Abstract
Background
Animal research indicates that oxytocin is involved in social behavior, stress regulation, and positive physiologic adaptation. This study examines whether oxytocin enhances adaptive responses to social stress and compares effects between men and women.
Methods
Hypotheses were tested with a placebo-controlled, double-blind experiment. Social stress was induced. Changes in cardiovascular reactivity, affect, and behavior were assessed.
Results
Participants given oxytocin, relative to placebo, responded to social stress with a challenge orientation characterized by a benign pattern of cardiovascular reactivity. Gender differences emerged. Men given oxytocin reported less negative affect and had greater vagal rebound, while women given oxytocin reported more anger and had better math performance following social stress.
Discussion
Findings indicate oxytocin stimulates an approach-oriented cardiovascular profile during social stress, suggesting mechanisms by which oxytocin might improve physical health. However, before considering oxytocin as therapeutic or uniformly enhancing health, greater understanding of possible gender differences in effects is needed.
Keywords: social stress, oxytocin, gender, cardiovascular, experiment, health
1. Introduction
Positive social relationships have consistently been associated with better health, although the neurobiological underpinnings of these observed effects are not well understood. Animal research has indicated that oxytocin, a neuropeptide produced in the supraoptic and paraventricular nuclei (PVN) of the hypothalamus, is involved in social bonding, stress regulation, and positive physiologic adaptations that may be linked with greater longevity and successful aging (Depue & Morrone-Strupinsky, 2005; Knox & Uvnas-Moberg, 1998; Taylor et al., 2000; Uvnas-Moberg, 1997). Because of its potential role in promoting positive human social behavior, recent research has focused on whether oxytocin may lead to improved social and emotional functioning for a range of disorders including autism, schizophrenia, and anxiety or depression (Averbeck, 2010; Bartz & Hollander, 2008; Bartz et al., 2010). Moreover, given its apparent anti-stress effects, some investigators have posited that oxytocin may reduce stress-related physiologic consequences such as decreasing heart rate and blood pressure, and have speculated important links between oxytocin and cardiovascular health (Knox & Uvnas-Moberg, 1998). However, knowledge of the effects of oxytocin in healthy humans remains limited and some recent research has suggested that effects may not be uniformly prosocial or stress-reducing (De Dreu et al., 2010; Miller, 2010; Shamay-Tsoory et al., 2009). Moreover, gender differences in these stress-related effects have been speculated but never directly tested in humans. Therefore, in this study we provide one of the first direct tests of the hypothesis that oxytocin facilitates a positive, adaptive profile of physiological responses during highly stressful situations in healthy men and women.
Interest in a clinical application for oxytocin comes from positive results in basic science studies. Pre-clinical research on the neurobiology underlying social bonding and engagement suggests that oxytocin facilitates bonding and approach behaviors partly by inhibiting stress-related maladaptive cognitive, affective, and biological activation (Depue & Morrone-Strupinsky, 2005; Porges, 2003a). In animals, oxytocin modulates sympathetic and parasympathetic nervous system activity (SNS, PNS), and more generally is involved in cardiovascular regulation (Higa, Mori, Viana, Morris, & Michelini, 2002; Petersson & Uvnas-Moberg, 2007; Uvnas-Moberg, 1997). Such effects, if applicable to humans, could promote health by reducing potentially toxic direct effects of stress-related neuroendocrine and autonomic activation.
Several recent studies in men found that exogenously administered oxytocin reduced fear-related activation in the amygdala, reduced cortisol levels and distress in response to social stress, increased prosocial behaviors, and modulated social memory (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Heinrichs, Meinlschmidt, Wippich, Ehlert, & Hellhammer, 2004; Kirsch et al., 2005; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005). These results led investigators to propose oxytocin as a key hormone underlying positive social experience and prosocial behavior, and their related health benefits, which could perhaps portend a role as a therapeutic agent (Uvnas-Moberg, 1997).
However, despite early promising evidence, one recent study found that exogenously administered oxytocin increased parasympathetic and sympathetic cardiac control under resting conditions only among individuals who did not report feeling lonely (Norman et al., 2011). Other research has found that under certain conditions oxytocin may actually enhance negative responses to social stress in humans (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005; Shamay-Tsoory, et al., 2009). The animal literature has suggested that oxytocin is more potent in the presence of higher estrogen levels, leading investigators to hypothesize that effects of oxytocin will be stronger in women than men. However, among female animals, higher levels of oxytocin are associated with more aggression and willingness to attack along with less fear (Campbell, 2008). Further, a number of studies in humans suggest that higher peripheral levels of oxytocin serve as a marker of greater distress among women (Cyranowski et al., 2008; Hoge, Pollack, Kaufman, Zak, & Simon, 2008; Taylor, Saphire-Bernstein, & Seeman, 2010). To date, little experimental data are available on the effects of exogenously administered oxytocin in women or whether oxytocin alters subjective, hormonal, cardiovascular, and behavioral responses to social stress uniformly in both men and women.
One critical distinction that might assist in the interpretation of whether oxytocin is stress-buffering or not is a more precise definition of what are the features of harmful versus beneficial stress responses. Not all stress reactions are equal. By differentiating acute stress reactions that facilitate goal-directed behavior and are characterized by benign physiological reactions from stress reactions that impair performance and have harmful consequences, we might gain traction on the question of how oxytocin modulates acute stress reactions.
Several theories have identified psychological antecedents and physiological consequences that differentiate “good” stress from “bad” stress responses (e.g., Dienstbier, 1989; Frankenhaeuser, 1986; Henry, 1986). One theory that integrates Dienstbier’s “physiological toughness” theory and Lazarus and Folkman’s stress appraisal theory in the context of acute stressful situations is the biopsychosocial model of challenge and threat (for a review see Blascovich & Mendes, 2010). In this model both challenge and threat states occur during acute “stressful” situations; however, the states differ in their antecedent appraisal process and subsequent downstream cardiovascular reactivity. For example, “challenge states” occur when individuals appraise their resources as exceeding the demands of the task, whereas “threat states” occur when situational demands are perceived to exceed resources. Importantly, these psychological states have different profiles of cardiovascular responses. Even though both states are characterized by SNS activation, challenge is characterized by increases from a resting state in cardiac output (CO, the total volume of oxygenated blood the heart pumps in a minute) and decreases in total peripheral resistance (TPR)—vasodilation. Threat is characterized by little or no increase in CO and increases in TPR—vasoconstriction. Furthermore, in some contexts, SNS activation is greater in challenge than threat states, consistent with Dienstbier’s idea of physiological toughness, which suggests that larger increases in SNS activation to novel situations is related to effective coping and better performance (see also Jamieson, Mendes, Blackstock, & Schmader, 2010).
