Abstract
Application of 3.6 mm silicon (Si+) to the rose (Rosa hybrida) cultivar Smart increased the concentration of antimicrobial phenolic acids and flavonoids in response to infection by rose powdery mildew (Podosphaera pannosa). Simultaneously, the expression of genes coding for key enzymes in the phenylpropanoid pathway (phenylalanine ammonia lyase, cinnamyl alcohol dehydrogenase, and chalcone synthase) was up-regulated. The increase in phenolic compounds correlated with a 46% reduction in disease severity compared with inoculated leaves without Si application (Si−). Furthermore, Si application without pathogen inoculation induced gene expression and primed the accumulation of several phenolics compared with the uninoculated Si− control. Chlorogenic acid was the phenolic acid detected in the highest concentration, with an increase of more than 80% in Si+ inoculated compared with Si− uninoculated plants. Among the quantified flavonoids, rutin and quercitrin were detected in the highest concentrations, and the rutin concentration increased more than 20-fold in Si+ inoculated compared with Si− uninoculated plants. Both rutin and chlorogenic acid had antimicrobial effects on P. pannosa, evidenced by reduced conidial germination and appressorium formation of the pathogen, both after spray application and infiltration into leaves. The application of rutin and chlorogenic acid reduced powdery mildew severity by 40% to 50%, and observation of an effect after leaf infiltration indicated that these two phenolics can be transported to the epidermal surface. In conclusion, we provide evidence that Si plays an active role in disease reduction in rose by inducing the production of antifungal phenolic metabolites as a response to powdery mildew infection.
Miniature potted roses (Rosa hybrida) have become an increasingly popular ornamental crop for the floriculture industry (Pemberton et al., 2003). Powdery mildew caused by Podosphaera pannosa is one of the most widespread diseases of potted roses (Horst, 1983; Eken, 2005), and the white colonies, together with leaf distortion, curling, and premature defoliation caused by the pathogen, lead to poor marketing value (Eken, 2005). Powdery mildew is typically managed through the use of synthetic fungicides (Horst, 1983; Eken, 2005). However, environmental considerations have necessitated increasing restrictions on the use of pesticides; therefore, eco-friendly production methods for plant disease suppression need to be developed. One of the most promising methods is to increase the level of silicon (Si) in the growth medium (or soil), as this has been able to reduce the growth of a number of plant pathogens, such as Magnaporthe oryzae infecting rice (Oryza sativa; Rodrigues et al., 2001, 2004), Blumeria graminis f. sp. tritici infecting wheat (Triticum aestivum; Bélanger et al., 2003; Rémus-Borel et al., 2005), and Podosphaera fuliginea infecting cucumber (Cucumis sativus; Fawe et al., 1998). Recently, we have shown that this effect is also seen in roses, since application of 3.6 mm Si significantly reduced powdery mildew severity (Shetty et al., 2011).
Si is the second most abundant element in the crust of the Earth and is regarded as a semiessential nutrient for plant growth (Epstein, 1994). Si is readily absorbed by plant roots in the form of silicic acid [Si(OH)4; Ma and Yamaji, 2006]. The soluble silicic acid is transported through the xylem to the vegetative tissues, concentrated through transpiration, polymerized as amorphous Si, and deposited in intracellular and intercellular spaces (Ma and Takahashi, 2002). We demonstrated that application of 3.6 mm Si in roses, which is considered a nonaccumulator of Si, increased leaf Si content to 14 ppm in the dry matter compared with no application of Si (Si content of 3 ppm), and confocal microscopy showed that Si deposition mainly occurred in the apoplast, particularly in epidermal cell walls (Shetty et al., 2011). Earlier studies on Si have documented the ability of Si to alleviate abiotic and biotic stress by acting as a physical barrier to infection and also by inducing active defense mechanisms (Ma, 2004; Fauteux et al., 2005). In accordance with this, Shetty et al. (2011) found that the Si-induced protection against P. pannosa in roses was accompanied by the increased formation of papillae and fluorescent epidermal cells (FEC) as well as the accumulation of callose and hydrogen peroxide, especially at the sites of penetration and in FEC, which are believed to represent the hypersensitive response.
