel splice junctions. The stacks of mRNA-Seq reads on the left identify the novel first exon in both CR and control muscle samples (isoform-a). The mainstream transcript databases are shown, with no coding sequences annotated in the new exon region. Arrows beneath the first 3 exons of the novel transcript denote the positions of forward and reverse PCR primers used for validation in Fig. 3D. In addition control muscle contains a novel splice variant that lacks exon 2 (isoform-b).
Fig. 5.
A novel isoform of the Slc37a4 gene mRNA, featuring an exon not present in the annotated transcript databases. Screenshots from the UCSC genome browser, displaying the mRNA-Seq reads from control (blue) and CR muscle (red) samples mapping to annotated and novel exons in the Slc37a4 gene locus. Novel splice junctions detected by Cufflinks are also depicted. The stack of sequence reads in the middle identifies a novel exon present in both CR and control muscle samples (novel transcript in green). UCSC genome browser tracks for mainstream annotated transcript databases are shown, with no coding sequences annotated in the new exon region. The mammalian conservation track is at the bottom, showing conservation of the novel exon. Arrows beneath the exons flanking the novel exon represent position of forward and reverse PCR primers.
Fig. 6.
A novel isoform of Lmo7 mRNA. Screenshots from the UCSC genome browser, displaying mRNA-Seq reads from control and CR muscle samples mapping to annotated and novel exons in the Lmo7 gene locus. The reads aligned to the Lmo7 locus are shown at top (control, blue; CR, red). The novel junctions detected by splice junction reads are also depicted. Lmo7 transcripts annotated in the mainstream transcript databases are shown in the lower portion of the figure. The middle stacks of sequence reads identify the novel exon in both CR and control muscle samples, which is conserved as indicated by the mammalian conservation track (bottom). Arrows beneath the first and second exons of the novel transcript represent positions of the forward and reverse PCR primers used to verify the isoform.
Regulation Dynamics of the Genes Differentially Expressed by CR
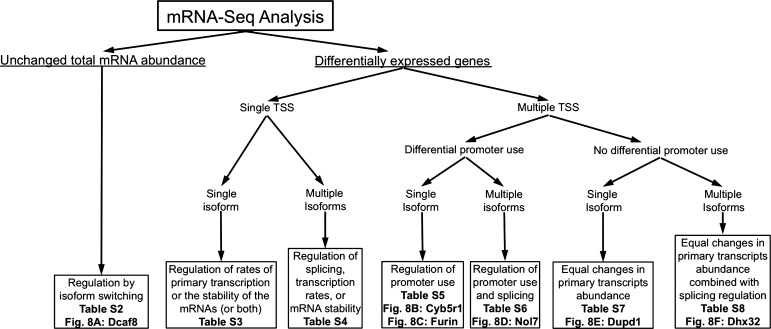
To characterize the types of regulation governing the gene expression patterns induced by CR in the muscle, we classified the differentially expressed genes into groups based on whether a gene featured a single or multiple primary transcripts and whether there was differential promoter usage within genes with multiple transcription start sites and/or differential splicing within primary transcripts. This scheme (Fig. 7) allows classification of the differentially expressed genes according to the underlying regulatory mechanism: change in mRNA abundance, alternative splicing, or both. Cufflinks/Cuffdiff quantifies differential splicing by measuring the relative abundance of transcripts with a common transcription start site, while differential promoter usage is quantified by measuring the relative abundance of primary transcripts within a gene. We have classified types of regulation into the following categories.
Fig. 7.
Scheme for regulation type classification of CR-regulated genes. Gene expression patterns induced by CR in muscle result from changes in transcript abundance, promoter usage, and splicing. This scheme classifies genes according to which changes are induced by CR. Genes classified by the scheme can be found in the referenced tables and figures.
Genes with unchanged total mRNA abundance, but exhibiting isoform switching.