Benefits in cognitive performance, emotional status and health have been identified as following from challenge states. For example, challenge, relative to threat, states have been associated with better decision making (Kassam, Koslov, & Mendes, 2009), higher status (Scheepers, De Wit, & Ellemers, In press), more approach-oriented behavior and increased positive affect (Mendes, Major, McCoy, & Blascovich, 2008). Larger increases in sympathetic activation (commonly measured using changes in ventricle contractility, a relatively pure measure of sympathetic activation, or changes in catecholamine levels) also tend to produce better performance in physical and cognitive tasks. For example, a recent study of students preparing to take the GRE assigned participants to either a stress reappraisal manipulation, which encouraged participants to interpret their physiological arousal during test-taking as a beneficial reaction that would help them perform better during the exam, or a no-instruction control condition. Reappraisal participants exhibited a larger increase in sympathetic activation (measured with salivary alpha amylase) immediately before taking a practice GRE and performed better at the math exam than those who were assigned to the control condition. Indeed, consistent with the challenge and threat framework, the greater the SNS increase the better the math performance. Furthermore, the reappraisal participants, compared to control, earned higher GRE exam scores when they took the actual test in the following months (Jamieson, et al., 2010).
Individual differences also align in expected directions with experiencing threat or challenge during acute stress episodes. People who believe more strongly in a just world (Tomaka & Blascovich, 1994), individuals with high, stable self-esteem (Seery, Blascovich, Weisbuch, & Vick, 2004), and non-lonely individuals (Cacioppo et al., 2002) typically exhibit cardiovascular reactivity consistent with challenge states during acute stressful tasks more than individuals who self-report lower on these constructs. Individual differences in the neurobiological milieu have also been linked to challenge and threat states. In a recent paper, individuals with higher left, relative to right, frontal cortical activity (a neurological pattern previously linked to positive affect and well-being) were more likely to respond to a stressful situation with challenge appraisals and “challenge” reactivity (higher CO and lower TPR) (Koslov, Mendes, Pajtas, & Pizzagalli, 2011).
These patterns of benign “challenge” responses may accumulate over time to more positive health outcomes. In a recent examination of more than 1500 participants from the Framingham sample (Jefferson et al., 2010), increased cardiac output, one of the primary cardiovascular determinants of “challenge” states, was associated with decelerated brain aging- -individuals with greater cardiac output had increased brain volume and showed increased cognitive processing speed in older adulthood. These researchers speculated that increased oxygenated blood produced by the heart may have long-term protective effects in the brain.
Recovery from stressful situations might also provide a more precise picture of the stress modulating role of oxytocin. One promising measure of recovery from stressful situations is vagal rebound or the extent to which vagus nerve activity returns to resting levels or even “over-shoots” resting levels after suppression of the vagal brake (Porges, 2007). Whereas lower levels of heart rate variability during a stressful task might indicate attentional control or distress, once a stressor has ended, an adaptive response is for the vagus nerve to re-establish control and slow down heart rate. Vagal rebound is measured with heart rate variability responses during recovery from an acute stressor.
In our experimental paradigm, we rely on these distinctions in cardiovascular responses during and recovery from acute stressful situations to examine the role of oxytocin in modulating stress responses. Using a placebo-controlled double-blind experiment with a sample of healthy men and women recruited from the community, we tested the hypothesis that oxytocin facilitates adaptive stress responses during and following an acute social evaluation by examining: 1) changes from baseline to stress exposure for ventricle contractility (VC: measured as pre-ejection period multiplied by -1 so that increases in sympathetic nervous system are represented as increased VC) and cardiac output (CO); and 2) vagal rebound. We hypothesized that if oxytocin promotes positive responses to social stress, individuals exposed to exogenously administered oxytocin relative to placebo would exhibit a pattern of cardiovascular reactivity consistent with challenge states (increased VC and CO) during a highly stressful social evaluation. To provide converging evidence of challenge states we videotaped participants during the stress task, and trained observers (blind to condition) to code these recordings for specific challenge and threat behavior that occurred during the stress task. We predicted that oxytocin participants would exhibit more positive affect and behavior and possibly better performance than placebo participants. Following the stressor, we expected that oxytocin participants would show greater vagal rebound immediately following the completion of the stress task. Finally, we hypothesized that oxytocin would be associated with less self-reported negative affect under conditions of stress. Although we predicted these general positive effects of oxytocin for both men and women, we also tested whether gender moderated any of the responses.
2. Methods
2.1. Overview
This placebo-controlled, double-blind experiment employed a between subjects factorial design, using a 2 (men vs. women) X 2 (oxytocin vs. placebo) design. Participants were randomized to receive either intranasal oxytocin spray or placebo (saline) nasal spray. Social stress was induced using the Trier Social Stress Test (TSST), which prior work has suggested reliably activates the hypothalamic-pituitary-adrenal axis and the SNS (Kirschbaum, Pirke, & Hellhammer, 1993). Baseline measures of estradiol were obtained via saliva samples. Primary outcomes were cardiovascular (CV) reactivity, objective behavior during the stress task coded by observers unaware of the oxytocin condition, and self-reported affective responses. Details of the study design have been previously published (Kubzansky, Mendes, Appleton, Block, & Adler, 2009). This protocol was approved by the Institutional Review Boards at the two institutions overseeing the research: Harvard School of Public Health and Brigham and Women’s Hospital.
2.2. Participants
Participants between the ages of 25 and 65 years were recruited from the community. Interested participants completed an initial telephone screening and were scheduled for a lab appointment. Upon arrival participants completed a face-to-face screening, designed to determine individuals’ health status and adherence to study day instructions. Individuals who had any known medical condition (including mental disorders) or were taking any type of medication (including birth control pills) were excluded. Further exclusions included obesity, pregnancy, breastfeeding, smoking, heavy alcohol use, and drug abuse (as assessed by validated instruments, the CAGE and the RAGS, Ewing, 1984; Sobell et al., 1999). Premenopausal women were asked to participate during the follicular phase of the menstrual cycle, a time when hormone levels are relatively low and stable (Symonds, Gallagher, Thompson, & Young, 2004). Participants were asked to abstain from food and liquids (except water) for 4 hours prior to participation in the experiment and from exercise, caffeine, and alcohol during the 12 hours prior to participation. Eligible individuals completed consenting procedures and a brief final screen after arrival at the laboratory. This was conducted by a physician who obtained a medical history, performed a physical exam, and administered a pregnancy test for women. Eligible participants who continued with the protocol received monetary compensation for participation as well as transportation costs.
