Abstract
Memory/effector T cells traffic efficiently through extralymphoid tissues, entering from the blood and leaving via the afferent lymph. During inflammation, T cell traffic into the affected tissue dramatically increases; however, the dynamics and mechanisms of T cell exit from inflamed tissues are poorly characterized. Here we show, using both a mouse and a sheep model, that large numbers of lymphocytes leave the chronically inflamed skin. Many T cells capable of producing IFN-γ and IL-17 also entered the draining afferent lymph, demonstrating that memory/effector T cells egress from sites of inflammation. Whereas efficient egress from acutely inflamed skin required lymphocyte-expressed CCR7, chronic inflammation promoted significant CCR7-independent exit as well. Lymphocyte exit at late time points of inflammation was sensitive to pertussis toxin but only partially affected by the drug FTY720, implying the contribution of alternative chemoattractant receptors other than S1P1. Our data show that CCR7 is an important receptor for lymphocyte egress from both resting and inflamed extralymphoid tissues, but that alternative exit receptors come into play during chronic inflammation.
Introduction
Memory/effector T cells migrate efficiently from the bloodstream into extralymphoid tissues and sites of inflammation and infection (reviewed in (1, 2)) not only providing an effective defense against invading pathogens, but also contributing to local inflammation. Studies of lymphocyte recirculation pathways in sheep showed that memory/effector T cells exit extralymphoid tissues through afferent lymphatic vessels and travel to local lymph nodes in the afferent lymph (3). Approximately 10% of all lymphocytes (4) and a major fraction of antigen-experienced T cells (3) that enter a resting lymph node do so via the afferent lymph. After a period of residency in lymph nodes, naïve and antigen-experienced T cells enter efferent lymph sinuses, which drain into efferent lymph vessels, and via the thoracic duct, back into the blood. On average, lymphocytes require 24h to migrate from blood into and through extralymphoid tissue and back into afferent lymph (5). Parabiotic mouse models also illustrate that memory CD8 T cells rapidly turn over in many extralymphoid tissues (6).
Inflammation is the response to microbial, physical or chemical injury. Acute inflammation is histologically characterized by polymorphonuclear leukocyte infiltration and, if not resolved, progresses into chronic inflammation. In contrast to acute inflammation, chronic inflammation is typified by a mononuclear tissue infiltrate composed mainly of lymphocytes and macrophages (7). Other hallmarks of prolonged chronic inflammation, particularly in response to foreign bodies or non-specific adjuvants, include fibrosis with possible granuloma formation, angiogenesis, and areas of tissue necrosis. Importantly, autoimmune and chronic inflammatory diseases are characterized by a chronic infiltration of lymphocytes in extralymphoid tissues; however, the precise mechanisms that promote the development of chronic inflammation remain unknown (7). It has become clear that Th1 and Th17-polarized T cell subsets that produce the prototypical cytokines IFN-γ and IL-17, respectively, are responsible for the development and severity of inflammation in many autoimmune diseases (8, 9). Despite their importance, once inflammatory T cell subsets enter the inflamed site, it is not known if they can subsequently exit the site of inflammation, enter the afferent lymphatics and return to the draining lymph node and the blood circulation.
A major feature of inflammation is the drastically increased recruitment of leukocytes from blood into the affected tissue. Blood vascular endothelium regulates lymphocyte extravasation from blood into tissues via the expression of inflammation- and organ-specific chemoattractants and adhesion molecules (reviewed in (2, 10, 11)). Thus, T cell-expressed chemoattractant receptors are crucial in guiding T cells into inflamed and uninflamed extralymphoid tissues. In inflamed sites, concomitant with enhanced recruitment, there is an increase in the permeability of afferent lymphatic endothelium, the rate of lymph flow, and the numbers of cells in the regional afferent lymph (12, 13). Consequently, lymphatic endothelial cell-expressed chemoattractants may regulate T cell exit via the afferent lymph from inflamed tissues. Indeed, lymphatic endothelial cells of afferent lymphatics constitutively express the CCR7 ligand CCL21 (14-16) as well as adhesion molecules (17-19). Consistent with the lymphatic expression of CCL21, we and others recently showed that CCR7 expression on CD4 and CD8 T cells mediates their egress from resting extralymphoid tissues into the draining lymph node via the afferent lymph (20, 21). In contrast to lymphocyte exit from extralymphoid tissues via the afferent lymph, egress from lymph nodes via the efferent lymph is regulated by spingosine-1-phosphate (S1P) and its receptors (22-24). An extended role for CCR7 in lymphocyte exit from inflamed extralymphoid tissues is supported by studies demonstrating that CCL21 expression is upregulated in lymphatic endothelial cells under inflammatory conditions in vitro (25) and in vivo (26, 27). However, recent data has shown that chemokines other than CCL21 are induced in lymphatic endothelial cells upon inflammatory stimulation (19, 25, 28), thus, additional chemoattractant-chemoattractant receptor interactions may regulate the relative rate of lymphocyte egress from inflamed extralymphoid tissues.
T cell exit from extralymphoid tissues through the afferent lymph removes potentially dangerous cells and is therefore likely a crucial control point of the inflammatory response. Despite this, lymphocyte egress from inflamed extralymphoid tissues is only poorly characterized. In this study, we show that chronic inflammation promotes a large flux of T cells, including Th1 and Th17 cells, from the affected tissue into afferent lymph. Lymphocyte egress from inflamed tissue requires G protein-coupled receptor signaling. Moreover, the chronicity of inflammation determines the chemoattractant receptor expression requirements for T cell egress from the affected site with CCR7 being crucial in the acute but not late phases of the inflammatory response.
Materials and Methods
Animals, lymph cannulation, and induction of cutaneous inflammation
Sex- and age-matched BALB/c wild-type (The Jackson Laboratory) and CCR7-deficient mice (29), kindly provided by Martin Lipp (Max-Delbrück Center, Berlin, Germany), were used for cell transfer experiments. Donor mice for cell transfer experiments were between 4 and 12 months of age; recipient mice were between 6 and 12 weeks old. Intact female or wether mixed breed sheep 5-10 months of age were purchased from 3/D Livestock (Woodland, CA), the University of California, Davis (Davis, CA), or Animal Biotech Industries (Danboro, PA). Pseudoafferent lymph vessels were induced in sheep by surgically removing the subiliac lymph nodes as previously described (30). Following lymphectomy, the afferent lymph vessels anastomose with the larger efferent vessels, carry afferent (prenodal) lymph, and are termed “pseudoafferent” vessels (30). 6-12 weeks after lymphectomy, pseudoafferent lymph vessels were surgically cannulated using heparin-coated catheters (Carmeda) and afferent lymph was continuously collected into sterile bottles containing heparin (APP Pharmaceuticals) as described (30). Every 1 to 12h, lymph collection bottles were changed, and leukocyte as well as lymphocyte numbers and composition analyzed by flow cytometry. Lymphocyte output for different T lymphocyte subsets (CD4, CD8) was calculated and averaged per hour of collection. The cannulated lymphatics drained the skin, muscles and bones of the rear flank. In sheep, inflammation was induced by subcutaneous injection of 0.3 – 0.5 ml CFA emulsified with saline into 2 injection sites either less than 48 hours (acute inflammatory phase) or more than 3 weeks (chronic inflammatory phase) before experimentation as described (5, 31-34). In mice, inflammation was induced by subcutaneous injection of 10μl non-emulsified CFA into the rear footpads using a 10μl Hamilton syringe. Experiments were performed either 6 hours (acute inflammation) or on days 10 or 21 (chronic inflammation) after induction of inflammation. All animal experiments were approved by the institutional animal care and use committees of the University of Pennsylvania and the Veterans Affairs Palo Alto Health Care System.