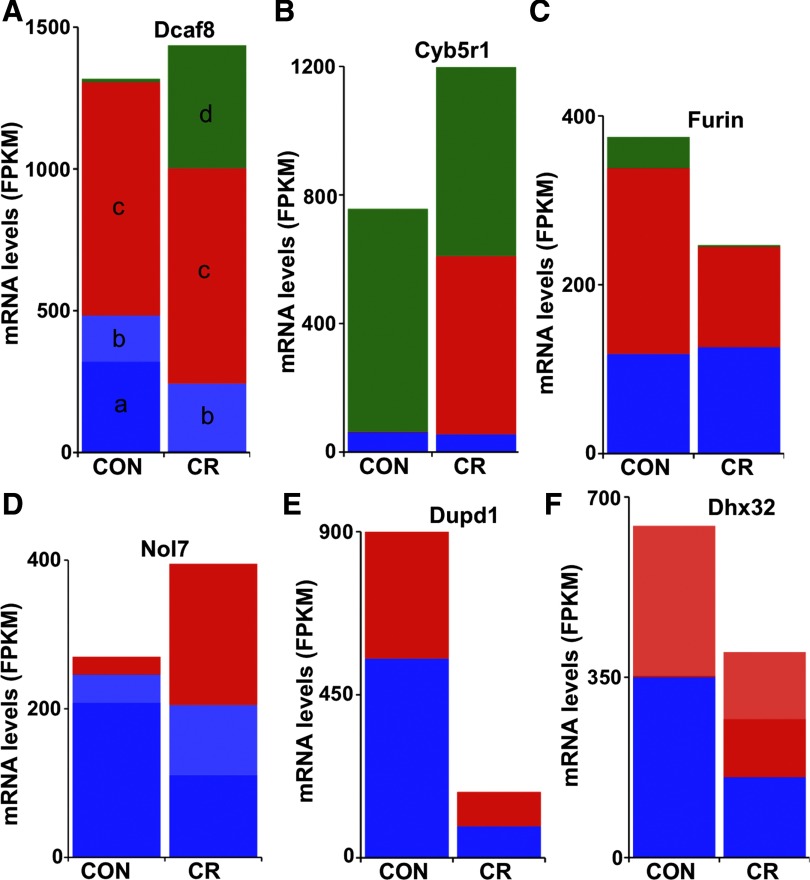
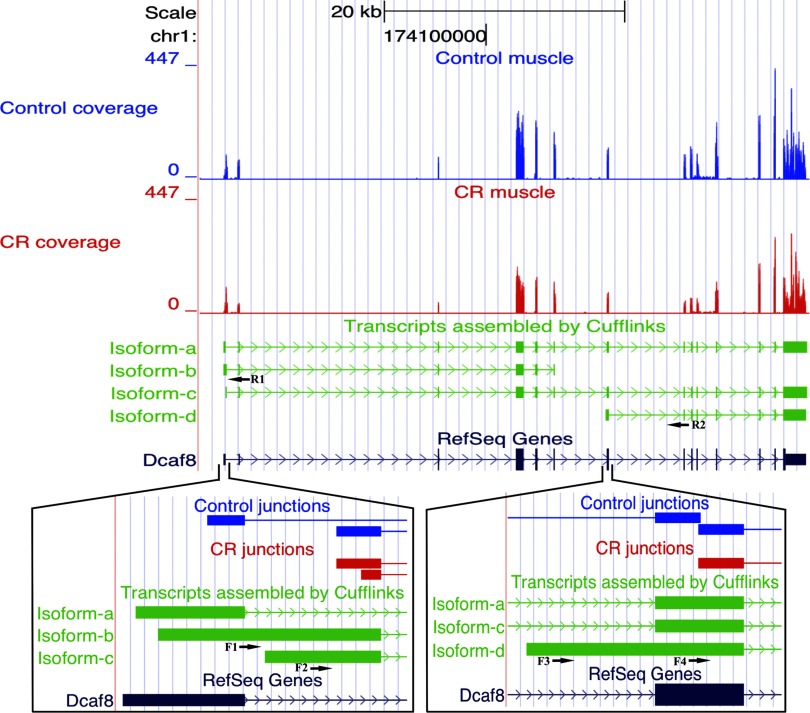
CR did not change the abundance of mRNAs derived from many genes. However, Cufflinks/Cuffdiff revealed that transcripts of some of these genes underwent considerable change (Supplemental Table S2). The underlying mechanism for isoform switching could be differential splicing, promoter switching, or a more complex mix of both mechanisms. For example, the abundance of Dcaf8 mRNA does not change with CR (Fig. 8A), but the isoforms of Dcaf8 mRNA change markedly. The Dcaf8 gene generates three primary transcripts: TSS1, the canonical transcription start site, produces two alternatively spliced isoforms (isoform-a and -b), TSS2, and TSS3, which are processed into a single isoform each (isoform-c and -d, respectively) (Fig. 9). CR is associated with the presence of a novel mRNA isoform that is nearly absent in control muscle; this novel isoform (isoform-d) is transcribed from a region just 5′ to the seventh exon of the RefSeq canonical isoform and so lacks the first six exons of the canonical isoform. Moreover, CR induced the disappearance of one of the alternative splicing isoforms of TSS1 (isoform-a) (Fig. 8A, Supplemental Table S2; P = 0.0), while it slightly increases the levels of isoform-b. Expression of the novel leader exons of isoform-b and -d was confirmed by appropriately sized PCR product (Fig. 3G). CR changed the usage of two of the transcription start sites: TSS1 declined in activity, while TSS3 that is barely used in control muscle was readily apparent in CR (Fig. 8A, Supplemental Table S2; P = 0.0). Thus while the abundance of total Dcaf8 mRNA did not change with CR, its isoforms underwent a combination of significant changes that resulted in isoform switching: disappearance of isoform-a and appearance of isoform-d.
Fig. 8.