A total of 99 healthy participants completed the study, including 49 men and 50 women. Participants were 68% Whites and 32% non-Whites with an age range of 21 to 63 years (mean = 33, standard deviation {SD} = 10.7). Levels of educational attainment ranged from high school degree or GED equivalent to graduate school degree (83% had a college degree or higher).
2.3. Procedure
Primary informed consent was obtained directly from participants upon arrival at the laboratory; however, consent for the social stress tasks was delayed until just prior to oxytocin or placebo administration to avoid anticipatory stress in the baseline resting period. After the brief final screening eligible participants completed baseline questionnaires assessing demographics and relevant individual attributes (e.g., trait anxiety). Participants then had a quiet period, followed by a baseline measure of cardiovascular responses and collection of a saliva sample for determination of estradiol (Jennings, Kamarck, Stewart, Eddy, & Johnson, 1992; Piferi, Kline, Younger, & Lawler, 2000). We used the cardiovascular baseline responses to create physiological “reactivity” scores (see below).
After a brief description of the stress tasks, consent to continue with the protocol was obtained. Then participants received a single dose of either 24 IU oxytocin or placebo intranasally. Studies with other peptides (melanocortin, vasopressin which is closely related in structure to oxytocin, and insulin) administered intranasally have found that they are absorbed within 30 minutes as assessed by peptide levels in the cerebrospinal fluid (Born et al., 2002). Other experimental work with intranasal oxytocin in humans has consistently utilized an absorption period ranging between 40 and 50 minutes (Heinrichs, et al., 2003; Kosfeld, et al., 2005; Shamay-Tsoory, et al., 2009). The stress tasks were initiated 40 minutes after oxytocin or placebo administration with a brief 5-minute period during which participants prepared for the first stress task. Immediately prior to performing this task, a state mood measure was obtained.
The TSST generally requires participants to perform two separate tasks in front of an audience: a 5-minute public speaking task and a 5-minute mental arithmetic exercise. Both tasks were conducted in front of a stoic audience (consisting of two women) and in the presence of a video camera conspicuously videotaping the performance. Participants were told that their performance would be evaluated by audience members and reviewed (via videotape) by other trained professionals. After performing the speech task, participants were asked to perform an arithmetic task (counting backwards from 996 by steps of 7), which was described as being related to mental ability and general intelligence. Prior to performing this task, state mood measures were administered again. State mood and post-task measures were also obtained a final time after completion of the math task. Autonomic nervous system responses were monitored throughout the procedure. At the completion of the protocol, the experimenter debriefed participants and paid them for participation.
2.4. Measures
Autonomic Nervous System (ANS)
Sympathetic and parasympathetic measures were recorded noninvasively according to established guidelines (Sherwood et al., 1990). We measured electrocardiography (ECG100C) and impedance cardiography (NICO100C) and integrated the signals with an MP150 system (BIOPAC Systems Inc, Goleta, CA). Sensors to measure ECG were applied in a modified lead II configuration and impedance cardiography was obtained using four mylar bands that completely encircled the neck and chest area. A 4 mA AC current at 100kHz was passed through the outer bands, and z0 and dZ/dt were recorded from the inner bands. All signals were filtered on-line and collected at 1000Hz.
We used HRV (2.5) and IMP (3.0) modules from Mindware Technologies (Gahanna, OH) to edit and score the physiological parameters of interest, specifically respiratory sinus arrhythmia (RSA, a measure of heart rate variability), cardiac output (CO), and pre-ejection period (PEP). PEP, a chronotropic measure of the strength of the contractile force of the heart, provides a sensitive index of sympathetic control of the heart (Brownley, Hurwitz, & Schneiderman, 2000). Impedance data were ensemble averaged with 1-minute epochs and each waveform was verified and edited as needed. Visual inspection was conducted by research assistants who completed extensive training in the second author’s lab and all scored data were confirmed by a post-doc level research scientist and the second author. Inspection of the waveforms focused on detecting ectopic beats and accurate Q, R, S placement in the ECG trace, and accurate detection of the B-, X-, and Z-points (aortic valve opening, aortic valve closing, and dz/dt max, respectively) on the dZ/dT waveform. We did not rely on available B-detection algorithms (e.g.Lozano et al., 2007), but rather only accepted waveforms in which we could visually confirm a B-point.
Stroke volume (SV) was estimated using the Kubicek equation (see Sherwood, et al., 1990) and the subsequent cardiac output (CO) in liters per minute was calculated by multiplying heart rate (HR) X (SV/ 1000). PEP was calculated as the time interval in ms between the Q-point of the ECG and the B-point of the dZ/ dt signal, such that greater PEP decreases indicate a sympathetic nervous system response. For ease of interpretation, we calculated ventricle contractility by multiplying PEP reactivity by -1 so that increases in ventricle contractility indicate increases in sympathetic activation or faster time between contraction and valve opening.
HRV was also scored in 1-minute bins. The HRV module detrended the data using a first order polynomial to remove the mean and any linear trends, cosine tapered the data, submitted it to Fast Fourier Transformation, and took the natural log integral of the high frequency power (.15-.40 Hz) as an index of RSA. This method is generally accepted as an optimal noninvasive measure of cardiac vagal control (Berntson et al., 1997; Berntson, Cacioppo, & Quigley, 1993; Cacioppo, Uchino, & Berntson, 1994). After scoring all data, a random subsample of data was selected to re-score. Inter-scorers reliability exceeded 90% for all measures of interest.
We also attempted to collect continuous blood pressure responses given the previous data on TPR differences between challenge and threat states. Unfortunately, we used a tonometric blood pressure device that estimated blood pressure from the radial artery using a sweep technology. Though the device met hospital standards, it is extremely sensitive to movement; when participants move their arms or wrists, it can produce highly variable estimates of questionable validity (see Berntson, Quigley, & Lozano, 2007; Mendes, 2009). Even with our best efforts to restrain participants’ wrists during the TSST, we observed highly variable and invalid blood pressure responses (ranging from 40 mmHg to 190 mmHg) due to movement artifact. Because of these problems we did not analyze the blood pressure responses nor could we estimate TPR, which requires blood pressure responses.
Based on our hypotheses and the valid data collected, we focused on reactivity (i.e., changes from baseline to stress task) of three autonomic nervous system parameters: CO, VC, and RSA. We calculated change scores for each minute at baseline and during the stress tasks. We used the last minute of the baseline period because that is typically the time when autonomic parameters are at their most quiescent state. To examine post-stress autonomic changes, we subtracted the last minute of the stress task period from the first minute of the post-stress exposure period (i.e., recovery time after completion of stress tasks).