Cell isolation and culture, PTX and FTY720 treatment
Lymphocytes collected from ovine lymph were washed with RPMI 1640 medium (Invitrogen) with 5% newborn calf serum (Hyclone). Ovine blood was collected by venipuncture and mixed with heparin. Cells were released from mouse spleens by grinding through a 40μm cell strainer (BD Bioscience). Mononuclear cells were further separated from mouse spleen and from sheep blood by gradient centrifugation with Histopaque (Sigma). For the Th1 culture, splenocytes were sorted by positive selection with CD4 microbeads (Miltenyi Biotec) to a purity of ≥96%, and plated at 2×106/ml on 24-well plates (Corning Costar) coated with anti-CD3 (145-2c11; Ebioscience) and anti-CD28 (37.51; Ebioscience) in RPMI containing 10% fetal bovine serum, 20 ng/ml IFN-γ, 5 ng/ml IL-12 (R&D Systems), and 5 μg/ml anti-IL-4 antibody (Ebioscience). The cells were cultured for 5 to 6 days. The resulting Th1 cells on wild-type and CCR7-deficient background produced ≥20% IFN-γ upon restimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin and intracellular cytokine staining. For PTX treatment, splenic mononuclear cells were incubated for 2h at 37°C in RPMI 1640 medium containing 5% normal calf serum and 200 ng/ml PTX (Calbiochem) prior to extensive washing, labeling with fluorescent dyes (see below) and injection. Based on published FTY720-treatment regimens (35, 36), recipient mice were treated by intraperitoneal injection with 1 mg/kg FTY720 (Cayman Chemical Company) dissolved in saline containing 2% Hydroxypropyl-beta-cyclodextrin (Sigma Aldrich) or carrier only 8 hours prior to adoptive transfer of cells into inflamed or uninflamed footpads. To determine lymphocyte numbers in blood of each FTY720- and control-treated mouse, mice were deeply anesthetized by intraperitoneal injection with pentoparbital (Virbac Animal Health) and blood was collected by cardiopuncture and mixed with heparin. Collected blood volume was measured and red blood cells removed using dextran cross-linking and sedimentation with subsequent lysis as described (37).
Cell labeling and transfer
Cell labeling with PKH26 was performed according to the manufacturer's instructions (Sigma Aldrich). CFSE (Molecular Probes) labeling was achieved by incubating cells at a concentration of 5×106 ml in HBSS containing 25mM Hepes and 0.2μM CFSE for 5 min at 37°C. Both the PKH26 and CFSE labeling reactions were stopped by adding fetal bovine serum, and washed three times in RPMI containing 5% serum and once in PBS. Adoptive transfer skin egress studies were performed as described (20). Briefly, between 1×106 and 5×106 cells in 10μL PBS were injected subcutaneously into the inflamed or uninflamed skin of mouse footpads. At 12h after transfer, single-cell suspensions of the draining and as a control, the non-draining popliteal lymph nodes, were analyzed for transferred cells (identified by fluorescent labels), and total cell numbers were determined by flow cytometry with a fixed number of polystyrene beads (Polybead; Polysciences). In experiments comparing the relative migration efficiency of CCR7-deficient cells with that of wild-type cells of a specific lymphocyte subsets, the ratio of migrated CFSE+ cells to PKH26+ cells of the various subsets was determined by flow cytometry. The migration of CCR7-deficient CFSE+ cells relative to that of PKH26+ wild-type cells was based upon the ratios of migrated CFSE/PKH26 to injected CFSE/PKH26 cells in each lymphocyte subset. To control for variability in the overall recovery, results were normalized to the mean ratio of CFSE+ wild-type cells to PKH26+ wild-type cells for each subset. The following formulas were used: 1) ratio of migrated (M) to ratio of injected (I) cells (M/I) = (MCFSE /MPKH)/(ICFSE /IPKH); 2) mean ratio of migrated wild type (WT) to ratio of injected WT (mean M/I WT/WT) = [mean (MCFSE-WT/MPKH-WT)]/(ICFSE-WT/IPKH-WT); 3) migration of CCR7-deficient or WT CFSE+ cells (% of mean WT) = (M/I)/(mean M/IWT/WT)*100.
Flow cytometry and histology
To reduce unspecific staining, mouse cells were preincubated with rat IgG (Jackson Immunoresearch) and antibody to CD16/CD32 (2.4G2; BD Biosciences); sheep cells were preincubated with mouse and sheep IgG (Jackson Immunoresearch). After blocking, the cells were labeled with the following biotinylated or fluorochrome-conjugated (fluorescein isothiocyanate, phycoerythrin, Alexa Fluor 647, allophycocyanin, phycoerythrin-cyanine 7; Alexa Fluor 700) rat anti-mouse monoclonal antibodies from Ebioscience: CD4 (RM4-5), CD8 (53-6.7), CD45RB (16A), CD19 (1D3); or mouse anti-sheep monoclonal antibodies: CD4 (44.38; Serotec), CD8 (38.65; Serotec), CD62L (DU1-29, VMRD). Some mouse monoclonal antibodies were directly labeled prior to staining using Zenon labeling kits according to the manufacturer's instructions (Invitrogen). Staining for E-selectin (CD62E) ligand was performed using an E-selectin-human IgM chimera (38) kindly provided by Daniel Campbell (Benaroya Research institute, Seattle, WA). Staining in tissue culture supernatant containing E-selectin-human IgM was followed with biotinylated polyclonal F(ab)2 goat anti-human IgM (Jackson Immunoresearch). For cell surface staining, allophycocyanin- (BD Biosciences) or Alexa Fluor 405-conjugated streptavidin (Invitrogen) were used as second or third step reagents. To detect intracellular cytokines in ovine T cells, afferent lymph cells were stimulated for 4h with 10 ng/ml PMA and 500 ng/ml ionomycin. For the last 2h of stimulation 10 μg/ml brefeldin A was added. Intracellular cytokine staining for IL-17A (eBio64DEC17; Ebioscience) and IFN-γ (CC302; Serotec) of ovine T cells was performed of fixed cells in saponin buffer. Samples were acquired on a BD LSRII or Calibur using Diva or CellQuest software, respectively, and analyzed with FlowJo. Gates were set according to appropriate isotype control staining. Mouse feet were manually deboned and fixed in 10% neutral buffered formalin. Subsequently, the skin samples were processed into paraffin, and 6μm sections were stained with haematoxylin and eosin at Histo-Tec Laboratories (Hayward, CA). Histology images were acquired on a Nikon Eclipse E600 microscope using a Nikon digital sight DS-Fi1 camera and NIS-Elements BR 3.0 software. Images with 100x objective were acquired using oil immersion on an Olympus BX40F4 microscope with a CC12 camera.
Statistical analysis
The non-parametric Kruskal-Wallis test followed by Dunn's Multiple Comparison post test, or the non-parametric Mann-Whitney U test were employed using GraphPad Prism software analyzing combined data from all experiments performed, and p<0.05 was considered statistically significant.
Results
Chronicity of inflammation determines the relative rate of T cells entering the draining afferent lymph
We utilized the sheep lymph cannulation model established by Morris and colleagues (39, 40) to analyze CD4 and CD8 T cell migration from resting and inflamed skin into draining afferent lymph at different time points of a local inflammatory response. A pseudoafferent skin draining lymph vessel was cannulated and the output of total cells and lymphocyte subsets recorded over time (Fig. 1A). Subsequently, we induced inflammation in the drainage site of the cannulated vessel by subcutaneous injection of Complete Freund's Adjuvant (CFA). Cutaneous inflammation induced by CFA is an established model to study both acute and chronic inflammation in the sheep and is a relevant model for chronic inflammatory diseases in humans (13). While acute inflammation (<48h after induction of inflammation) increased the total output of leukocytes from the inflamed skin, the numbers of total lymphocytes (based on forward-side scatter properties) and CD4 and CD8 T cells were largely unchanged (Fig. 1A). A transient flux of neutrophils in afferent lymph was mainly responsible for the increased total cell output during the acute inflammatory response in this inflammation model (32-34), and data not shown).