Complex isoform switching in muscle mRNA is induced by CR. Examples of alternative transcript isoform switching induced by CR in skeletal muscle. A: complex isoform switching in Dcaf8 transcripts. While CR does not change total Dcaf8 mRNA levels, it induces the appearance of one isoform and disappearance of another. Dcaf8 generates primary transcripts from three alternative promoters (TSS1, blue; TSS2, red; TSS3, green). The primary transcript TSS1 is alternatively spliced into 2 isoforms (dark blue and light blue: a and b), while TSS2 and TSS3 are processed into a single isoform each (c and d, respectively). Total Dcaf8 mRNA level is not changed by CR; TSS2 (red) is also unchanged, but proportions of TSS1 (blue) and TSS3 (green) change markedly. Although total TSS1-derived mRNA declines by only about half, this change is completely accounted for by the disappearance of one of the TSS1-derived isoforms (isoform-a); the other isoform (isoform-b) slightly increases in abundance. B: CR is associated with promoter switching in the Cyb5r1 gene. Cyb5r1 generates 3 transcripts each, arbitrarily labeled TSS1, TSS2, and TSS3, that are initiated at 3 different transcription start sites (TSS). The TSSs mark the isoforms initiated at each of the three promoters. The y-axis shows total mRNA abundance, with the colors within the bars indicating the proportion of the total made up by each TSS isoform (TSS1, blue; TSS2, red; TSS3, green). Use of the TSS marker shows that the difference in Cyb5r1 expression levels between control (CON) and CR resulted from differential promoter usage. Changes in absolute Cyb5r1 gene expression in the CR muscle are due to increased use of TSS2: TSS1 and TSS3 do not change, but use of TSS2 is dramatically increased in the CR muscle. C: Furin gene also uses 3 alternative TSSs (TSS1, blue; TSS2, red; TSS3, green). Changes in absolute Furin gene expression in CR muscle result from decreased use of TSS2 and TSS3: TSS1 levels are stable, while TSS2 makes up a smaller proportion of the total and TSS3 is nearly absent. D: CR regulates both promoter preference and alternative splicing of Nol7 transcripts. Nol7 generates 2 primary transcripts, TSS1 and TSS2, from alternative promoters (TSS1, blue; TSS2, red). The primary transcript TSS1 is alternatively spliced into 2 isoforms (dark blue and light blue), while TSS2 is processed into a single isoform. Total Nol7 mRNA is upregulated in CR muscle; the increase is accounted for by increased TSS2, indicating that expression has been increased by use of an alternative promoter. The total TSS1-derived transcript does not change significantly with CR, but there is a large decline in abundance of one TSS1-derived isoform (dark blue), and a corresponding increase in the abundance of the other (light blue). E: regulation of the Dupd1 gene by CR involved reduced abundance of both alternative transcripts. Dupd1 generates 2 primary transcripts (TSS1, blue; TSS2, red), which are processed into a single isoform each. CR downregulates both Dupd1 mRNA isoforms. F: regulation of the Dhx32 gene by CR. Dhx32 generates 2 primary transcripts (TSS1, blue; TSS2, red) from alternative promoters. TSS2 is alternatively spliced into 2 isoforms (dark red and light red), while TSS1 is processed into a single isoform. Total Dhx32 mRNA is downregulated in CR muscle: both TSS1 (blue) and TSS2 (red) are decreased, but the decrease in TSS2 is accounted for entirely by decrease in one of the TSS2 isoforms (light red), while the other TSS2 isoform (dark red) actually shows a dramatic increase.
Fig. 9.
Isoforms of Dcaf8 mRNA change with CR. Screenshots from the UCSC genome browser, displaying mRNA-Seq reads from control and CR muscle samples mapping to the Dcaf8 gene locus, with insets enlarging 2 regions. Reads aligned to the Dcaf8 locus are shown at top (control, blue; CR, red). The canonical RefSeq Dcaf8 mRNA is at bottom. Dcaf8 mRNA isoforms assembled by Cufflinks are in green. Isoform-b has the same transcription start site as -a, but a longer leader exon and an alternative 3′ end that drops the last 8 exons. Isoform-c has an alternative TSS. Isoform-d is a novel isoform initiated upstream of the 7th RefSeq canonical exon and thus lacking the first 6 exons. Arrows beneath exons of the novel transcripts represent positions of the forward and reverse PCR primers used to verify the isoforms as shown in Fig. 3G.
Differentially expressed genes featuring a single primary transcript processed into a single isoform.
CR differentially regulated 116 genes featuring a single primary transcript processed into a single isoform (Supplemental Table S3); 56 genes were upregulated and 60 downregulated. Since each single primary transcript produced a single isoform (i.e., no alternative splicing), CR changed the expression levels of these genes by modulating either their rates of primary transcription or the stability of the mRNAs (or both).
Differentially expressed genes featuring a single primary transcript processed into multiple isoforms.
CR differentially regulated 59 genes featuring a single primary transcript processed into more than one isoform (Supplemental Table S4). However, only two of these genes were differentially spliced; the CR-induced change in the expression of these two genes could be accounted for by changes in splicing, transcription rates, or mRNA stability. In the remaining 57 genes, there was no significant differential splicing; thus, the differential gene expression between control and CR muscles resulted from changes in rates of primary transcription or mRNA stability.
Differentially expressed genes with multiple primary transcripts processed into a single isoform each: differential promoter usage.
For some genes with multiple primary transcripts, Cufflinks/Cuffdiff also revealed significant differential promoter usage induced by CR. The mode of regulation of these genes was further characterized according to whether their primary transcripts were processed into single or multiple isoforms; this identifies possible differential splicing in addition to the differential promoter usage. For those genes in which the primary transcripts were processed into a single isoform each (i.e., no alternative splicing; Supplemental Table S5), the difference in the whole gene expression level between control and CR arose from differential promoter usage only. For example, Cyb5r1 and Furin genes each generate three primary transcripts (Fig. 8). The overall level of Cyb5r1 mRNA increased in CR muscle (Fig. 8B, Supplemental Table S5; P = 0.0003), while the expression of Furin gene decreased (Fig. 8C, Supplemental Table S5; P = 0.00002). Furthermore, CR significantly changed the promoter preference in both Cyb5r1 and Furin genes (Supplemental Table S5, P = 0.00 for Cyb5r1 and P = 0.01 for Furin). Differential promoter usage is indicated by the significant changes in relative abundances of the primary transcripts (Fig. 8, B and C). Expression of the primary Cyb5r1 transcript TSS2 increased significantly in the CR muscle, while the expression of the other two primary transcripts remained unchanged (Fig. 8B). The switch in promoter preference made TSS2 the dominant primary transcript of Cyb5r1 gene in the CR state. The Furin TSS3 promoter became less preferred in the CR state, followed by TSS2, while the expression of Furin TSS1 was not affected by CR (Fig. 8C). Thus, the CR-associated promoter switch produced transcripts that could be functionally specific to CR and potentially important for mediating its effects.