Estradiol
Saliva samples were collected using IBL sampling devices and the methodology described by Kirschbaum and Hellhammer (Kirschbaum & Hellhammer, 1989). Estrogen was evaluated from a saliva sample obtained at baseline. Before assaying for free estradiol, samples were thawed and spun at 3000 rpm for 10 min to obtain clear saliva with low viscosity. The free estradiol concentrations in saliva were analyzed with a time-resolved immunoassay with fluorescence detection. The limit of detection was 0.3 pg/mL and the inter- and intra-assay coefficients of variance were below 14% and 15%. A random sample of 20% of the samples was assayed in duplicate and reliability was 0.98.
Psychological Measures
Anger and anxiety were assessed with the Spielberger State-Trait Personality Inventory (STPI) (Spielberger, 1998). Trait measures assessed stable individual differences in the frequency and intensity with which individuals experience these negative emotions. State measures assessed how anxious, angry or depressed individuals felt while in the laboratory. Subscales all have demonstrated validity and had high internal consistency reliability in this study (all trait α > 0.77; all state α > 0.84). State positive and negative affect were measured using subscales from the Positive and Negative Affect Schedule which prior studies have indicated is both valid and reliable (Watson, Clark, & Tellegen, 1988). These subscales had high internal reliability in this study (all α > 0.84). State measures were obtained at four time points: baseline, immediately prior to performing the speech task, immediately prior to performing the math task, and then directly after completion of the math task. For each state measure, scores from the baseline condition were compared with task condition or post-task scores to indicate affective response to the stress exposure.
Behavioral Observation and Coding
Research assistants (N = 4; 2 men and 2 women) were trained to code the videotaped speech and math tasks. Training consisted of a group instructional session, followed by everyone scoring the same 30 participants and determining reliability across those participants. Inter-rater reliability was high (agreement within 2 points ranged from 82% to 100% for all variables). Once consistency was established, each study participant’s performance was scored by a single coder. Each participant’s behavior was scored during the first stress task using established and reliable coding schemes for challenge and threat emotions and behaviors (Mendes, et al., 2008). The challenge construct comprised 11 questions including how comfortable, strong, confident, enthusiastic, interested, clear, and alert the participant appeared, as well as how much eye contact they made with the evaluators, how animated they were during the speech, the intensity of their gestures, and how much somatic activity they exhibited during the speech (α = .92). The threat construct was created using 8 items including how anxious, agitated, distressed, jittery, and tense the participant appeared, as well as how rigid the participants held their body, how curled into themselves they became during the speech (trying to appear smaller), and how much they froze (α = .80).
Performances were also scored according to the quality of speech and performance on the math task. Speech quality was operationalized according to whether it seemed well structured and given in a clear and animated way (α= 0.90). An objective measure of math performance quality was derived by rating participants on how many numbers they were able to get correct and how clear and consistent they were while engaging in the counting task (α= 0.85).
Covariates
Height and weight was obtained by the physician during the final screening, with participants wearing socks and clothing, from which body mass index (weight/height2) was calculated. Age, race/ethnicity, and years of education were obtained by self-report on the baseline questionnaires.
2.5. Data analysis
Initial analyses evaluated effectiveness of the randomization procedure by considering distribution of demographic and trait anger, anxiety, negative and positive affect across oxytocin and placebo groups, using t-tests or chi-square tests as appropriate. Affective, cardiovascular, and behavioral outcomes were considered using a series of two-way (oxytocin X gender) analyses of variance (ANOVA) or analyses of co-variance (ANCOVA) when inclusion of covariates in the models was required. As most covariates did not differ across gender or oxytocin condition, they were not included in the reported models with several exceptions. Because cardiovascular outcomes may be particularly sensitive to age, all reported analyses with this outcome use ANCOVA and adjust for age. Similarly, because the performance tasks are highly sensitive to education levels, all reported analyses of performance outcomes use ANCOVA and adjust for education level.
Subjective responses were measured once per time period, and thus we assessed change between relevant time periods using each time period score. Of note is that cardiovascular and affective measures were taken at each of the two stress exposure tasks. However, findings at the two time points were virtually identical. Thus for parsimony when considering effects of stress exposure, we report the findings taken during the initial stress exposure period (the speech task).
For each analysis, we considered whether the interaction between gender and oxytocin was significant. In addition, we report effect sizes as Cohen’s d statistic, calculated as the difference between the two relevant group means divided by the pooled standard deviation (i.e., the root mean square of the two standard deviations). Small, medium, and large effects sizes for d are estimated as .20, .50, and .80 respectively (Cohen, 1992). We also conducted all analyses using ANOVAs with repeated measures, but findings were identical, and for ease of interpretation we report the two-way ANOVAs or ANCOVAs. Statistical tests were two-tailed and conducted at the .05 level of significance. In addition, to identify potential outliers on each outcome, all variables were examined and individuals who had extreme values (> 2.5 SDs above the mean) were set to missing for that particular outcome (n’s range from 0 to 4 depending on the outcome). Additionally some physiological data were unscorable because of faulty sensors, movement artifacts, loss of signal, or noisy signals (8 to 22% of data are missing for each study interval; impedance parameters have more missing data than others). Thus, data for each outcome have variable degrees of freedom, but missing data were not related to oxytocin administration and thus should be considered to be missing completely at random. Data presented in figures include means and standard error of the means.
3. Results
3.1. Initial analyses
Participants in the oxytocin and placebo groups did not differ significantly according to any of the baseline (pre-oxytocin manipulation) variables, although there was a slight trend for individuals randomly assigned to receive oxytocin to have slightly higher negative affect at baseline (p = 0.09) than those receiving placebo (see Table 1). Men and women also did not differ significantly on any of the baseline demographic or affect variables with the exception that men had a trend toward higher trait anger than women (men M = 22.0, SD = 7.6 versus women M = 19.5, SD = 6.3 t(96) = 1.76 p = 0.08). Women had higher estradiol levels than men, although the difference was not statistically significant (men M = 3.6, SD = 2.8 versus women M = 4.3, SD = 3.4, t(97) = -1.19, p = 0.24). Men also had significantly higher CO, higher PEP and lower RSA than women (CO: men M = 8.1, SD = 1.8 versus women M = 6.0, SD = 2.0, t(82) = 4.93, p < 0.01; PEP: men M = 114.8, SD = 11.1 versus women M = 108.2, SD = 10.8, t(82) = 2.75, p < 0.01; RSA: men M = 6.1, SD = 1.4 versus women M = 6.6, SD = 1.0, t(89) = −2.16, p = 0.03). A manipulation check suggested that all participants found the TSST “stressful” with a mean score above the median of the range (1-5), M = 2.89, SD = 1.05, significantly different from 0, t = 27.35, p < .001, and a marginal gender difference whereby women found the task slightly more stressful than men (p = .09).