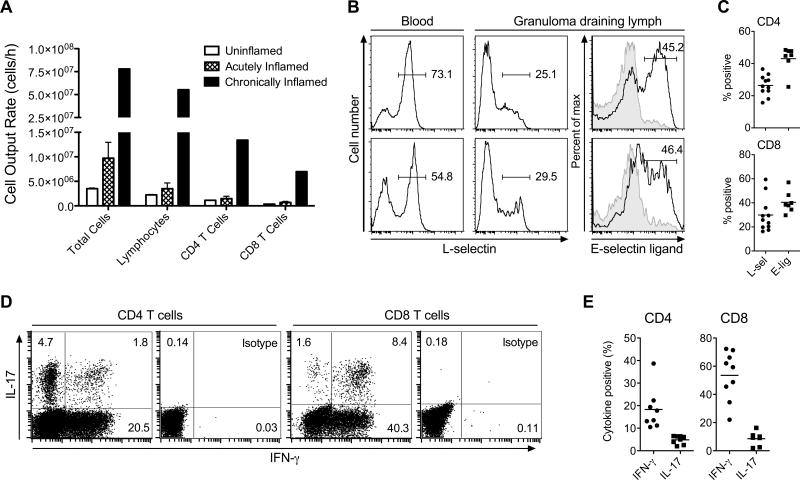
Figure 1. Chronic inflammation enhances memory/effector T cell egress from the affected skin via the afferent lymph.
Different stages of cutaneous inflammation were elicited by subcutaneous injection of CFA emulsified with saline into sheep flanks. Ovine peripheral blood or lymph was collected after venipuncture or catheterization of afferent lymph vessels draining uninflamed (control), acutely (≤48h after induction of inflammation) or chronically (3-5 weeks after induction of inflammation) inflamed skin. (A) Number of cells collected from the skin draining afferent lymph vessel over time (cell output) was determined for total cells, total lymphocytes, and CD4 and CD8 T cells. Data points show the mean ± SD of multiple time points analyzed for cell output from acutely inflamed and control (uninflamed) skin, and one time point for cell output from chronically inflamed skin of one animal. Data are representative of one animal out of a minimum of four individually analyzed sheep per time point of inflammation. (B) Flow cytometric analysis of L-selectin or E-selectin ligand expression by gated CD4 (top row) and CD8 (bottom row) T cells from afferent lymph draining chronically inflamed skin. Gray areas indicate isotype control staining. Numbers indicate the percent positive T cells in the specified gates. Data are representative of at least seven individually analyzed sheep. (C) Scatter plot of all analyzed animals, showing percentages of L-selectin (L-sel) and E-selectin ligand (E-lig) expressing CD4 and CD8 T cells in granuloma draining lymph. Data points represent individually analyzed sheep and horizontal lines indicate the mean of each group. (D) Intracellular cytokine staining for IFN-γ and IL-17 of gated afferent lymph CD4 and CD8 T cells draining chronically inflamed skin after stimulation with PMA and ionomycin. One representative staining of eight individually analyzed animals is shown. (E) Scatter plot of all analyzed animals, showing percentages of IFN-γ and IL-17 expressing CD4 and CD8 T cells in granuloma draining lymph. Data points represent individually analyzed sheep and horizontal lines indicate the mean of each group.
To study lymphocyte egress from the chronically inflamed skin, we first induced inflammation with CFA and then cannulated the draining pseudoafferent lymph vessel 3-4 weeks later. At this time point, typical skin granulomas, 3-5 cm in diameter, had formed at the CFA injection sites (data not shown). In contrast to acute inflammation, chronic inflammation dramatically increased the numbers of exiting total lymphocytes including CD4 and CD8 T cells (Fig. 1A). To determine the phenotype of exiting T cells, we stained for the adhesion molecule L-selectin (CD62L), which is expressed by naïve T cells and subsets of memory T cells and is required for homing into peripheral lymph nodes via high endothelial venules. Additionally, we tested T cell expression of ligands for the skin-selective endothelial adhesion molecule E-selectin; E-selectin binding ligands are expressed selectively on skin homing memory/effector T cells (reviewed in (11)). Unlike T cells in peripheral blood, most CD4 and CD8 T cells draining the skin granuloma were L-selectinneg (28.4±11%, mean±SD) and many expressed E-selectin ligand (42.7±7.8%; Fig. 1B and C), indicating that large numbers of memory/effector T cells exit the chronically inflamed site. Moreover, polyclonal stimulation with PMA and ionomycin revealed that the CD4 and CD8 T cells that egress the chronically inflamed site and enter afferent lymph are capable of producing inflammatory cytokines. Specifically, depending on the individual animal, 10.5 - 39% of the CD4 T cells produced IFN-γ and 2 – 6.5% IL-17, and among CD8 T cells, 22 - 72% produced IFN-γ and 2 - 16% IL-17 (N=7-9 animals; Fig. 1D and E). Thus, memory/effector T cells, including Th1 and Th17 cells, not only migrate from blood into sites of inflammation but also exit, leaving inflammatory sites via afferent lymph.
Chronicity of inflammation determines the efficiency of lymphocyte egress from extralymphoid tissue
Having established that chronic inflammation boosts the lymphatic exit of T cells from the affected site in sheep, it was unclear if this finding was also true for the mouse. To test lymphocyte egress from the inflamed skin at different phases of inflammation in the mouse, we induced localized inflammation by subcutaneous injection of a small volume (10 μl) of CFA into the footpads. Within 30 minutes following injection, swelling and hyperemia, hallmarks of acute inflammation, were visible (data not shown). At 6h after injection, the inflammation was histologically characterized by edema and polymorphonuclear infiltrates in the subcutis consistent with acute inflammation (Fig. 2B). Over time, the inflammation developed a chronic character with reduced swelling and hyperemia (not shown), and at days 10 and 21 the inflammatory response had progressed into a subcutaneous granuloma consisting of mostly mononuclear cells (lymphocytes and macrophages) (Fig. 2C). Thus, CFA injection into either mouse or sheep skin induced a similar inflammatory reaction.
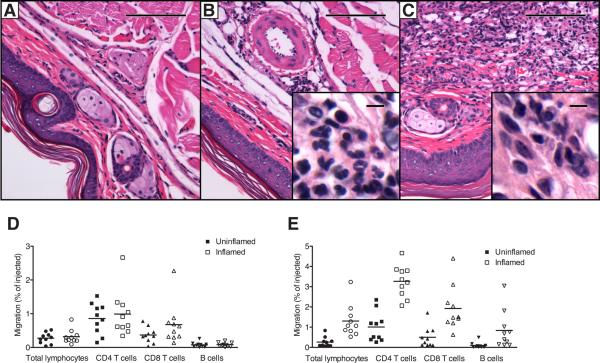
Figure 2. Chronic inflammation enhances lymphocyte egress from inflamed skin in an adoptive transfer mouse model.
CFA was injected subcutaneously into the area of footpads of mice to induce cutaneous inflammation. At different time points of inflammation, histology was assessed by paraffin sections after H&E staining in control skin (A), acutely inflamed skin at 6h (B), and chronically inflamed skin 21d (C) after induction of inflammation. (D, E) CFSE-labeled splenic lymphocytes were transferred into acutely inflamed skin (D, open symbols) or chronically inflamed skin (E, open symbols) 6h and 21d after induction of inflammation, respectively, or into the skin of untreated control mice (closed symbols). 12h after transfer, migrated CFSE+ total lymphocytes, CD4 T cells, CD8 T cells, and B cells were enumerated in the draining popliteal lymph nodes by flow cytometry. Migration was expressed as the percent of injected cells of each respective lymphocyte subset that migrated to the draining lymph node. (A-C) One representative staining out of a minimum of 5 mice per condition is shown at 10x and 100x (insets) original magnification. Scale bars indicate 100μm and 5μm for insets (D, E). One representative out of a minimum of three experiments performed analyzing 8-10 mice per group is shown. Data points represent individually analyzed mice and horizontal lines indicate the mean of each group.