Due to the threat of infection by pathogens, plants have evolved and developed a multitude of chemical and structural barriers for their protection. Various antimicrobial compounds, which are synthesized by plants after infection, have been discovered (Osbourn, 1996). One group of compounds, phytoalexins, is formed de novo after invasion, whereas others, phytoanticipins, are preformed compounds that may undergo postinfection modifications in order to express full toxicity (Barz et al., 1990). Secondary metabolites of the phenylpropanoid pathway such as phenolic acids and flavonoids are well-known examples of compounds that may be produced by plants as phytoanticipins or phytoalexins in order to fight invading microorganisms (Dixon and Paiva, 1995; Dixon et al., 2002). Rapid and early accumulation of phenolic compounds at infection sites is a characteristic of phenolic-based defense responses. At the infection sites, the production of toxic phenolic compounds may result in effective inhibition of the pathogen (de Ascensao and Dubery, 2003). Production of such metabolites may also be involved in the increased formation of FEC in Si-treated roses after P. pannosa inoculation (Shetty et al., 2011). Only a few secondary metabolites have been implicated in Si-induced resistance against fungal diseases (i.e. flavonoid phytoalexins in cucumber as well as diterpenoid phytoalexins in rice; Fawe et al., 1998; Rodrigues et al., 2004; Rémus-Borel et al., 2005). Transcription analysis of genes encoding enzymes in the biosynthetic pathways of secondary metabolites, especially during the early stages of infection, could help explain the relationship between secondary metabolites and Si-mediated resistance. Transcriptome analysis in wheat and Arabidopsis (Arabidopsis thaliana) showed that inoculation of both Si-treated and untreated plants with powdery mildew induced alterations in expression levels of several hundred of genes (Fauteux et al., 2005; Chain et al., 2009). On the other hand, Si application alone only played a limited role in the transcriptomic changes in wheat, Arabidopsis, and tomato (Solanum lycopersicum), and no effect was seen for genes related to secondary metabolism (Fauteux et al., 2005; Chain et al., 2009; Ghareeb et al., 2011).
Investigations of modern rose cultivars have shown that they are a rich source of polyphenols in both leaves and petals (Biolley et al., 1994a, 1994b; Helsper et al., 2003). Our hypothesis is that secondary metabolites of the phenylpropanoid pathway such as flavonoids and phenolic acids are important defense compounds in roses against powdery mildew, acting either as phytoanticipins or phytoalexins, and that application of Si in the form K2SiO3 increases the production of these phenolic compounds.
RESULTS
Phenolic Acids and Flavonol Glycosides in Rose Leaves
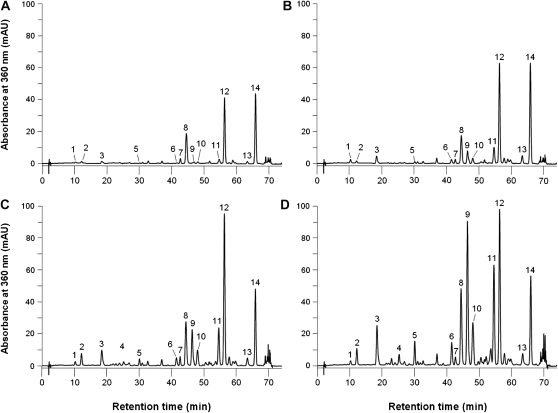
Several phenolic acids and flavonoids were identified in extracts of rose leaves (Supplemental Fig. S1). Typical HPLC chromatograms at 320 and 360 nm of an aqueous methanol extract of the leaves are shown in Supplemental Figure S2 and Figure 1, respectively. Based on the UV spectra, the compounds could be grouped into (1) caffeic acid derivatives, with an absorption band centered around 325 nm with a shoulder at around 300 nm, and (2) flavonol glycosides, with λmax values between 343 to 364 nm and 253 to 265 nm for peaks in bands I and II, respectively (Table I). The UV absorptions of compounds 2 and 3 clearly indicated that these compounds were derivatives of caffeic acid, which was also confirmed by their liquid chromatography-mass spectrometry (LC-MS) data. Compounds 2 and 3 had a pseudomolecular ion [M–H]– at mass-to-charge ratio (m/z) 353, compatible with caffeoylquinic acids, and were identified as 3-O-caffeoylquinic acid (neochlorogenic acid) and 5-O-caffeoylquinic acid (chlorogenic acid), respectively.