Differentially expressed genes with multiple primary transcripts processed into multiple isoforms each: combined differential promoter usage and differential splicing regulation.
Differences in the gene expression levels between control and CR can result from more complex regulation patterns combining promoter preference and differential splicing (Supplemental Table S6). This complex mechanism is illustrated by the regulation of the Nol7 gene (Fig. 8D). Nol7 generates two primary transcripts, TSS1 and TSS2. The overall abundance Nol7 mRNA increased in the muscle of CR mice (Fig. 8D, Supplemental Table S6; P = 0.00). CR also significantly changed Nol7 promoter usage (Supplemental Table S6, P = 0.0009) as reflected by the significant changes in relative abundances of its primary transcripts (Fig. 8D). Furthermore, the primary transcript TSS1 was alternatively spliced into two isoforms. CR induced differential splicing of the TSS1 primary transcript (Supplemental Table S6, P = 0.0004) as reflected by the significant change in relative abundances of its splicing isoforms (Fig. 8D). Thus, CR decreased the abundance of an isoform that was dominant in the control state, while it increased the abundance of another isoform which was scarce in the control state.
Differentially expressed genes with multiple primary transcripts exhibiting no promoter preference.
In some cases, genes with multiple primary transcripts were differentially regulated by CR, but without affecting promoter usage (Supplemental Table S7). For example, regulation of Dupd1 by CR involved decreased abundance of both alternative transcripts (Fig. 8E). CR also regulated genes in which multiple primary transcripts were processed into multiple isoforms each (Supplemental Table S8), resulting in more complex patterns that combine changes in transcript abundance and splicing as illustrated by the regulation of Dhx32 (Fig. 8F). Dhx32 generates two primary transcripts, TSS1 and TSS2. The abundance of total Dhx32 mRNA decreased in CR muscle (Fig. 8F, Supplemental Table S8; P = 0.00). CR did not alter the relative promoter usage as reflected by the equal changes in relative abundances of TSS1 and TSS2; thus the decreased expression of Dhx32 resulted from a parallel decrease in transcription of TSS1 and TSS2, or stability of these mRNAs. The primary transcript TSS2 is alternatively spliced into two isoforms. CR induced differential splicing of the primary transcript TSS2 (Supplemental Table S8, P = 0.000004) as reflected by the significant change in relative abundance of its splicing isoforms (Fig. 8F). CR increased the abundance of an isoform that is nearly absent in the control state, while it decreased the abundance of the second isoform.
Ontological Pathways Regulated by the Genes Differentially Expressed in the CR Muscle
To identify biological pathways potentially underlying the effects of CR on skeletal muscle physiology, we functionally annotated the genes that are differentially expressed between control and CR muscle (those listed in Supplemental Table S1), using DAVID and GO. This analysis took into account only the total abundance of mRNA from each gene, and not the alternative isoforms discussed above: because the distinct functions of these isoforms are not established, GO has no way to use information on isoform expression. Genes downregulated by CR produced six clusters that have enrichment scores >1.3 (Table 1). The most highly enriched downregulated pathways in the CR muscle included processes that control the actin cytoskeleton (arrangement, assembly, or disassembly of cytoskeletal structures containing actin filaments), pathways of protein breakdown initiated by ubiquitin attachment and mediated by the proteasome, and pathways involved in muscle tissue development. Other enriched biological processes suppressed by CR included processes that mediate cellular response to oxidative stress and pathways that control the breakdown of hydrogen peroxide.
Table 1.