Our data in the sheep system showed that chronic inflammation increased T cell exit from skin via the afferent lymph (Fig. 1); however, there are more tissue lymphocytes in sites of inflammation, and chronic inflammation also dramatically enhances lymphocyte migration from blood into the inflamed site (5). To test whether the efficiency of tissue egress is itself affected by chronic inflammation, or if enhanced exit could be explained solely by increased tissue lymphocyte numbers, we turned to the mouse model. We transferred CFSE-labeled splenocytes into the CFA-inflamed footpad skin of mice at 6h and 21d after induction of inflammation, or into the footpads of untreated control mice. 12h after cell transfer, CFSE-labeled cells were enumerated in draining and contralateral popliteal lymph nodes. During acute inflammation, transferred CFSE+ lymphocytes still exited the site and migrated to the draining lymph node, with no statistically significant difference to untreated mice for total lymphocytes, CD8 T cells, CD4 T cells, and B cells (Fig. 2D). In contrast, chronic inflammation significantly enhanced the egress of transferred total lymphocytes (p < 0.001), CD8 T cells (p < 0.05), CD4 T cells (p < 0.01) as well as B cells (p < 0.01) (Fig. 2E). Based on these findings, we conclude that chronic inflammation enhances lymphocyte exit from inflamed extralymphoid tissue in two mammalian species, and that enhanced lymphatic egress reflects in part an increase in the efficiency of exit mechanisms.
Chronic but not acute inflammation promotes CCR7-independent tissue exit of T cells
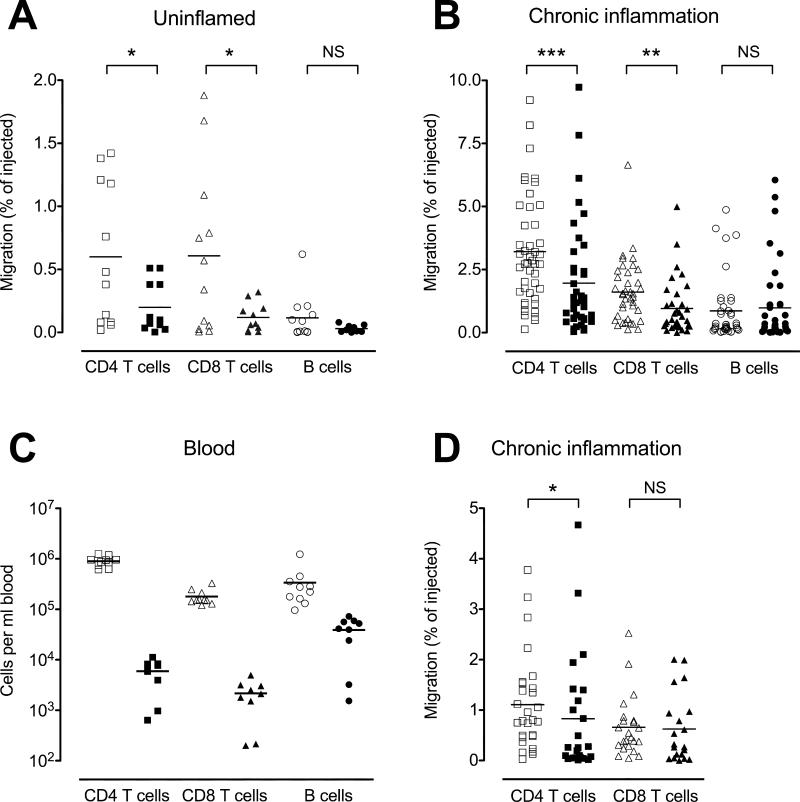
We have recently shown that CD4 and CD8 T cells require CCR7 for efficient egress from skin under steady-state (non-inflammatory) conditions (20). To test whether T cell egress from the inflamed skin is also CCR7-dependent, we transferred CFSE- and PKH26-labeled splenic lymphocytes from wild-type and CCR7-deficient mice into inflamed skin and monitored migration into the draining lymph node via the afferent lymph. 12h after splenocyte transfer into acutely inflamed skin (6h after induction of inflammation with CFA), CCR7-deficient total CD4 as well as naïve (CD45RBhi) and memory (CD45RBlo) CD4 T cells were reduced by ~80% and B cells by ~70% in the draining lymph node compared with their co-transferred wild-type counterparts (Fig. 3A). In this setting, numbers of recovered CD8 T cell were too low to be reliably analyzed (data not shown). These data indicate that CCR7 expression is critically important for lymphocyte exit from acutely inflamed skin (Fig. 3), akin to its role in lymphocyte egress from uninflamed skin (20).
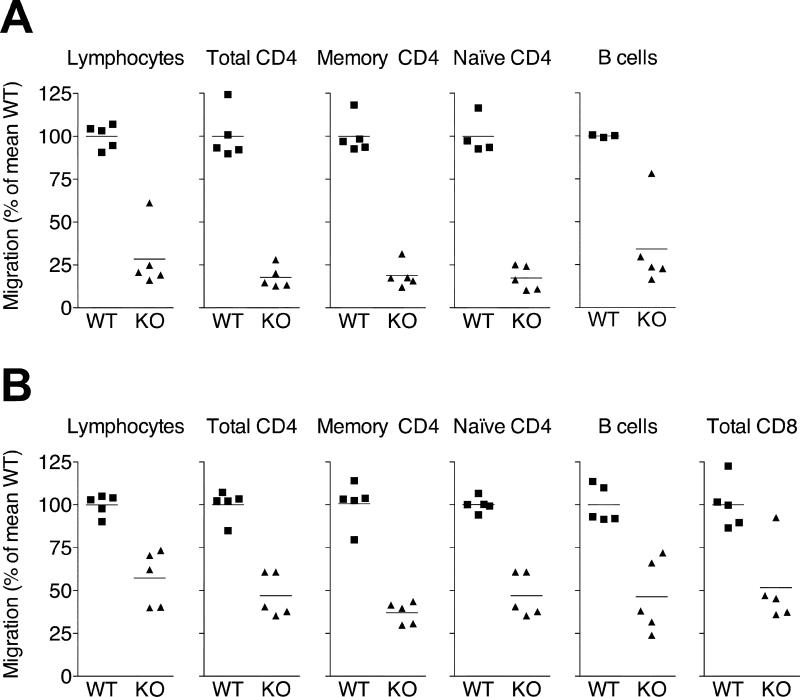
Figure 3. Chronic but not acute inflammation supports CCR7-indendent lymphocyte exit from inflamed skin.
PKH26-labeled WT lymphocytes were mixed with equal numbers of either WT or CCR7-deficient CFSE-labeled lymphocytes and injected into the inflamed footpads of recipient mice 6h (A) or 10d (B) after induction of cutaneous inflammation with CFA. 12h after cell transfer, the draining popliteal lymph nodes were analyzed for migrated CFSE+ and PKH26+ cells. The ratio of migrated CFSE/PKH26 to injected CFSE/PKH26 cells was determined for total lymphocytes, total CD4 T cells, memory (CD45RBlo) CD4 T cells, naïve (CD45RBhi) CD4 T cells, total CD8 T cells and B cells. In each case, results were normalized to the mean ratio of WT CFSE+ cells to WT PKH26+ cells (internal standard cells) for each subset (set as 100%). Data points represent individually analyzed mice of groups of 4-5 mice; horizontal lines indicate the mean of each group. One representative of a minimum of three experiments analyzing each cell type is shown. WT, wild-type; KO, CCR7-deficient.
In contrast, when transferred into chronically inflamed skin (d10 after induction of inflammation with CFA), CCR7-deficient splenic total lymphocytes, total CD4, memory (CD45RBlo) and naïve (CD45RBhi) CD4 T cells, CD8 T cells, and B cells were reduced by only 40 to 60% in their ability to exit the inflamed site and access the draining node (Fig. 3B). We could detect few if any of the adoptively transferred cells in non-draining contralateral lymph nodes or the spleen, indicating that cells had entered via the afferent lymph and not the blood. The same results were obtained when recipient mice were treated with the monoclonal antibody MEL-14 (anti-CD62L), which would block any residual migration from blood into lymph nodes via HEVs (data not shown). Our data suggest that CCR7 acts as a dominant exit receptor for T cells during inflammation but that CCR7-independent egress mechanisms develop during late stages of the inflammatory response.