Figure 1.
Typical HPLC-PDA chromatograms of aqueous 80% methanol extracts of leaves of rose at 360 nm for Si− uninoculated (A), Si+ uninoculated (B), Si− inoculated (C), and Si+ inoculated (D). Compounds are as follows: 1, unknown phenolic acid; 2, 3-O-caffeoylquinic acid (neochlorogenic acid); 3, 5-O-caffeoylquinic acid (chlorogenic acid); 4, quercetin-3-O-gentiobioside; 5, quercetin diglycoside; 6, quercetin derivative; 7, quercetin pentoside; 8, quercetin-3-O-galactoside (hyperoside); 9, quercetin-3-O-rutinoside (rutin); 10, quercetin-3-O-glucoside (isoquercitrin); 11, quercetin-3-O-arabinoside (avicularin); 12, quercetin-3-O-rhamnoside (quercitrin); 13, kaempferol-3-O-pentoside; and 14, kaempferol-3-O-rhamnoside (afzelin). The chromatographic conditions and the validation of the HPLC method are described in “Materials and Methods.” mAU, Milliabsorbance units.
Table I. LC-PDA-MS analysis (UV spectra, characteristic ions, and molecular masses) of phenolic acids and flavonoids in aqueous 80% methanol extracts of leaves of rose.
Data represent results from the analysis of samples from two independent experiments, each with three independent extractions. Compounds listed here were detected in all samples.
| Peak No.a | Rtb | LC-MS (Atmospheric Pressure Chemical Ionization, Negative Ion Mode) | HPLC-PDA, UV Spectra, λmax | Compound |
| min | m/z (% base peak) | nm | ||
| 1 | 10.5 | 347 [M–H]– (56), 301 (52), 139 (100) | 296sh,c 323 | Unknown phenolic acid |
| 2 | 12.4 | 353 [M–H]– (100), 191 (18), 179 (15) | 302sh, 329 | 3-O-Caffeoylquinic acid (neochlorogenic acid)d |
| 3 | 18.6 | 353 [M–H]– (100), 325 (29), 191 (19) | 298sh, 325 | 5-O-Caffeoylquinic acid (chlorogenic acid)d |
| 4 | 25.6 | 625 [M–H]– (100), 463 (28), 301 (9) | 253, 263sh, 353 | Quercetin-3-O-gentiobiosidee |
| 5 | 31.3 | 609 [M–H]– (100), 447 (21), 301 (8) | 265, 284sh, 348 | Quercetin diglycosideef |
| 6 | 41.8 | 615 [M–H]– (100), 493 (4), 463 (7), 441 (9), 301 (24) | 262, 291sh, 352 | Quercetin derivativef |
| 7 | 42.9 | 433 [M–H]– (7), 301 (100) | 255, 285sh, 348sh, 362 | Quercetin pentosidef |
| 8 | 44.7 | 463 [M–H]– (100), 301 (6) | 253, 299sh, 356sh, 364 | Quercetin-3-O-galactoside (hyperoside)d |
| 9 | 47.2 | 609 [M–H]– (100), 463 (67), 301 (19) | 256, 263sh, 298sh, 354 | Quercetin-3-O-rutinoside (rutin)d |
| 10 | 48.3 | 463 [M–H]– (100), 301 (22) | 256, 263sh, 299sh, 354 | Quercetin-3-O-glucoside (isoquercitrin)d |
| 11 | 54.7 | 433 [M–H]– (100), 301 (7) | 256, 263sh, 301sh, 352 | Quercetin-3-O-arabinoside (avicularin)d |
| 12 | 56.5 | 447 [M–H]– (100), 301 (10) | 256, 262sh, 305sh, 348 | Quercetin-3-O-rhamnoside (quercitrin)d |
| 13 | 63.5 | 417 [M–H]– (100), 285 (10) | 264, 294sh, 331sh, 345 | Kaempferol-3-O-pentosidef |
| 14 | 66.0 | 431 [M–H]– (100), 285 (9) | 263, 296sh, 324sh, 343 | Kaempferol-3-O-rhamnoside (afzelin)d |
Peak numbers correspond to the compound numbers in Figure 1 and Supplemental Figure S2.