Table 1.
Distribution of study variables across oxytocin and placebo groups at baseline
| Study Variable | Oxytocin n = 48 |
Placebo n = 51 |
p-value | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Demographics | |||||
| Age (yrs) | 32 | 10.2 | 34 | 11.2 | 0.34 |
| BMI (kg/m2) | 23.9 | 2.8 | 23.6 | 3.1 | 0.62 |
| Educational attainment (yrs) | 16.6 | 3.0 | 16.6 | 2.1 | 0.93 |
| Affect (sum score) | |||||
| Trait negative affect | 16.4 | 5.3 | 14.7 | 4.8 | 0.09 |
| Trait positive affect | 22.5 | 24.4 | 24.6 | 6.8 | 0.12 |
| Trait anxiety | 23.0 | 6.6 | 21.6 | 5.7 | 0.25 |
| Trait anger | 21.7 | 7.6 | 19.9 | 6.4 | 0.21 |
| Physiological* | |||||
| CO (L/min) | 7.2 | 2.3 | 6.8 | 2.1 | 0.44 |
| PEP (ms) | 112.2 | 11.8 | 110.1 | 10.9 | 0.38 |
| RSA (ms2) | 6.5 | 1.1 | 6.2 | 1.3 | 0.25 |
| Estradiol (pg/mL) | 3.9 | 3.0 | 4.1 | 3.3 | 0.76 |
Note. average over 6 minutes of baseline. CO indicates cardiac output; PEP indicates pre-ejection period (we use this measure to calculate ventricle contractility); RSA indicates respiratory sinus arrhythmia.
3.2. Effect of oxytocin on autonomic responses to stress exposure and stress recovery
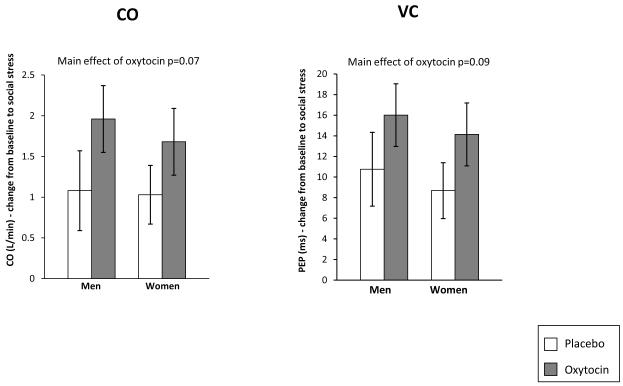
Consistent with our hypothesis that oxytocin participants would exhibit a challenge response to the stressful task, participants given oxytocin, compared to placebo participants, exhibited a trend toward greater increases in CO (F(1, 68) = 3.31, p = 0.07, d = 0.47), and VC (indicating more sympathetic activation; F(1, 71) = 2.98, p = 0.09, d = 0.45) (Figure 1). No effect of oxytocin on RSA reactivity during the stress task was observed. Neither the main effect for gender nor the gender by oxytocin interaction was significant for any of the stress reactivity variables.
Figure 1.
Effect of Oxytocin on Cardiac Activity During Stress Exposure
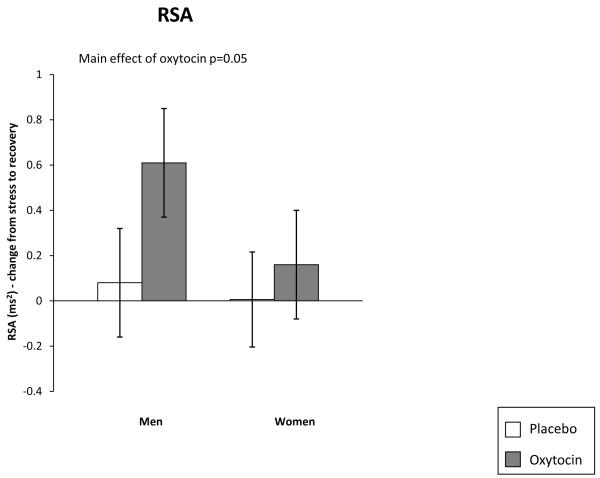
We then examined vagal rebound following the stress task. Oxytocin participants differed from placebo participants (F(1, 82) = 3.83, p < 0.05, d = 0.37) showing greater vagal rebound (Figure 2). Again the main effect for gender or the interaction was not significant.
Figure 2.
Effect of Oxytocin on Vagal Rebound During Stress Recovery
Given the possible potentiating role of estrogen on the influence of oxytocin we tested whether estradiol was an effect modifier of any of the observed effects. However, all effects of oxytocin on stress response reported above were unchanged when ANCOVA’s took account of estradiol. Thus, effects of oxytocin on autonomic response to stress did not depend on endogenous levels of estrogen either within or across gender.
3.3. Effect of oxytocin on task performance and behavior
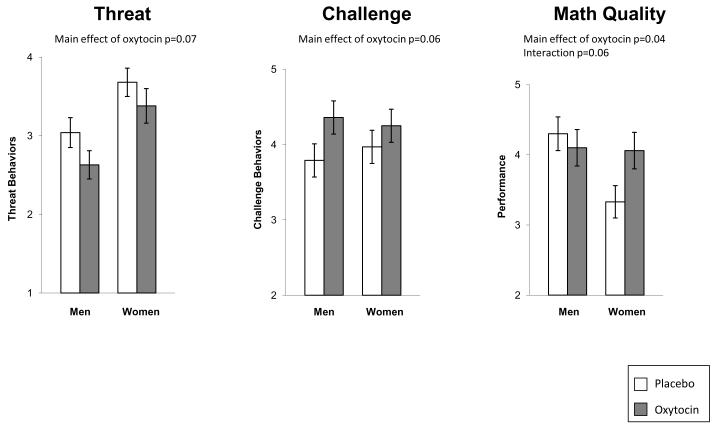
As challenge and threat states have been linked to more positive behavior and performance outcomes, we then turned to the behavioral coding to determine if the effects of OT were evident in participants’ behavior and performance. Using the challenge and threat constructs separately, we observed a trend for oxytocin to produce less threat and more challenge behavior (Figure 3): Threat: F(1,86) = 3.26, p = 0.07, d = 0.33; Challenge: F(1, 85) = 3.52, p = 0.06, d = 0.32. As in previous work, the threat construct was correlated with the challenge construct (r = 0.71, p < 0.001), so we created a single index in which higher scores indicated greater challenge behavior (α = 0.91). This combined index produced an effect significant for the oxytocin manipulation, F(1, 86) = 4.24, p = 0.04, d = 0.36. Participants who were given oxytocin were rated by observers as exhibiting more challenge and less threat behavior during the speech task than placebo participants (Figure 3).