Chronic inflammation promotes CCR7-independent exit of Th1 cells
Th1 cells are important in the maintenance of chronic inflammatory diseases and efficiently home to sites of cutaneous inflammation (41, 42). Having established that Th1 cells egress from chronically inflamed skin via lymph (Fig. 1), we asked whether this cell type required CCR7 for this process. To address this, we generated Th1 cells from wild-type and CCR7-deficient mice in vitro and labeled the cells with CFSE and PKH26. Wild-type Th1 cells expressed high levels of surface CCR7 and migrated to CCR7 ligands in an in vitro chemotaxis assay (data not shown). When transferred into the uninflamed or acutely inflamed footpad skin (6h after induction of inflammation), CCR7-deficient in vitro-polarized Th1 cells were drastically reduced (on average by >80%) in their capacity to exit the skin and migrate to the draining lymph node (Fig. 4), showing that, similar to resting splenic T cells, Th1 effector cells require CCR7 for efficient egress from uninflamed and acutely inflamed skin (Figs. 3 and 4, and (20)). In contrast, when in vitro polarized wild-type and CCR7-deficient Th1 cells were transferred into the chronically inflamed skin (d21 after induction of inflammation with CFA), CCR7-deficient Th1 cells exited the chronically inflamed site with up to ~75% of the efficiency of their wild-type counterparts (Fig. 4). Similar results were seen for in vitro-polarized (CD8) Tc1 cells (data not shown).
Figure 4. Th1 effector cell egress chronically inflamed is largely independent of CCR7.
Th1 effector cells were generated in vitro from WT and CCR7-deficient mice. PKH26-labeled WT Th1 cells were mixed with equal numbers of either WT or CCR7-deficient CFSE-labeled Th1 cells and injected into the uninflamed (A), acutely inflamed (B; 6h after induction of inflammation with CFA), or chronically inflamed (C; 21d after induction of inflammation with CFA) footpad skin of recipient mice. 12h after cell transfer, the draining popliteal lymph nodes were analyzed for migrated CFSE+ and PKH26+ T cells. The ratio of migrated CFSE/PKH26 to the ratio injected CFSE/PKH26 Th1 cells was determined. Results were normalized to the mean ratio of WT CFSE+ cells to WT PKH26+ cells (internal standard cells), which was set as 100%. Data points represent individually analyzed mice in groups of 5 mice; horizontal lines indicate the mean of each group. One representative of a minimum of three experiments for each condition (A and C) or one out of two with similar results (B) is shown. WT, wild-type; KO, CCR7-deficient.
The results imply that CCR7 expression is important for Th1 cells to egress from uninflamed skin, but that during the chronic phase of inflammation alternative factors can regulate effector T cell exit via afferent lymph.
Lymphocyte exit from the chronically inflamed skin is pertussis toxin sensitive
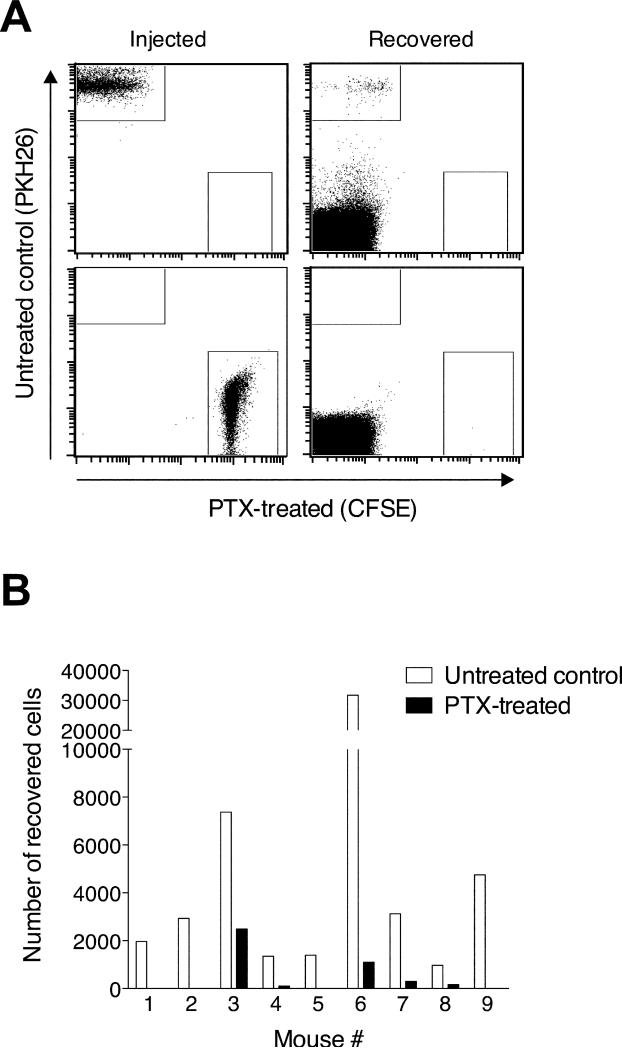
To test whether lymphocyte egress from the chronically inflamed skin is an active process that involves Gαi protein-coupled receptor signaling, we employed pertussis toxin (PTX) treatment of cells. PTX irreversibly modifies Gαi proteins, rendering chemokine receptors unresponsive to subsequent ligand binding. PTX-treated or untreated splenocytes were labeled with CFSE or PKH26 and transferred into chronically inflamed footpad skin on day 21 after induction of inflammation with CFA. The untreated control cells were transferred into the contralateral footpad (Fig. 5) or into a separate group of recipient mice (not shown). Treated and untreated control cells could not be mixed and co-injected because, even after extensive washing, PTX of treated cells was carried over to untreated cells (not shown). 12h after cell transfer, a population of transferred, untreated lymphocytes was detectable in the draining lymph node, while PTX-treatment efficiently blocked lymphocyte migration (p<0.0001; Fig. 5). When injected cells were re-isolated from skin 12h after transfer into CFA-induced skin granulomas (day 21 after induction of inflammation), no difference in the percentage of apoptotic or dead cells (determined by annexin V and propidium iodide binding) was seen between PTX-treated and untreated control lymphocytes (data not shown). Based on these findings, we conclude that egress from the chronically inflamed skin and migration into the draining lymph node is sensitive to PTX treatment, suggesting the involvement of Gγi-coupled chemoattractant receptors and excluding passive mechanisms of lymph flow as a principal means of lymphocyte egress from inflamed sites.
Figure 5. Lymphocyte egress from chronically inflamed skin depends on Gαi protein-coupled receptors signaling.
Splenic lymphocytes were incubated with PTX or control treated and subsequently labeled with CFSE or PKH26, respectively. 21d after induction of cutaneous inflammation with CFA in both hind footpads, PTX-treated CFSE+ cells were injected into the inflamed footpad skin of one side and PKH26+ cells into the inflamed skin of the contralateral footpad. 12h after cell transfer, fluorescently labeled lymphocytes that migrated into the draining lymph node were enumerated by flow cytometry. One representative staining (A) and experiment (B) out of three performed experiments analyzing 9-10 mice each are shown.