Rt, Retention time on HPLC.
sh, Shoulder.
Conclusively identified by comparison with authentic standard.
Identification based on comparison of retention time, UV, and LC-MS data with data from the literature (Masada et al., 2009).
Tentatively identified by UV and mass spectral data.
Compounds 4 to 14 showed typical UV spectra of flavonol glycosides, which were also confirmed by their LC-MS data (Table I). Compounds 4 to 12 all gave an ion at m/z 301 corresponding to the aglycone quercetin. Compounds 8 and 10 showed a pseudomolecular ion [M–H]– at m/z 463, clearly indicating that these compounds are quercetin hexose molecules, and they were identified as quercetin-3-O-galactoside (hyperoside) and quercetin-3-O-glucoside (isoquercitrin), respectively. Compounds 4 and 9 showed pseudomolecular ions [M–H]– at m/z 625 and 609, respectively, as well as ions at m/z 463 and 301 corresponding to the loss of two Glc moieties in compound 4 and a Rha and Glc moiety in compound 9. Consequently, compounds 4 and 9 were identified as quercetin-3-O-gentiobioside and quercetin-3-O-rutinoside (rutin), respectively (Table I). Compound 11 was identified as quercetin-3-O-arabinoside (avicularin) based on its pseudomolecular ion [M–H]– at m/z 433, which clearly indicated that it was a quercetin pentose. The pseudomolecular ion [M–H]– at m/z 447 for compound 12 indicated that it was a quercetin methyl-pentose; thus, it was identified as quercetin-3-O-rhamnoside (quercitrin). Compounds 13 and 14 both gave an ion at m/z 285 corresponding to the aglycone kaempferol. Compound 14 showed a pseudomolecular ion [M–H] – at m/z 431, clearly indicating that it was a kaempferol methyl-pentose; thus, it was identified as kaempferol-3-O-rhamnoside (afzelin).
Effects of Si Application and Inoculation on the Concentration of Phenolic Acids
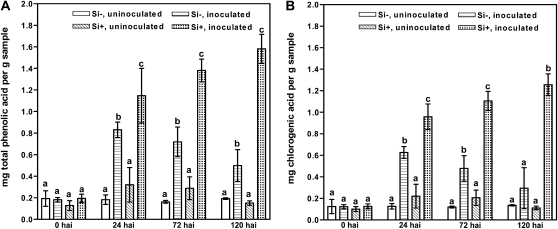
The concentrations of all phenolic acids were unaffected by Si treatment at 0 h after inoculation (hai). However, at 24 to 120 hai, the concentrations of total phenolic acids, chlorogenic acid, neochlorogenic acid, and an unknown phenolic acid increased in the inoculated Si+ and Si− compared with uninoculated Si− and Si+ leaves (Fig. 2; Supplemental Table S1). Furthermore, Si+ inoculated leaves had a higher level of phenolic acids than Si− inoculated leaves at 24 to 120 hai, except for an unknown phenolic acid at 24 and 72 hai. While the total concentration of total phenolic acids, chlorogenic acid, and neochlorogenic acid increased at 24 to 120 hai in Si+ inoculated leaves, the concentration of total phenolic acids and chlorogenic acid peaked at 24 hai in Si− inoculated leaves. For the uninoculated Si− and Si+ leaves, the concentrations of phenolic acids did not change over time, except for chlorogenic acid and neochlorogenic acid at 24 and 72 hai, respectively, with a higher level in Si+ uninoculated than in Si− uninoculated leaves. For all treatments and time points, chlorogenic acid occurred at the highest concentrations and made up more than 80% of the total phenolic acids in most cases (Supplemental Table S1).