Figure 3.
Effect of Oxytocin on Task Performance and Behavior
We then examined performance on the math task. We did not observe an oxytocin main effect but there was a gender main effect – on average men performed better than women on the math task, F(1, 87) = 4.22, p = 0.04, d = 0.46. However this main effect was qualified by a gender x oxytocin interaction trend, F(1, 87) = 3.54, p = 0.06. Women given oxytocin performed better (M = 4.06; SE = .26) than women given placebo (M = 3.33; SE = .23), d = 0.63, but men given oxytocin or placebo did not differ, d = 0.17 (Figure 3). No effect of oxytocin on speech quality was evident.
3.4. Effect of oxytocin on affective responses to stress exposure
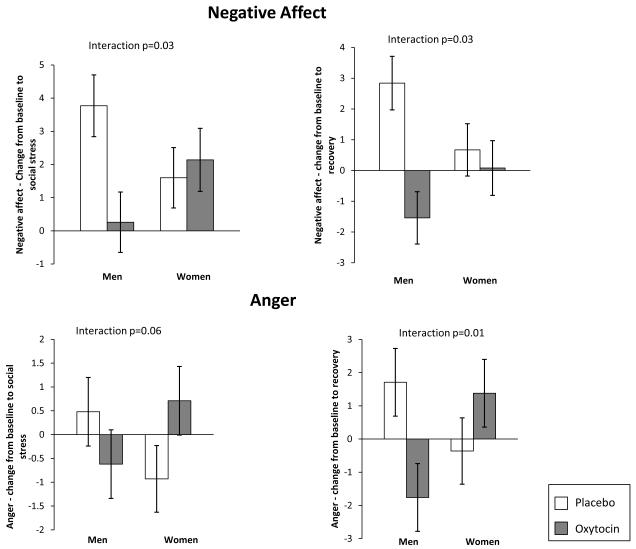
Effects of oxytocin on CO and VC during stress exposure suggest that oxytocin facilitates a more adaptive challenge response to an acute stressor and promotes healthy recovery as indicated by greater vagal rebound. Behavioral coding provided converging evidence that oxytocin promoted positive social behavior and for women better math performance. We then examined participants’ self-reported affective states. In response to oxytocin, men had significantly lower negative affect scores immediately following the stress task compared to men given placebo, d = 0.71. In contrast, women given either placebo or oxytocin exhibited similar increases in negative affect, d = 0.14 (Figure 4). This two-way interaction of gender-by-oxytocin on negative affect was significant comparing baseline to immediately prior to the stress exposure, F(1, 92) = 4.83, p = 0.03, or to post-stress, F(1, 92) = 4.78, p = 0.03.
Figure 4.
Effect of Oxytocin on Negative Affect and Anger During Stress Exposure and Recovery
Given the importance of specific negative affect for a range of health outcomes (Kawachi, Sparrow, Spiro, Vokonas, & Weiss, 1996; Kubzansky et al., 2006) we explored the patterning of specific emotions in more detail. Findings for anger followed the same pattern as those for negative affect and yielded a substantial gender by oxytocin interaction. For example, relative to men given placebo, men given oxytocin had greater decreases in anger, d = 0.24, whereas women given oxytocin had greater increases in anger than women given placebo, d = 0.65, comparing baseline to stress exposure F(1, 88) = 3.72, p = 0.06 and findings were similar when considering baseline to post-stress, F(1, 88) = 6.61, p = 0.01 (Figure 4). Findings for anxiety were similar to those of anger but less pronounced and less consistent. Finally, positive affect decreased over the course of the study in all groups, until recovery when women given oxytocin reported continued reduction in positive affect but men given oxytocin reported increased positive affect, gender x oxytocin interaction F(1, 94) = 6.89, p = 0.01. Overall, oxytocin appeared to mitigate self-reported distress in response to stress exposure among men, but amplified anger among women.
DISCUSSION
This is the first study to examine effects of oxytocin on response to social stress across a number of domains in both men and women. Findings indicate an effect of oxytocin on physiologic activation that is highly consistent with prior theorizing and empirical animal studies, suggesting that oxytocin enhances biological readiness to approach or engage in social interaction under stressful conditions. Other work has highlighted the importance of the ANS (both PNS and SNS) in facilitating social engagement (Porges, 2003b) and for providing a broad picture of the quality of an individual’s functioning. We observed cardiovascular and behavioral indicators consistent with the idea that oxytocin would be associated with a positive or challenge state under conditions of social stress. Additionally we found that following the stress task participants provided oxytocin showed a healthier recovery profile as indexed by greater vagal rebound. Taken together, the ANS and behavioral findings suggest that oxytocin facilitated a more positive response to a psychological stressful evaluation for both men and women. Recent work identifies oxytocin as a central cardiovascular modulatory peptide (Higa, et al., 2002; Martins, Crescenzi, Stern, Bordin, & Michelini, 2005) and indicates key cellular mechanisms by which oxytocin modulates sympathetic and parasympathetic activity in response to social stress. Animal studies indicate that oxytocin is involved in mediating cardiopulmonary input into the nucleus of the solitary tract (NTS) and integrating these inputs with centrally mediated responses like stress (Higa, et al., 2002; Peters et al., 2008). Oxytocin projections from the PVN of the hypothalamus selectively innervate the NTS and act on second-order neurons involved in regulating cardiac function (Higa, et al., 2002). Research examining effects of oxytocinergic neurons projecting to the NTS under various conditions demonstrates that oxytocin modulates baroreflex control of heart rate by augmenting vagal outflow (Braga, Mori, Higa, Morris, & Michelini, 2000; Higa, et al., 2002). Together with findings from the present study, these experiments suggest an important pathway by which oxytocin may modulate social stress response.