Functional annotation clusters of enriched GO biological processes downregulated in the CR skeletal muscle
| ES | GO Biological Process | Count | P Value |
|---|---|---|---|
| Cluster 1 | |||
| 3.5 | GO:0030029∼actin filament-based process | 12 | 2.2E-05 |
| GO:0030036∼actin cytoskeleton organization | 10 | 3.3E-04 | |
| GO:0007010∼cytoskeleton organization | 14 | 4.0E-04 | |
| Cluster 2 | |||
| 3.0 | GO:0006511∼ubiquitin-dependent protein catabolic process | 8 | 2.7E-03 |
| GO:0043161∼proteasomal ubiquitin-dependent protein catabolic process | 5 | 6.2E-04 | |
| Cluster 3 | |||
| 2.8 | GO:0044257∼cellular protein catabolic process | 18 | 6.4E-04 |
| GO:0051603∼proteolysis involved in cellular protein catabolic process | 18 | 7.3E-04 | |
| GO:0043632∼modification-dependent macromolecule catabolic process | 17 | 9.7E-04 | |
| GO:0006508∼proteolysis | 23 | 1.5E-02 | |
| Cluster 4 | |||
| 2.2 | GO:0044267∼cellular protein metabolic process | 49 | 3.2E-05 |
| GO:0044260∼cellular macromolecule metabolic process | 73 | 3.8E-02 | |
| GO:0043687∼posttranslational protein modification | 22 | 3.9E-02 | |
| Cluster 5 | |||
| 2.1 | GO:0014706∼striated muscle tissue development | 9 | 2.5E-04 |
| GO:0060537∼muscle tissue development | 9 | 4.0E-04 | |
| GO:0007517∼muscle organ development | 10 | 6.3E-04 | |
| GO:0042692∼muscle cell differentiation | 7 | 5.0E-03 | |
| GO:0048747∼muscle fiber development | 4 | 1.3E-02 | |
| GO:0007528∼neuromuscular junction development | 3 | 3.8E-02 | |
| Cluster 6 | |||
| 1.3 | GO:0042744∼hydrogen peroxide catabolic process | 3 | 1.0E-02 |
| GO:0070301∼cellular response to hydrogen peroxide | 3 | 1.1E-02 | |
| GO:0000302∼response to reactive oxygen species | 4 | 1.2E-02 | |
| GO:0034599∼cellular response to oxidative stress | 3 | 3.4E-02 | |
An enrichment score (ES) of 1.3 is equivalent to a nonlog scale value of 0.05. Count refers to the gene members that belong to an annotation term. Fisher Exact P value represents the degree of enrichment of the Gene Ontology (GO) term. CR, caloric restriction.
Genes upregulated in CR muscle were organized into four significant annotation clusters (Table 2); the most significant associations were with pathways that generate precursor metabolites from which cells derive energy by oxidation. CR also stimulated additional energy-related pathways in the muscle, including electron transport chain, cellular respiration, and the KEGG pathway “oxidative phosphorylation.” Another class of pathways that was stimulated in the CR muscle involved lipid biosynthetic processes, in particular, fatty acid biosynthesis.
Table 2.
Functional annotation clusters of enriched GO biological processes upregulated in the CR skeletal muscle
| ES | GO Biological Process | Count | P Value |
|---|---|---|---|
| Cluster 1 | |||
| 15.5 | GO:0006091∼generation of precursor metabolites and energy | 32 | 3.2E-26 |
| GO:0022900∼electron transport chain | 21 | 8.4E-21 | |
| GO:0055114∼oxidation reduction | 33 | 4.9E-15 | |
| Cluster 2 | |||
| 3.6 | GO:0015980∼energy derivation by oxidation of organic compounds | 10 | 2.4E-07 |
| GO:0006119∼oxidative phosphorylation | 6 | 1.5E-04 | |
| GO:0042775∼mitochondrial ATP synthesis coupled electron transport | 3 | 7.0E-03 | |
| Cluster 3 | |||
| 3.1 | GO:0019752∼carboxylic acid metabolic process | 15 | 3.2E-05 |
| GO:0006629∼lipid metabolic process | 18 | 2.0E-04 | |
| GO:0006633∼fatty acid biosynthetic process | 6 | 5.1E-04 | |
| GO:0008610∼lipid biosynthetic process | 10 | 1.1E-03 | |
| GO:0046394∼carboxylic acid biosynthetic process | 6 | 5.9E-03 | |
| Cluster 4 | |||
| 2.8 | GO:0006084∼acetyl-CoA metabolic process | 5 | 1.1E-04 |
| GO:0009109∼coenzyme catabolic process | 4 | 1.6E-03 | |
| GO:0006732∼coenzyme metabolic process | 7 | 1.9E-03 | |
| GO:0006099∼tricarboxylic acid cycle | 3 | 1.8E-02 | |
| GO:0046356∼acetyl-CoA catabolic process | 3 | 2.0E-02 | |
For abbreviations and definitions, see Table 1 footnote.
Ontological Pathways Regulated by the Genes That Display Isoform Switching in the CR Muscle Without a Change in Total mRNA Abundance
One group of genes, shown in Supplemental Table S2, showed changes in isoforms without a change in abundance of total mRNA from the gene. Functional annotation and clustering analysis of these genes (Supplemental Table S9) shows that many fall into the same ontological pathways associated with the genes that are differentially expressed at the level of total mRNA abundance. This is significant because it suggests that isoform switching itself is associated with functional changes related to the effects of CR.