Figure 2.
Contents of total phenolic acids (A) and 5-O-caffeoylquinic acid (chlorogenic acid; B) in leaf extracts of rose from plants either treated with (Si+) or without (Si−) Si followed by either inoculation with P. pannosa or no inoculation. Data represent results from one experiment, and each observation represents the mean from three extractions. All values are presented as means ± se. Means within each time point are comparable, and bars marked by different letters are significantly different. Further information on the results from this experiment is given in Supplemental Table S1. The findings of this experiment were confirmed in a second independent experiment.
Effects of Si Application and Inoculation on the Concentration of Flavonoids
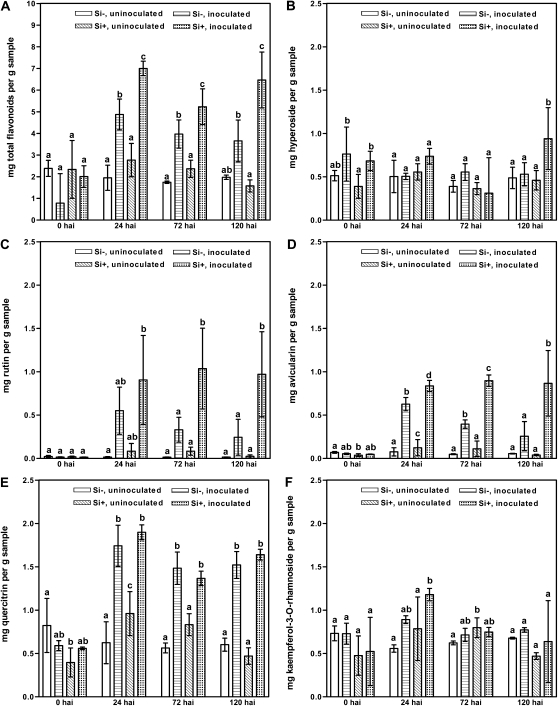
The total concentrations of all flavonoids were generally unaffected by Si treatment at 0 hai (Fig. 3; Supplemental Table S2). However, at 24 to 120 hai, significant changes in the concentrations of flavonoids occurred, depending on treatment and time point, except for a quercetin diglycoside (compound 5; Table I), where all treatments displayed a similar low content at all time points (Supplemental Table S2). For the other detected flavonoids, four main patterns were observed. (1) From 24 to 120 hai, the total content of flavonoids for Si+ inoculated leaves was higher than for Si− inoculated leaves, in which the concentration was higher than for both the uninoculated treatments. The same pattern was seen for a quercetin derivative (compound 6; Table I) at 24 hai (Supplemental Table S2). (2) For several of the detected flavonoids (e.g. avicularin and quercitrin), the inoculated leaves for both treatments had similar concentrations, especially at 24 and 72 hai, and these concentrations were higher than for the uninoculated leaves. (3) In several cases, especially at 120 hai, the concentrations in uninoculated Si+ and Si− leaves and inoculated Si− leaves did not differ, but the levels were lower than for the inoculated Si+ leaves (e.g. for hyperoside, rutin, and avicularin). (4) The levels of quercetin derivative, avicularin, quercitrin, and kaempferol-3-O-pentoside followed the order Si+ inoculated > Si− inoculated > Si+ uninoculated > Si− uninoculated. The concentration of total flavonoids and the 11 quantified flavonoids did not change over time (0–120 hai) for the uninoculated Si− and Si+ leaves, except that the levels of avicularin and quercitrin in Si+ uninoculated leaves were higher at 24 hai and that the quercetin derivatives, kaempferol-3-O-pentoside and afzelin, were higher at 72 hai compared with Si− uninoculated (Fig. 3F; Supplemental Table S2).