However, we also observed some gender differences, particularly when examining self-reported affect responses to the stress task. Consistent with prior work considering response to stress exposure, men given oxytocin reported less distress (Heinrichs, et al., 2003). However, we found a different effect in women. Women given oxytocin reported more distress and specifically more anger. Interestingly, among the negative emotions, anger is unique in that it is an approach-related emotion and part of the appetitive system (Carver & Harmon-Jones, 2009; Harmon-Jones & Allen, 1998), and has been linked to challenge profiles of reactivity (higher CO and lower TPR, Mendes, et al., 2008). Women given OT also performed objectively better on the math task than women given placebo. The performance finding might very well be expected given that anger is often associated with improved cognitive performance (see Lerner & Tiedens, 2006). Oxytocin may be associated with multiple approach-related emotion states. Thus, while oxytocin generally influenced a sense of challenge and readiness to engage, men and women may differ in how they engage with their social environment and in their subjective response to social demands. The increased cardiovascular activation that often characterizes challenge responses was associated with more positive states for men but with less positive states for women who reported more anger following the social evaluative stress. Because anger may be associated with short-term benefits in performance or other tasks (Lerner & Tiedens, 2006) but long-term harm when it extends to more ruminative processes, more work is needed to determine health-related effects of oxytocin and their similarity across men and women. However, the gender differences in anger may also be due to floor effects for women who presented with lower rates of anger than men at baseline allowing women more room to have an anger reaction.
Prior studies with intranasal oxytocin have suggested that oxytocin modulates activation of the amygdala and fear-related behavior and autonomic activity (Domes et al., 2007). Other work, however, has suggested that in certain situations (e.g., intergroup competition) this reduced threat response may correspond with more positive feelings toward one’s own group but more hostility and aggression toward non-group members (De Dreu, et al., 2010). Our finding supports this more complex understanding of oxytocin effects, suggesting that rather than uniformly increasing prosocial behavior and positive feelings, oxytocin may well increase sensitivity to and willingness to engage with the social context and social agents in part by attenuating a sense of social threat (Shamay-Tsoory, et al., 2009). If this is true, then perhaps in the present study under conditions of enhanced sensitivity, men and women – who both exhibited higher levels of social approach behavior - attended to different social cues or differentially interpreted similar cues, leading to a differentiated set of emotional and behavioral responses. Thus, it may be that men in our study perceived this Trier task more positively than women. An interesting test of this hypothesis might be to administer oxytocin to men and women under conditions of stress that men perceive more negatively and women perceive more positively. If oxytocin increases sensitivity to the social context, then under these conditions we might expect to see the gender difference observed in the present study reversed, such that women’s responses are more positive relative both to women with placebo or to the men. What is clear is that one should not assume either that the effects of oxytocin are uniform across multiple parameters or that they are similar for men and women.
Our study has some important limitations as well as strengths. Due to equipment malfunction we were unable to measure (and then calculate) two key aspects of cardiovascular function, blood pressure and total peripheral resistance. This tempers our strong interpretation that OT was associated with a challenge relative to threat profile of stress reactivity. However, in most challenge and threat studies, CO and TPR show similar effect sizes and given that CO is used to calculate TPR (the TPR formula is [mean arterial pressure]/cardiac output]) this is not particularly surprising. We have no reason to believe that valid blood pressure data would lead us to a different conclusion here, but given the failure of our blood pressure device, we acknowledge that this is a critical limitation. Moreover we were unable to assess peripheral levels of oxytocin and compare those with levels of oxytocin absorbed via nasal spray administration because assays of peripheral oxytocin are thus far somewhat unreliable. Further, peripheral oxytocin levels may not correlate with cerebrospinal fluid levels of oxytocin. The design of the current study did not allow for measurements of oxytocin levels in the cerebrospinal fluid. Some of our findings showed trends but fell just short of conventional significance perhaps due to limited cell sizes. Finally due to concerns about habituation to the stress task we used a between-subjects design, which meant we could not compare effects of oxytocin and placebo within the same participants.
However, this study also has significant strengths. Effect sizes were generally in the moderate range (trend or significant Cohen’s d effect sizes ranged from 0.32 to 0.71). In addition, the experimental double-blind placebo-controlled design enhances our confidence that oxytocin genuinely modulates cardiovascular response to acute stress in humans. Our volunteer sample was relatively highly educated and therefore may not be fully representative of the general population. However, recruitment from the community and inclusion of participants with a broad age range, suggests that results may be generalizable beyond young men, the population most frequently considered in other experimental studies with oxytocin. In fact, we view our inclusion of both men and women to be a particular strength of this investigation. Finally, effects of oxytocin across a range of biological, behavioral, and subjective responses were considered, thereby permitting a more comprehensive consideration of the range and consistency of effects.
In sum, our results suggest that oxytocin strongly influences CO and sympathetic activation during a stressful task, and heart rate variability following a stressful task in humans, supporting the idea that the oxytocinergic system modulates responses to stress and influences the social engagement system. This finding has interesting implications when considering oxytocin as a potential therapeutic agent, especially when coupled with research linking higher levels of CO to slower brain aging (Jefferson, et al., 2010) and research linking higher heart rate variability and lower levels of distress with reduced risk of cardiovascular and other diseases (Chida & Hamer, 2008; Everson-Rose & Lewis, 2005; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Thayer & Lane, 2007). However, our findings that in the context of social evaluative stress, oxytocin facilitated a generally positive social experience for men, but a less uniformly positive experience for women, add complexity when considering the potential health benefits of oxytocin.
Because of its potential for mitigating maladaptive biological and psychological responses to stress, oxytocin has been proposed as a key biological substrate that may underlie resilience. Our results show that oxytocin may underlie resilience processes and the ability to build and sustain social relationships, but its effects may not be uniformly positive and prosocial. Before broadly considering oxytocin as a therapeutic or uniformly enhancing health, greater understanding of the conditions under which it is most beneficial is needed.
Highlights.
○This experiment tests if oxytocin uniformly enhances adaptive response to stress
○Oxytocin stimulated a salutary cardiovascular profile during social stress
○Gender differences emerged in affective and behavioral response to stress
○Effects of oxytocin on stress response were unchanged when accounting for estradiol
○Oxytocin enhances biological readiness to approach under stressful conditions
Acknowledgements
This work is supported by a grant from NIH 1R21AG030632-01A2. Seed funds were provided by the Robert Wood Johnson Foundation Health and Society Scholars Seed Grant Program. We are grateful to Dr. Markus Heinrichs for conceptual and methodological advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors declare they have no competing interests.
Authors’ Contributions All authors contributed to the design and coordination of the study and have read, commented on, and approved the manuscript.