Validation of CR-associated Changes in Gene Expression
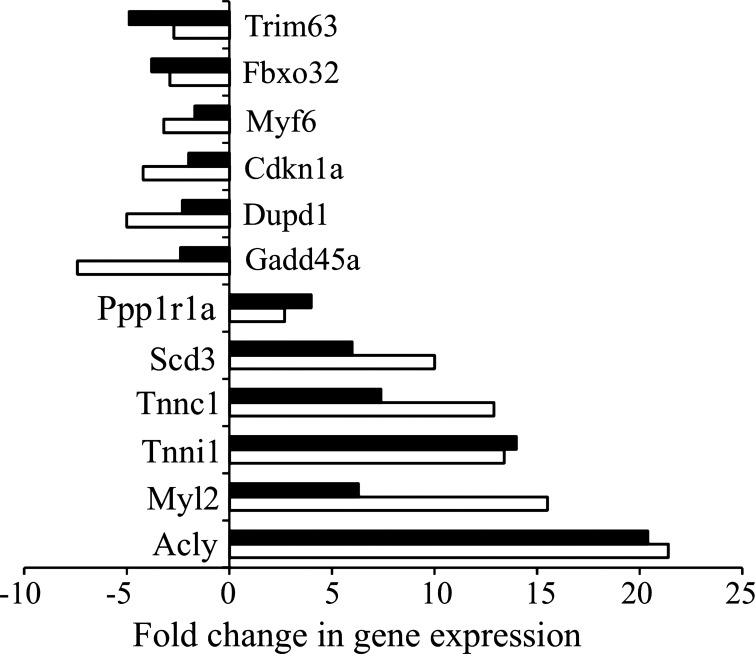
Our mRNA-Seq studies were carried out on muscle from one control and one CR mouse. The version of Cufflinks/Cuffdif used in our analysis [Tophat v1.2.0 (29)] is designed to compare single specimens, by assuming that there is no true differential expression for most genes and that a valid mean-variance relationship can be estimated from treating the two samples as if they were replicates. While this approach may yield fewer differentially expressed genes due to loss in statistical power, as described above it seems to reveal CR-associated changes in gene expression occurring in certain ontological pathways. To validate changes in these pathways with an independent method, we used qPCR to assay CR-associated changes in mRNA levels of 14 genes. We selected at least one gene from each of the functional annotation clusters (listed in Tables 1 and 2) of GO biological processes regulated in the skeletal muscle by CR and carried out qPCR in the same muscle samples used for the Illumina sequencing and in additional muscle samples from aged control and aged CR mice (n = 3) (Fig. 10). This analysis confirms the up- and downregulation seen in the RNA-Seq analysis, although the magnitude in changes is not precisely the same.
Fig. 10.
Validation of RNA-Seq data with qPCR. The expression of 12 genes was analyzed by qPCR. Muscle RNA samples from 3 aged control and 3 CR mice, including the ones used for the RNA-Seq study, were analyzed by qPCR. The solid bars representing the qPCR data are the fold change between control and CR groups as determined by the delta-delta comparative threshold method. The open bars representing the RNA-Seq data are the fold change in the specific mRNA for control and CR mice as determined by the Cuffcompare and Cuffdiff utilities included in the Cufflinks package. The genes analyzed were ATP citrate lyase (Acly), cyclin-dependent kinase inhibitor 1A (P21) (Cdkn1a), dual specificity phosphatase and pro isomerase domain containing 1 (Dupd1), F-box protein 32 (Fbxo32), growth arrest and DNA-damage-inducible 45 alpha (Gadd45a), myogenic factor 6 (Myf6), myosin, light polypeptide 2, regulatory, cardiac, slow (Myl2), protein phosphatase 1, regulatory (inhibitor) subunit 1A (Ppp1r1a), stearoyl-coenzyme A desaturase 3 (Scd3), troponin C, cardiac/slow skeletal (Tnnc1), troponin I, skeletal, slow 1 (Tnni1), tripartite motif-containing 63 (Trim63), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Atrophy-induced Gene Expression Changes Potentially Hindered by CR in Skeletal Muscle
Muscle atrophy occurs during aging as well as following prolonged bed rest or hind-limb suspension in experimental animals (2). To determine any potential effects of CR on gene expression counteracting those associated with atrophy, we contrasted genes whose expression is increased during atrophy to genes that were downregulated by CR in mouse skeletal muscle and vice versa. We used the limma Bioconductor package to generate gene expression profiles from Agilent whole genome array raw data files of skeletal muscle from control and mice with hind-limb suspension obtained from the GEO repository GSE9802 (22). Even though microarrays have a narrower dynamic range than mRNA-Seq and detect fewer expressed genes, we found 97 genes that were inversely regulated in atrophied skeletal muscle and muscle from CR mice (Supplemental Table S10).
DISCUSSION
We have used mRNA-Seq to compare the transcriptomes of skeletal muscle from old mice and old mice subjected to CR; this analysis considerably broadens the set of genes whose expression is affected by CR, in terms of either total mRNA expression or the expression of alternative mRNA isoforms. mRNA-Seq measures transcript abundance, determines differential gene expression between experimental conditions, and resolves the abundance of multiple isoforms of an mRNA, allowing judgments about changes in mRNA abundance and posttranscriptional regulatory events (30). Compared with microarrays, mRNA-Seq yields more accurate and sensitive measurement of mRNA levels, identifies novel transcripts and isoforms, and detects expression of rare mRNAs. Despite these advantages, mRNA-Seq has its limitations and is still a work in progress (26). Various confounding factors, experimental and computational, are still being resolved: these include transcript length bias in estimating mRNA abundance, sequence base composition, and the handling of reads that align to multiple positions in the genome (14, 19, 32). Our mRNA-Seq results in general support findings made with microarrays but reveal previously unsuspected complexity in the effects of CR on gene expression. In a very large number of cases, the most prominent change is not the total abundance of mRNA derived from a gene, but the proportions of isoforms that may encode different functions (when they involve protein coding regions) or different mRNA metabolism (when they involve 5′ and 3′ UTRs).