Figure 3.
Contents of total flavonoids and selected flavonoids in leaf extracts of rose from plants either treated with (Si+) or without (Si−) Si followed by either inoculation with P. pannosa or no inoculation. A, Total flavonoids. B, Quercetin-3-O-galactoside (hyperoside). C, Quercetin-3-O-rutinoside (rutin). D, Quercetin-3-O-arabinoside (avicularin). E, Quercetin-3-O-rhamnoside (quercitrin). F, Kaempferol-3-O-rhamnoside (afzelin). Data represent results from one experiment, and each observation represents the mean from three extractions. All values are presented as means ± se. Means within each time point are comparable, and bars marked by different letters are significantly different. Further information on the results from this experiment is given in Supplemental Table S2. The findings of this experiment were confirmed in a second independent experiment.
Disease Reduction Mediated by Si Application
Assessment of disease on the remaining inoculated plants from the metabolite experiment showed that Si+ plants had a disease severity score of 48.1% compared with 81.4% of Si− plants (P < 0.001). The Si application thus resulted in a 46% disease reduction (Supplemental Fig. S3).
Disease Reductions after Chlorogenic Acid and Rutin Treatment
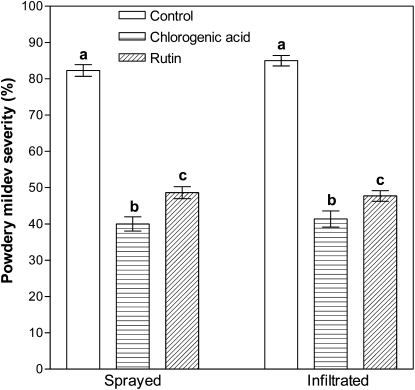
Treatment of roses either by spray application or leaf infiltration with 1 mg mL−1 chlorogenic acid or rutin resulted in an overall reduction in powdery mildew severity (P < 0.001) compared with their respective water-treated controls at 9 d after inoculation (dai; Fig. 4). The application of chlorogenic acid decreased the disease severity in both sprayed and infiltrated leaves by 51%. Likewise, rutin also reduced the disease severity following both treatments, although at slightly lower levels (41% for spraying and 44% for infiltration). For both types of treatments, chlorogenic acid was more effective than rutin (P < 0.001).
Figure 4.
Powdery mildew severity in leaves of rose after spraying or leaf infiltration with chlorogenic acid and rutin. Control plants were treated with water. Disease severity was scored 9 d after inoculation with P. pannosa. Data represent results from one experiment, and each observation represents the mean from 22 leaves. All values are presented as means ± se. Means within each application method are comparable, and bars marked with different letters are significantly different. The findings of this experiment were confirmed in a second independent experiment.
Table II shows results from the quantitative bright-field and epifluorescence microscopy study of the interaction at 72 hai after spraying or infiltration with chlorogenic acid, rutin, or water (control). The different infection steps are calculated based on the number of germinated conidia. The percentages of germinated conidia (having a primary germ tube) and of conidia forming appressoria were reduced by both spray application and infiltration of the phenolics compared with the water controls. On the other hand, penetration and formation of haustoria and elongating secondary hyphae (ESH) were not altered in plants treated with either phenolic compound. None of the host responses examined (formation of papillae and FEC) was affected by chlorogenic acid or rutin by either application method.