References
- Averbeck BB. Oxytocin and the salience of social cues. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9033–9034. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Progress in Brain Research. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychological Bulletin. 1993;114(2):296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd ed Cambridge University Press; New York, NY: 2007. pp. 182–210. [Google Scholar]
- Blascovich J, Mendes WB. Social psychophysiology and embodiment. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of social psychology. 5th ed John Wiley & Sons Inc; Hoboken, NJ: 2010. pp. 194–227. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. Journal of Neuroscience. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga DC, Mori E, Higa KT, Morris M, Michelini LC. Central oxytocin modulates exercise-induced tachycardia. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278(6):R1474–1482. doi: 10.1152/ajpregu.2000.278.6.R1474. [DOI] [PubMed] [Google Scholar]
- Brownley KA, Hurwitz BE, Schneiderman N. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd ed Cambridge University Press; New York, NY: 2000. pp. 224–264. [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosomatic Medicine. 2002;64(3):407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: the psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31(4):412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2008;77(1):1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134(6):829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70(9):967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28(3):313–350. doi: 10.1017/S0140525X05000063. discussion 350-395. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96(1):84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annual Review of Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism: The CAGE Questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M. A psychobiological framework for research on human stress and coping. In: Appley M, Trumbull R, editors. Dynamics of stress: Physiological, psychological, and social perspectives. Plenum Press; New York, NY: 1986. pp. 101–116. [Google Scholar]
- Harmon-Jones E, Allen J. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. Selective amnesic effects of oxytocin on human memory. Physiology and Behavior. 2004;83(1):31–38. doi: 10.1016/j.physbeh.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Henry JP. Neuroendocrine patterns of emotional response. In: Plutchik R, Kellerman H, editors. Emotion, theory, research, and experience. Vol. 3. Academic Press; Orlando, FL: 1986. pp. 37–60. [Google Scholar]
- Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2002;282(2):R537–545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Pollack MH, Kaufman RE, Zak PJ, Simon NM. Oxytocin levels in social anxiety disorder. CNS Neuroscience and Therapeutics. 2008;14(3):165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson JP, Mendes WB, Blackstock E, Schmader T. Turning the knots in your stomach into bows: Reappraising arousal improves performance on the GRE. Journal of Experimental Social Psychology. 2010;46(1):208–212. doi: 10.1016/j.jesp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122(7):690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29(6):742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Kassam KS, Koslov K, Mendes WB. Decisions under distress: stress profiles influence anchoring and adjustment. Psychological Science. 2009;20(11):1394–1399. doi: 10.1111/j.1467-9280.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Spiro A, Vokonas P, Weiss ST. A prospective study of anger and coronary heart disease. The Normative Aging Study. Circulation. 1996;94(9):2090–2095. doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23(8):877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Koslov K, Mendes WB, Pajtas PE, Pizzagalli DA. Asymmetry in resting intracortical activity as a buffer to social threat. Psychological Science. 2011;22(5):641–649. doi: 10.1177/0956797611403156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton A, Block J, Adler GK. Protocol for an experimental investigation of the roles of oxytocin and social support in neuroendocrine, cardiovascular, and subjective responses to stress across age and gender. BMC Public Health. 2009;9:481. doi: 10.1186/1471-2458-9-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Sparrow D, Jackson B, Cohen S, Weiss ST, Wright RJ. Angry breathing: a prospective study of hostility and lung function in the Normative Aging Study. Thorax. 2006;61(10):863–868. doi: 10.1136/thx.2005.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner JS, Tiedens LZ. Portrait of the angry decision-maker: How appraisal tendencies shape anger’s influence on cognition. Journal of Behavioral Decision Making. 2006;19:115–137. [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG. Where to B in dZ/dt. Psychophysiology. 2007;44(1):113–119. doi: 10.1111/j.1469-8986.2006.00468.x. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Martins AS, Crescenzi A, Stern JE, Bordin S, Michelini LC. Hypertension and exercise training differentially affect oxytocin and oxytocin receptor expression in the brain. Hypertension. 2005;46(4):1004–1009. doi: 10.1161/01.HYP.0000175812.03322.59. [DOI] [PubMed] [Google Scholar]
- Mendes WB. Assessing the autonomic nervous system. In: Harmon-Jones E, Beer J, editors. Methods in Social Neuroscience. Guilford Press; New York: 2009. pp. 118–147. [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94(2):278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. Psychology. The prickly side of oxytocin. Science. 2010;328(5984):1343. doi: 10.1126/science.328.5984.1343-a. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biological Psychology. 2011;86(3):174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. Journal of Neuroscience. 2008;28(45):11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Uvnas-Moberg K. Effects of an acute stressor on blood pressure and heart rate in rats pretreated with intracerebroventricular oxytocin injections. Psychoneuroendocrinology. 2007;32(8-10):959–965. doi: 10.1016/j.psyneuen.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Piferi RL, Kline KA, Younger J, Lawler KA. An alternative approach for achieving cardiovascular baseline: viewing an aquatic video. International Journal of Psychophysiology. 2000;37(2):207–217. doi: 10.1016/s0167-8760(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiology and Behavior. 2003a;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Annals of the New York Academy of Sciences. 2003b;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers D, De Wit FRC, Ellemers NS, K. Social power makes the heart work more efficiently. Evidence from cardiovascular markers of challenge and threat. Journal of Experimental Social Psychology. (In press) [Google Scholar]
- Seery MD, Blascovich J, Weisbuch M, Vick SB. The Relationship Between Self-Esteem Level, Self-Esteem Stability, and Cardiovascular Reactions to Performance Feedback. Journal of Personality and Social Psychology. 2004;87(1):133–145. doi: 10.1037/0022-3514.87.1.133. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biological Psychiatry. 2009;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Levin C, Cleland P, Ellingstad T, Toll B. RAGS: A new brief drug abuse screening instrument. Paper presented at the Association for Advancement of Behavior Therapy.1999. [Google Scholar]
- Spielberger CD. Preliminary Manual for the State-Trait Personality Inventory. University of South Florida; Tampa, FL: 1998. [Google Scholar]
- Symonds CS, Gallagher P, Thompson JM, Young AH. Effects of the menstrual cycle on mood, neurocognitive and neuroendocrine function in healthy premenopausal women. Psychological Medicine. 2004;34(1):93–102. doi: 10.1017/s0033291703008535. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blascovich J. Effects of justice beliefs on cognitive appraisal of and subjective physiological, and behavioral responses to potential stress. Journal of Personality and Social Psychology. 1994;67(4):732–740. doi: 10.1037//0022-3514.67.4.732. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin linked antistress effects - the relaxation and growth response. Acta Psychologica Scandinavica. 1997;640(Supplement):38–42. [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]