Our mRNA-Seq analysis identified novel exons and splice junctions not previously annotated in transcript databases, which adds a layer of information lacking in prior gene expression studies that relied on annotated gene models to design microarray features. We detected 871 novel isoforms of known genes that were differentially expressed in CR and control muscle; the generation of these isoforms involves alternative promoter usage, alternative splicing, and alternative cleavage and polyadenylation. The use of alternative promoters creates flexibility and complexity in the regulation of gene expression and can yield protein isoforms that differ at the amino terminus and respond differently to extracellular signals (4). The significance of these novel isoforms is supported by functional annotation of genes that undergo CR-associated isoform switching without a change in total mRNA abundance: many of these genes fall into the same functional clusters as the genes that undergo changes in mRNA level (Supplemental Table S9).
A gene may undergo substantial transcript-level dynamics that are not reflected into its overall expression pattern as reflected in total mRNA abundance and so would not be discovered by methods other than mRNA-Seq. For example, while the global expression level of Dcaf8 did not change, Cufflinks/Cuffdiff analysis of the mRNA-Seq data revealed that CR is associated with the presence of a novel mRNA isoform that is nearly absent in control muscle (Figs. 8A and 9; isoform-d) and also with the absence of a Dcaf8 mRNA isoform (Figs. 8A and 9; isoform-a) that is prominent in control muscle. Each of these isoforms may have some functional difference from the canonical form. Dcaf8 is a member of the DCAF protein family, whose members serve as substrate-recognition subunits in the ubiquitin ligase complex to target specific proteins for ubiquitination and proteasome-mediated degradation. The targets recruited by Dcaf8 have not yet been identified, but other DCAF proteins that recruit targets such as c-Jun and Cdt1 have been described (18). The upregulation of one isoform of Dcaf8, concurrently with the suppression of another, may reflect a change in protein function with significant biological impact. Dcaf8 is merely one example of this CR-associated isoform switching, which often takes place along without a change in total message abundance (Supplemental Table S2). mRNA-Seq analysis thus reveals complex changes that may mediate, or result from, the effects of CR.
To further understand the biological meaning of the genes differentially expressed by CR in the skeletal muscle, we used functional annotation to identify the most relevant biological processes and pathways associated with the differentially expressed genes. Functional annotation and clustering analysis are powerful tools to understand the biological significance of changes in gene expression on large datasets (16). Nevertheless it is important to keep in mind the limits of this method: a focus on functional annotation and clustering can obscure the significance of expression changes in genes that may have important functions but do not fit into a cluster because other functionally related genes are not affected. Of the 264 genes downregulated in CR muscle, 93 map to one or more of the clusters in Table 1, leaving 171 genes whose expression is downregulated but do not have known functions in common with other genes downregulated in this dataset. Furthermore, only 56 of the 171 genes upregulated in CR muscle map to one or more of the clusters in Table 2, leaving 115 that do not have known functions in common with other genes upregulated in this dataset. Thus the majority of the genes whose expression is changed in association with CR do not have obvious functions in common; however, they might still have functions that are critical, and even causal, in the functional changes associated with CR.
While much of our functional annotation serves to confirm and extend earlier work that used microarray-based expression profiling, there are differences and additions that add to the picture, and as discussed above the ability to resolve isoform switching adds new complexity. The only pathways that are significantly upregulated (enrichment score >1.3) function in the generation of energy by oxidation and other energy-related pathways including electron transport, cellular respiration, and oxidative phosphorylation; fatty acid synthesis is also upregulated. Upregulation of lipid biosynthesis pathways in CR muscle was also observed in skeletal muscle microarray profiling studies (17). Muscle is a nonlipogenic tissue; conversely, in the liver, CR has been shown to decrease the liver enzymatic capacity for fatty acid biosynthesis and increase fatty acid oxidation (8, 9). Measurement of whole body fatty acid synthesis and oxidation rates revealed that CR induced a cyclic pattern of fatty acid synthesis and oxidation with an increase of fatty acid synthesis after feeding followed by an increase of fatty acid oxidation, leading to the conclusion that whole body fatty acid oxidation in CR mice exceeds the dietary fat intake, and CR increases fatty acid synthesis to balance this excess (7). Our finding that CR stimulated lipid biosynthesis pathways in the muscle of CR mice is compatible with this conclusion; it may be part of this fatty acid synthesis balancing mechanism. The cyclic pattern of increased fatty acid synthesis and oxidation may reflect the highly efficient metabolic flexibility of the CR state. Consistently, CR is known to postpone metabolic disorders (obesity, insulin resistance syndrome, and diabetes) that are associated with metabolic inflexibility, i.e., inability of skeletal and cardiac muscles to shift readily back and forth between carbohydrate and fat as oxidative energy sources (28).