Table II. Quantitative recordings of infection biology of P. pannosa and defense responses in the fifth developed leaves of rose cv Smart.
Plants were treated with chlorogenic acid and rutin applied by spraying or leaf infiltration. Control plants were similarly treated with water. Observations were made at 72 hai, and values were calculated on the basis of the number of germinated conidia. Data represent results from one experiment, and each observation represents the mean from three leaves. All values are presented as means ± se. The findings of this experiment were confirmed in a second independent experiment.
| Application | Treatment |
Odds Ratioa |
||||
| Chlorogenic Acid | Rutin | Control | Chlorogenic Acid | Rutin | Control | |
| Sprayingb | ||||||
| Germinated | 15.3 ± 0.33 | 14.7 ± 0.33 | 25.3 ± 0.33 | 0.53*** | 0.51*** | 1.00 |
| With appressoria | 10.7 ± 0.33 | 10.2 ± 0.58 | 22.0 ± 0.58 | 0.43*** | 0.40*** | 1.00 |
| With haustoria | 8.7 ± 0.67 | 9.2 ± 0.33 | 13.3 ± 0.33 | 0.52NS | 0.56NS | 1.00 |
| With ESH | 8.7 ± 0.67 | 9.2 ± 0.33 | 13.3 ± 0.33 | 0.52NS | 0.56NS | 1.00 |
| With FEC | 8.0 ± 0.00 | 8.1 ± 0.00 | 7.9 ± 0.67 | 1.00NS | 1.02NS | 1.00 |
| With papillae | 8.7 ± 0.67 | 8.6 ± 0.33 | 9.3 ± 0.33 | 0.92NS | 0.93NS | 1.00 |
| Infiltrationc | ||||||
| Germinated | 15.3 ± 0.33 | 16.7 ± 0.33 | 24.7 ± 0.33 | 0.55*** | 0.60*** | 1.00 |
| With appressoria | 10.7 ± 0.33 | 11.9 ± 0.58 | 21.4 ± 0.67 | 0.44*** | 0.50*** | 1.00 |
| With haustoria | 8.0 ± 0.00 | 10.0 ± 0.00 | 11.9 ± 0.33 | 0.65NS | 0.82NS | 1.00 |
| With ESH | 8.0 ± 0.00 | 10.0 ± 0.00 | 11.9 ± 0.33 | 0.65NS | 0.82NS | 1.00 |
| With FEC | 9.3 ± 0.33 | 10.7 ± 0.33 | 9.4 ± 0.33 | 0.99NS | 1.15NS | 1.00 |
| With papillae | 9.3 ± 0.33 | 10.7 ± 0.33 | 10.0 ± 0.00 | 0.93NS | 1.07NS | 1.00 |
Odds ratio for comparison of treatments (control used as a reference; odds ratio = 1.00). NS, Nonsignificant difference; *** significant at P < 0.001; * significant at P < 0.05.
Sprayed with a solution (1 mg mL−1) of chlorogenic acid or rutin until runoff.
Infiltrated with a solution (1 mg mL−1) of chlorogenic acid or rutin.
Expression of Phenylpropanoid Pathway Genes
The expression of genes encoding the key enzymes phenylalanine ammonia lyase (PAL), cinnamyl alcohol dehydrogenase (CAD), and chalcone synthase (CHS) in the phenylpropanoid pathway were often affected both by Si application and powdery mildew inoculation (Table III). Compared with Si− uninoculated leaves, the transcript levels of PAL were elevated at 24 hai for the treatments Si− inoculated, Si+ uninoculated, and Si+ inoculated. However, at 72 hai, elevation of PAL transcript was only found for Si+ inoculated plants, with a 39-fold increase followed by a decrease to only a 3-fold up-regulation at 120 hai. For CHS, elevated transcript levels were seen for all three treatments at 24 and 72 hai. In contrast, accumulation of CHS transcript was only seen for the two Si+ treatments at 120 hai. The transcription of CAD followed a pattern differing markedly from the two other genes. Thus, CAD only showed elevated transcript levels in the Si+ inoculated plants at 24 and 72 hai, while levels were elevated for all three treatment at 0 hai (i.e. immediately after inoculation).