T cell exit from the chronically inflamed skin is mediated in part by S1P receptors
Having shown a requirement for Gαi-coupled receptors in lymphocyte egress from chronically inflamed tissue, we next tested for a role of G protein-coupled S1P receptors in the process. The drug FTY720 is phosphorylated in vivo and then acts as an agonist for the S1P receptors S1P1, S1P3, S1P4, and S1P5 and down-regulates the expression and subsequent function of these receptors (43). This “functional antagonism” prevents T cell egress from lymphoid tissues and induces lymphopenia via inhibition of S1P1 (24, 43). FTY720 accumulates in tissues relative to blood (44) and can affect lymphocytes in vivo for >100h following a single dose (36). As described for hematopoietic stem cells (45) and T cells (46), treatment of recipient mice with FTY720, reduced the egress of adoptively transferred wild-type CD4 (p<0.05) and CD8 T cells from the uninflamed skin (Fig. 6A) Next, we induced cutaneous inflammation with CFA and on day 21 of the inflammatory response, treated recipient mice systemically with FTY720 prior to adoptive transfer of wildtype splenocytes into the chronically inflamed footpads. While the drug treatment reduced migration of adoptively transferred wild-type CD4 (p<0.001) and CD8 T cells (p<0.05) from the chronically inflamed skin via the afferent lymph (Fig. 6B), the inhibition of migration was less than in the absence of inflammation (Fig. 6A). Differences in B cell egress from the inflamed or uninflamed skin between drug-treated and untreated animals did not attain statistical significance (Fig. 6A and B). As expected, the FTY720 treatment induced lymphopenia in recipient animals confirming activity of the drug (one example is shown in Figure 6C). The same treatment protocol was also able to drastically reduce transferred and endogenous lymphocytes in blood compared with untreated mice when CFSE-labeled cells were transferred IV 12-20h following the drug treatment and analyzed 12-20h later (=20-28h following the drug treatment) (Supplemental Figure 1). This indicates that our treatment protocol is sufficient to sequester transferred lymphocytes in tissues as well as induce lymphopenia. In additional control experiments, we continuously treated recipient mice with FTY720 via the drinking water (1mg/kg/day) starting 32h prior to transfer of lymphocytes from donor mice that were FTY720-treated for >24h; no additional reduction in T or B cell egress from the chronically inflamed skin was seen when compared to the IP-treated animals (Supplemental Figure 2 and Fig. 6B). Thus, FTY720-sensitive S1P receptors play a statistically significant but minor role in T cell exit from the chronically inflamed skin.
Figure 6. FTY720 treatment reduces T cell egress from chronically inflamed skin.
Naïve control mice (A) or mice with 21d-old CFA-induced cutaneous inflammation in both hind footpads (B-D) were treated intraperitoneally with FTY720 (closed symbols) or saline (open symbols). 8h post treatment, CFSE- and/or PKH26-labeled splenic lymphocytes from wild-type (A, B) or CCR7-deficient (D) mice were injected into the uninflamed (A) or inflamed (B, D) footpad skin of the FTY720-treated and control-treated mice. 12h after cell transfer, fluorescently labeled lymphocytes that migrated into the draining popliteal lymph node (A, B, D) and endogenous lymphocytes in blood (C) were enumerated by flow cytometry. Data points represent individually analyzed recipient mice and horizontal lines indicate the mean of each group. The combination of data from 2 experiments analyzing 5-10 mice per group (A), 5 experiments analyzing 8-10 mice per group (B), 3 experiments analyzing 10 mice per group (D), and one example of a corresponding endogenous blood lymphocyte count (C) is shown. *, p<0.05; **, p<0.01; ***, p< 0.001; NS, not significant (p>0.05).
To determine whether inhibition of CCR7 and S1P receptors was sufficient to prevent exit, we tested the ability of FTY720 treatment to block lymphocyte egress of CCR7-deficient T cells. When fluorochrome-labeled CCR7-deficient lymphocytes were transferred into the chronically inflamed skin on day 21 after induction of inflammation with CFA, FTY720 treatment led to minimally reduced egress of CCR7-deficient CD4 T cells (p<0.01), while the migration of CCR7-deficient CD8 T cells was not significantly affected (Fig. 6D).
The ability of PTX treatment but not combined inhibition and deficiency of CCR7 and S1P receptor signaling to abrogate lymphocyte egress suggests that S1P receptors and CCR7 can mediate exit from chronically inflamed skin, but that additional Gαi-coupled chemoattractant receptors must contribute as well.
Chronicity of inflammation determines the relative rate of CCR7-dependent and -independent lymphocyte egress from extralymphoid tissue
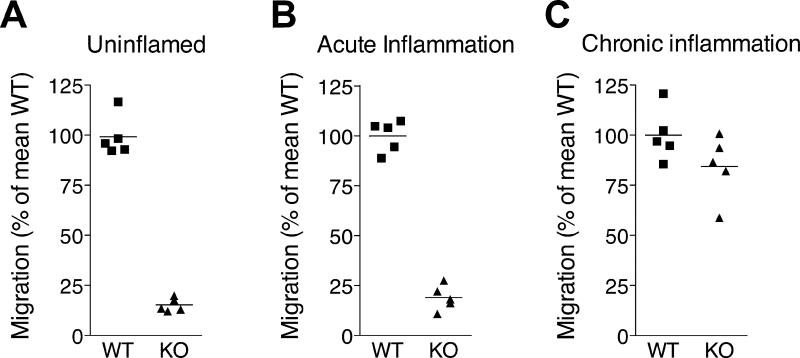
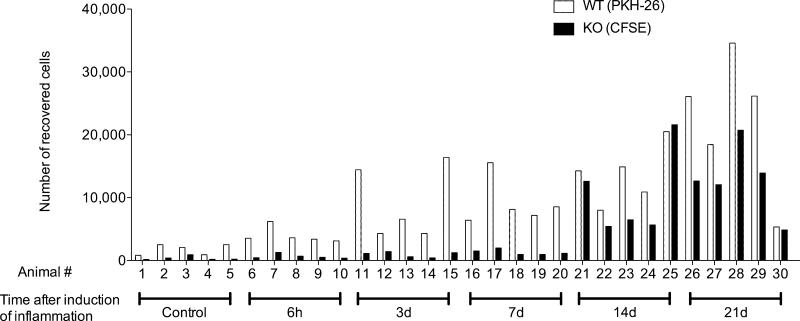
Our results showing that chronic, but not acute inflammation enhanced lymphocyte egress from the affected skin via lymph (Figs. 1 and 2), suggested that the chronicity of inflammation dictates the efficiency of lymphocyte exit from inflamed extralymphoid tissues. Moreover, our finding that CCR7 was required for lymphocyte egress from uninflamed and acutely inflamed skin (20) (Figs. 3 and 4) but less critical in T cell egress from chronically inflamed tissues (Figs. 3, 4, and 6) implies that the chronicity of inflammation additionally governs the receptor requirements for exit. To test whether the capacity to exit is indeed a function of the time that inflammation has progressed, we induced cutaneous inflammation by subcutaneous injection of CFA into the footpad of mice at different time points (i.e. 6h to 21d). Next, we transferred splenic lymphocytes from wild-type and CCR7-deficient mice after labeling with PKH26 and CFSE, respectively, into the inflamed footpads and uninflamed footpads of control mice, and monitored cell arrival in the draining popliteal lymph nodes 12h later. Adoptively transferred wild-type lymphocytes exited more efficiently from 3-day or older sites of inflammation relative to uninflamed skin (Fig. 7). Interestingly, the numbers of lymphocytes that exited the inflamed skin and migrated into the draining lymph node within the 12-hour migration period increased correspondingly with the duration of inflammation, with the greatest exit occurring at days 14 and 21. Moreover, large numbers of CCR7-deficient lymphocytes emigrated the inflamed skin on days 14 and 21 but not at the earlier time points (6h, 3d, and 7d after induction of inflammation). Thus, chronic inflammation promoted CCR7-independent exit. Based on these findings, we conclude that the chronicity of inflammation dictates the relative rate of lymphocyte exit from the inflamed tissue as well as the mechanisms involved.
Figure 7. Chronicity of inflammation determines the efficiency of CCR7-dependent and independent lymphocyte egress from extralymphoid tissue.
Different stages of cutaneous inflammation were induced by subcutaneous injection of CFA into the footpads of mice at different time points. CCR7-deficient and WT splenocytes were labeled with CFSE and PKH26, respectively, mixed, and injected into inflamed footpad skin of mice at the different indicated time points of a CFA-induced inflammation or into footpads of untreated mice (Control). 12h after cell transfer, migrated CFSE+ and PKH26+ lymphocytes were enumerated in the draining popliteal lymph nodes. One out of two experiments with similar results, analyzing four to five mice per time point, is shown.
Discussion
Memory/effector T cells recirculate through extralymphoid tissues entering from the blood and leaving via the afferent lymph. During inflammation, T cell traffic into the affected tissue dramatically increases, and its mechanisms have been well studied and proven key to the inflammatory process. Intuitively, T cell egress from the inflamed site is as important as entry in determining the size and quality of the inflammatory infiltrate; however, the dynamics and mechanisms of T cell exit from inflamed tissues are poorly characterized.