On the other hand, pathways downregulated in CR muscle included processes that control actin cytoskeletal structures, protein breakdown, cellular response to oxidative stress, and pathways involved in muscle tissue development. CR effects on genes involved in protein breakdown and oxidative stress were noted in previous studies (17), but the other pathways were not; the discrepancy is probably accounted for by the greater sensitivity of mRNA-Seq and functional annotation. CR is associated with increased expression of genes that encode thin filament proteins, including elements of the troponin complex and its associated tropomyosin (Tnni1, Tnni2, Tnnc1, Tnnc2, Tnnt3, and Tpm1). The troponin complex acts as the calcium-sensitive molecular switch-regulating contraction. Other thin filament gene products affected by CR include Capza2, a capping protein that regulates actin assembly, and Nrap, a striated muscle-specific scaffolding protein involved in myofibril assembly. CR also increased the expression of genes that encode thick filament proteins, including components of the myosin motor (Myl1, Myl2, Myl3, Myh7, and Mylpf) and Mybph, which encodes for an accessory protein that contributes to the assembly and stabilization of thick filaments. The increased expression of numerous contractile apparatus genes may suggest that CR is associated with skeletal muscle contractile properties that enhance muscle strength and function. The CR-associated expression changes in some of these genes (Tnni1, Tnnc1, and Myl2) were validated with qPCR in the same muscle samples used for the Illumina sequencing and in additional muscle samples from aged control and aged CR mice (n = 3) (Fig. 10). CR also downregulated the expression of genes known to mediate muscle atrophy, which results from a variety of conditions including muscle inactivity, multiple disease states, and aging. Mainly, CR decreased the expression of the muscle-specific ubiquitin ligases, Fbxo32 (also known as atrogin 1 or MAFbx) and Trim63 (also called MuRF1), which are members of E3 ubiquitin ligases that bind particular substrates to induce ubiquitin binding and degradation of the substrates through the proteasome. The CR-associated downregulation of both Fbxo32 and Trim63 were validated with qPCR in the same muscle samples used for the Illumina sequencing and in additional muscle samples from aged control and aged CR mice (n = 3) (Fig. 10). MuRF1 and MAFbx are markers of the atrophy process; they have been shown to be induced in multiple models of skeletal muscle atrophy and cachexia (6, 12, 13). As for substrates, MuRF1 acts by degrading components of the contractile apparatus. Interestingly, the expression of Myl2, a substrate of MuRF1, was higher in the CR than in the control muscle. Myl2, a regulatory protein essential for myofibril integrity and function, was shown to undergo ubiquitin-proteasome-mediated degradation in atrophying muscle (10). Thus, the lowered expression of MuRF1 combined with the enhanced expression of Myl2 may reflect a well-maintained myofibrillar apparatus in the muscle of CR mice and suggests that CR may delay the age-associated sarcopenia by, at least in part, regulating the expression of these genes.
Taken together, the effects of CR on muscle gene expression may as a whole be reflective of a healthier myocytes, consistent with its antisarcopenic effects. The evidence suggestive of improved function in CR muscle raises the issue of causality. None of the CR-associated changes in gene expression that we and others have described have been established as causing the beneficial effects on muscle physiology; it is simpler to assume that they are part of improved muscle function rather than its cause. Functional annotation and clustering are useful for identifying common pathways affected by an insult or intervention. But all of the changes we have identified could reflect merely the effects of changes in as yet unidentified functions performed by the products of genes that are not included in any of the clusters we find. As discussed above, many genes whose levels or isoforms are changed in association with CR do not fall into a functional cluster, but this does not mean that they have no important function. One or more of these genes might have a more direct role in mediating CR's effects on muscle physiology. The deeper understanding of gene regulation facilitated by mRNA-Seq and functional annotation may suggest new approaches that will advance the goal of identifying the primary effects of CR on muscle and other tissues.
GRANTS
This work was supported by National Institutes of Health Grants ES-016581, CA-115768, DK-080428 (D. I. K. Martin), and HL-084474 (D. Boffelli). J. M. Dhahbi was a Scholar of the California Institute of Regenerative Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.D., H.A., D.B., D.I.M., and S.R.S. conception and design of research; J.M.D. and H.A. performed experiments; J.M.D. and H.A. analyzed data; J.M.D., H.A., D.B., and D.I.M. interpreted results of experiments; J.M.D. prepared figures; J.M.D. drafted manuscript; J.M.D., D.B., D.I.M., and S.R.S. approved final version of manuscript; D.I.M. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Noel Guerrero, Amy Yamakawa, and Patricia Mote for their help.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. van Kan Abellan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging 13: 708–712, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95: 2185–2201, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J 11: 573–581, 1997. [DOI] [PubMed] [Google Scholar]
- 4. Ayoubi TA, Van De Ven WJ. Regulation of gene expression by alternative promoters. FASEB J 10: 453–460, 1996. [PubMed] [Google Scholar]
- 5. Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab 286: E852–E861, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab 298: E108–E116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA 98: 10630–10635, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22: 1753–1757, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185: 1083–1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci 63: 556–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37: 1974–1984, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucl Acids Res 38: e131, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Huang da W, Sherman BT, Zheng X, Yang J, Imamichi T, Stephens R, Lempicki RA. Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinformatics Chapter 13: Unit 13.11, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell 26: 775–780, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26: 493–500, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansilla F, Dominguez CA, Yeadon JE, Corydon TJ, Burden SJ, Knudsen CR. Translation elongation factor eEF1A binds to a novel myosin binding protein-C-like protein. J Cell Biochem 105: 847–858, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masoro EJ. Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology 7: 153–155, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Mazzatti DJ, Smith MA, Oita RC, Lim FL, White AJ, Reid MB. Muscle unloading-induced metabolic remodeling is associated with acute alterations in PPARdelta and UCP-3 expression. Physiol Genomics 34: 149–161, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Nair KS. Aging muscle. Am J Clin Nutr 81: 953–963, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12: 87–98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sendler E, Johnson GD, Krawetz SA. Local and global factors affecting RNA sequencing analysis. Anal Biochem 419: 317–322, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev 9: 324–353, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35: W71–W74, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng W, Chung LM, Zhao H. Bias detection and correction in RNA-Sequencing data. BMC Bioinformatics 12: 290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.