Using sheep lymph cannulation and mouse adoptive transfer models, we found that chronic inflammation efficiently increased lymphocyte egress from the affected tissue via the afferent lymph. The enhanced lymphatic lymphocyte egress was not simply a reflection of enhanced entry from the blood into the tissue because, bypassing recruitment from blood, lymphocytes adoptively transferred into chronically inflamed skin emigrated more efficiently than did lymphocytes adoptively transferred into uninflamed skin (Fig. 2). This finding is in line with recent reports that chronic inflammation and infection induce expansion of draining lymphatics, mainly through lymphangiogenesis, at the site of inflammation (47, 48). Thus, in addition to facilitating transport of interstitial fluid, antigen, and antigen presenting cells, such an expanded afferent lymphatic network also appears well suited to enhance egress of memory/effector T cells from inflamed sites.
Within hours after inflammatory stimulation with cytokines or microbes, or following antigenic re-challenge, lymphocyte output via efferent lymph from the regional draining lymph node is temporarily decreased by up to 90% (49, 50), a phenomenon referred to as “lymph node shutdown”. Interestingly, Liao and Ruddle showed that early inflammatory events transiently impair afferent lymphatic drainage (51), pointing to the possibility of a “shutdown” at the level of afferent lymph draining the site of inflammation. However, consistent with previous studies (13), at all time points following induction of inflammation, we did not detect a drop in lymphocyte numbers traveling in the draining afferent lymph (Fig. 1A). Consequently, throughout inflammation, T cells (along with antigen-presenting cells) continue to egress from extralymphoid tissue and migrate to the draining lymph node, where they can participate in the initiation or maintenance of primary and secondary adaptive immune responses. Even though we did not detect a “shutdown” at the level of afferent lymph, tissue edema at early time points of acute inflammation (Fig. 2) indicated that lymphatic drainage was temporarily insufficient to remove excess interstitial fluid and possibly large cell numbers. The process of lymphangiogenesis takes >1 week (52), so perhaps there are no newly formed or expanded vessels that could support enhanced cellular exit during the acute phase of inflammation, explaining why the egress of adoptively transferred and endogenous lymphocytes from acutely inflamed skin was not different from egress from uninflamed skin (Figs. 1 and 2).
Classic studies in the sheep showed that large numbers of lymphocytes leave the site of inflammation via the afferent lymph (5, 34). Our experiments extend these early studies by revealing that chronic inflammation dramatically boosts the egress rate of memory/effector (L-selectinlo/- and E-selectin ligand+) CD4 and CD8 T cells from the affected site (Fig. 1). Importantly, many of the T cells leaving the chronically inflamed skin are capable of secreting the inflammatory cytokines IFN-γ and/or IL-17 (Fig. 1), proving that polarized T cell subsets not only traffic from blood into inflamed sites, but also leave the inflammatory site via the afferent lymph. The finding that effector T cell subsets actively recirculate has several implications for inflammatory diseases. For example, the fact that Th1 and Th17 cells leave the inflamed site and reenter the circulation makes their trafficking back to inflammatory sites a promising therapeutic target; in contrast to permanently accumulating cells, which would not be affected by drugs interfering with their recruitment. By removing proinflammatory cells such as Th17 cells, egress via afferent lymph may help limit the local inflammatory response. Conversely, impaired egress of inflammatory T cell subsets might exacerbate local inflammatory responses and contribute to chronic inflammation. In support of this idea, Rockson and colleagues found that in a mouse model of lymph edema, both the interstitial fluid drainage and leukocyte egress from the affected site are impaired, and suggested that reduced leukocyte exit via lymph could contribute to the development of localized inflammation that is associated with the disease (53).
Intriguingly, our data showed that the chronicity of inflammation not only influenced the exit rates of lymphocytes, but also the receptor requirements. CCR7, the best characterized “exit receptor“ for dendritic cells (54) as well as T cells (20, 21) became less essential for lymphocyte egress from inflamed tissue during the course of inflammation. Specifically, CCR7 expression by T cells was required for their egress from acutely inflamed or uninflamed skin, while egress from chronically inflamed skin had CCR7-dependent and –independent components. Comparing the egress capacity of CCR7-deficient and wild-type lymphocytes over the course of inflammation revealed that the inflammatory response to CFA had to progress for at least two weeks to support robust CCR7-independent egress of lymphocytes (Fig. 7). Egress at these late time points of inflammation depended upon the signaling of Gαi-coupled receptors because cell migration was sensitive to PTX treatment (Fig. 5). Ledgerwood et al. recently suggested that S1P and its G protein-coupled receptor S1P1 regulate T cell egress from and retention in extralymphoid tissues (46). In our hands, blocking the function of S1P receptors with FTY720 significantly reduced T cell egress from the uninflamed skin (Fig. 6A). However, the drug treatment had only a small effect on the egress of wild-type T cells from chronically inflamed skin (Fig. 6B) and, under all conditions tested, did not affect the egress of B cells from the skin (Fig. 6A and B). Consequently, FTY720-sensitive S1P receptors are only one participant in lymphocyte egress from chronically inflamed skin. Importantly, FTY720 treatment did not abrogate exit of adoptively transferred CCR7-deficient T cells from a site of chronic skin inflammation when these were co-transferred with wild-type cells (Fig. 6D). Specifically, the drug treatment only slightly reduced migration of CCR7-deficient CD4 T cells and had no statistically significant effect on egressing CCR7-deficient CD8 T cells (Fig. 6D). Congruently, others have also observed a smaller effect of FTY720 on CCR7-deficient lymphocytes relative to their wild-type counterparts (55, 56). Thus, S1P receptors and CCR7 act, at least in part, cooperatively in the process of lymphocyte egress from inflamed skin.
Collectively, these findings imply that alternative (Gαi) chemoattractant receptors mediate CCR7-independent, S1P receptor-independent lymphocyte egress from the chronically inflamed skin. Most likely, additional chemoattractants that are expressed by lymphatic endothelium at the inflamed site support the removal of CCR7-negative cell subsets, allowing for more efficient flux of lymphocytes through the site of inflammation.
In support of a role of alternative chemoattractants in lymphocyte egress from extralymphoid tissues, lymphatic endothelium expresses chemokines other than CCR7 ligands upon inflammatory stimulation (19, 25) and in vivo (28, 57). However, the induction of chemokines in lymphatic endothelial cells occurs rapidly (within hours to days) following inflammatory stimulation (19, 25), while it took weeks until we detected maximal egress of CCR7-deficient lymphocytes (Fig. 7). Thus, besides alternative chemoattractants, structural changes at the site of inflammation may also contribute to CCR7-independent lymphocyte exit. Some of the possible structural changes supporting CCR7-independent egress could be due to the changing leukocytic infiltrate; from granulocytes at early time points to mononuclear cells at late time points of the inflammatory response (Fig. 2). For example, due to their expression of subset-specific cytokines and other mediators, infiltrating leukocytes could induce different repertoires of chemoattractants by lymphatic endothelial cells as well as influence the expression and/or function of chemoattractant receptors on infiltrating lymphocytes. It is also conceivable that, as a result of the ongoing lymphangiogenesis at the inflamed site, newly formed lymph vessels have a different profile of chemoattractant expression relative to ‘old’ vessels. Finally, lymphocyte or lymphatic endothelial cell contact with other factors characteristic of chronic inflammation such as tissue fibrosis or necrosis could influence the expression and subsequent usage of chemoattractant receptors and chemoattractants.
Interestingly, during the course of inflammation, receptor requirements for lymphocyte recruitment from the blood into lymphoid and extralymphoid tissue change too. For instance, the blood vascular endothelium of chronically inflamed skin often expresses molecules that are usually absent in skin but are associated with homing into lymphoid tissues such as peripheral node addressin (PNAd) (58) or CCL21 (59, 60). Similar to our observations of CCR7-independent lymphocyte migration from inflamed extralymphoid tissue into lymph nodes via afferent lymph (Figures 3 and 4), effector CD8 T cell migration from blood into reactive lymph nodes via high endothelial lymph nodes becomes also independent of CCR7 with inflammation, and T cells require CXCR3 instead (61).
The finding that chronic inflammation supports robust lymphatic egress of CCR7-deficient lymphocytes is highly relevant for the receptor requirements in the tissue egress of cell types other than lymphocytes. For example, CCR7-negative dendritic cells or malignant cells could, through usage of alternative exit receptors, present antigen in draining lymph nodes maintaining the chronic inflammatory response or efficiently metastasize via the lymphatics, respectively. We are currently testing alternative chemoattractant receptors that may mediate CCR7-independent egress from chronically inflamed extralymphoid tissue. Based on their induction in lymphatic endothelial cells by inflammatory stimuli (19, 25, 28, 57, 62), and the expression of corresponding receptors by lymphocytes, the following chemokine receptor–ligand pairs are potential candidates for mediating CCR7-independent exit: CCR2–CCL2, CCR5–CCL3/CCL5, CCR6–CCL20, CCR10–CCL27, and CXCR4–CXCL12; and possibly CXCR3–CXCL9/CXCL10 (63). As already established for lymphocyte entry into sites of inflammation, it is likely that chemokines expressed by lymphatic endothelium exert redundant roles in mediating lymphocyte egress from inflamed tissues. In addition, future studies are needed to address how individual components of chronic inflammation influence trafficking of different leukocyte subsets into and out of the inflamed site.
In summary, the phase of inflammation in extralymphoid tissue determines the magnitude of lymphocyte exit from the site and the mechanisms employed in the process. Potentially deleterious Th1 and Th17 cells egress from sites of chronic inflammation via afferent lymph, suggesting T cell exit as a key regulator of inflammation and a promising future therapeutic target.
Supplementary Material
Acknowledgements
The authors thank Laura Gigliello and Christine Chapman for excellent surgical assistance, Penelope Collins and Norman Wiltshire for veterinary support of our sheep experiments, Karen Jackson for taking high magnification histology pictures, and Dave Allman, Malissa Diehl, Daniel Campbell, and Alf Hamann for critical reading of the manuscript.
Footnotes
This work was supported by NIH grants AI073682 and AR056730 to GFD, NIH grant T32AI007532 to SAG, and a Merit Award from the Department of Veterans Affairs, and NIH grants AI47822 and AI72618 to ECB.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 3.Mackay CR, Marston WL, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall JG, Morris B. The Origin of the Cells in the Efferent Lymph from a Single Lymph Node. J Exp Med. 1965;121:901–910. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin W, Hay JB. A comparison of lymphocyte migration through intestinal lymph nodes, subcutaneous lymph nodes, and chronic inflammatory sites of sheep. Gastroenterology. 1980;79:1231–1242. [PubMed] [Google Scholar]
- 6.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Abbas AK, Fausto N. Acute and Chronic Inflammation. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran: Pathologic Basis of Disease. Elsevier Saunders; Philadelphia: 2005. [Google Scholar]
- 8.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188–211. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 11.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seabrook T, Au B, Dickstein J, Zhang X, Ristevski B, Hay JB. The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin Immunol. 1999;11:115–123. doi: 10.1006/smim.1999.0167. [DOI] [PubMed] [Google Scholar]
- 13.Hay JB, Issekutz TB, Chin WG. Immunological Aspects of Rheumatology. MTP Press; Lancaster: 1981. Lymphocyte traffic through chronic inflammatory lesions: relevance to rheumatic diseases. pp. 29–40. [Google Scholar]
- 14.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 16.Nakano H, Gunn MD. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J Immunol. 2001;166:361–369. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- 17.Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104:3849–3857. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- 18.Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemela J, Martinez-Pomares L, Salmi M, Jalkanen S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood. 2008;112:64–72. doi: 10.1182/blood-2007-10-118984. [DOI] [PubMed] [Google Scholar]
- 19.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 22.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 23.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 24.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 25.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberhard Y, Ortiz S, Ruiz Lascano A, Kuznitzky R, Serra HM. Up-regulation of the chemokine CCL21 in the skin of subjects exposed to irritants. BMC Immunol. 2004;5:7. doi: 10.1186/1471-2172-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burman A, Haworth O, Hardie DL, Amft EN, Siewert C, Jackson DG, Salmon M, Buckley CD. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693–1700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 30.Young AJ, Hein WR, Hay JB. Cannulation of lymphatic vessels and its use in the study of lymphocyte traffic. In: Levkovits I, editor. Manual of immunological methods: the comprehensive source book of techniques. Academic Press; San Diego: 1997. pp. 2039–2059. [Google Scholar]
- 31.Issekutz TB, Chin W, Hay JB. The characterization of lymphocytes migrating through chronically inflamed tissues. Immunology. 1982;46:59–66. [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston MG, Hay JB, Movat HZ. Kinetics of prostaglandin production in various inflammatory lesions, measured in draining lymph. Am J Pathol. 1979;95:225–238. [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston MG, Hay JB, Movat HZ. The distribution of prostaglandins in afferent and efferent lymph from inflammatory sites. Am J Pathol. 1980;99:695–714. [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JB, McIntosh GH, Morris B. The migration of cells through chronically inflamed tissues. J Pathol. 1970;100:21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- 35.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 36.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 37.Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, Song CH, Galli SJ, Butcher EC. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205:2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- 39.Lascelles AK, Morris B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- 40.Hall JG, Morris B. The lymph-borne cells of the immune response. Q J Exp Physiol Cogn Med Sci. 1963;48:235–247. doi: 10.1113/expphysiol.1963.sp001660. [DOI] [PubMed] [Google Scholar]
- 41.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 42.Debes GF, Höpken UE, Hamann A. In vivo differentiated cytokine-producing CD4(+) T cells express functional CCR7. J Immunol. 2002;168:5441–5447. doi: 10.4049/jimmunol.168.11.5441. [DOI] [PubMed] [Google Scholar]
- 43.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 44.Sensken SC, Bode C, Graler MH. Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2009;328:963–969. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]
- 45.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, Bromberg JS. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 47.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Hall JG, Morris B. The output of cells in lymph from the popliteal node of sheep. Q J Exp Physiol Cogn Med Sci. 1962;47:360–369. doi: 10.1113/expphysiol.1962.sp001620. [DOI] [PubMed] [Google Scholar]
- 50.Young AJ, Marston WL, Dudler L. Subset-specific regulation of the lymphatic exit of recirculating lymphocytes in vivo. J Immunol. 2000;165:3168–3174. doi: 10.4049/jimmunol.165.6.3168. [DOI] [PubMed] [Google Scholar]
- 51.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 52.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R, Rockson SG. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254. doi: 10.1371/journal.pmed.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 55.Henning G, Ohl L, Junt T, Reiterer P, Brinkmann V, Nakano H, Hohenberger W, Lipp M, Forster R. CC chemokine receptor 7-dependent and -independent pathways for lymphocyte homing: modulation by FTY720. J Exp Med. 2001;194:1875–1881. doi: 10.1084/jem.194.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wick N, Haluza D, Gurnhofer E, Raab I, Kasimir MT, Prinz M, Steiner CW, Reinisch C, Howorka A, Giovanoli P, Buchsbaum S, Krieger S, Tschachler E, Petzelbauer P, Kerjaschki D. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am J Pathol. 2008;173:1202–1209. doi: 10.2353/ajpath.2008.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchimura K, Rosen SD. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol. 2006;27:559–565. doi: 10.1016/j.it.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 61.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 62.Sawa Y, Tsuruga E. The expression of E-selectin and chemokines in the cultured human lymphatic endothelium with lipopolysaccharides. J Anat. 2008;212:654–663. doi: 10.1111/j.1469-7580.2008.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancardi S, Vecile E, Dusetti N, Calvo E, Stanta G, Burrone OR, Dobrina A. Evidence of CXC, CC and C chemokine production by lymphatic endothelial cells. Immunology. 2003;108:523–530. doi: 10.1046/j.1365-2567.2003.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.