Table III. Quantitative real-time RT-PCR analysis of PAL, CHS, and CAD gene expression in leaves of rose from plants either treated with (Si+) or without (Si−) Si followed by either inoculation with P. pannosa or no inoculation.
Values shown represent fold up- or down-regulation in Si− inoculated, Si+ uninoculated, and Si+ inoculated plants relative to Si− uninoculated plants (relative expression ratio = 1) at each time point, after normalization of all treatments to 18S rRNA. Data represent results from one experiment, and each observation represents the mean from three extractions. All values are presented as means ± se. The findings of this experiment were confirmed in a second independent experiment. * Significant change; ns, nonsignificant change.
| Genes | Si Supply | Pathogen | Fold Change |
|||
| 0 hai | 24 hai | 72 hai | 120 hai | |||
| PAL | Si− | Uninoculated | 1.0 | 1.0 | 1.0 | 1.0 |
| Si− | Inoculated | −1.7 ± 0.17ns | 3.7 ± 1.25* | 1.5 ± 0.17ns | 1.1 ± 0.40ns | |
| Si+ | Uninoculated | −1.6 ± 0.14* | 2.6 ± 0.60* | 1.2 ± 0.27ns | −1.0 ± 0.25ns | |
| Si+ | Inoculated | −1.4 ± 0.11* | 5.2 ± 1.49* | 39.3 ± 11.6* | 3.0 ± 0.58* | |
| CHS | Si− | Uninoculated | 1.0 | 1.0 | 1.0 | 1.0 |
| Si− | Inoculated | 1.3 ± 0.46ns | 2.8 ± 1.06* | 1.5 ± 0.30* | 1.2 ± 0.14ns | |
| Si+ | Uninoculated | −1.4 ± 0.24* | 2.6 ± 0.55* | 2.6 ± 0.30* | 2.2 ± 0.15* | |
| Si+ | Inoculated | −1.0 ± 0.32ns | 4.5 ± 0.45* | 2.7 ± 0.38* | 2.1 ± 0.36* | |
| CAD | Si− | Uninoculated | 1.0 | 1.0 | 1.0 | 1.0 |
| Si− | Inoculated | 1.3 ± 0.21* | 1.6 ± 0.59ns | −1.8 ± 0.04ns | −1.2 ± 0.23* | |
| Si+ | Uninoculated | 2.1 ± 0.40* | −0.3 ± 0.26ns | −1.1 ± 0.12ns | 1.1 ± 0.39ns | |
| Si+ | Inoculated | 1.2 ± 0.28* | 2.0 ± 0.17* | 2.4 ± 0.55* | 2.5 ± 0.40ns | |
DISCUSSION
Active Role of Si in Disease Resistance of Rose: Induction of the Production of Antifungal Phenolics against Powdery Mildew Infection
This study provides evidence that root application of 3.6 mm Si+ to the miniature rose cv Smart increases the concentration of phenolic acids and flavonoids in response to P. pannosa infection, some of which, to our knowledge, have not been reported in roses before. This was accompanied by an increased expression of genes encoding enzymes in the phenylpropanoid pathway. This was particularly prominent in Si+ inoculated plants, but there were also elevated transcript levels in Si− inoculated plants. Thus, according to the definition of Ghareeb et al. (2011), most of the responses observed represent induced resistance. However, the contents of phenolics and flavonoids represent priming, since Si+ uninoculated and Si− uninoculated plants were not different. The level of potassium was different between the two nutrient solutions, due to the extra potassium present in SiKal compared with the control solution. It was only possible to partly compensate for the extra potassium present in the 3.6 mm Si treatment without affecting other nutrients. Therefore, it cannot be ruled out that the extra potassium potentially could have some influence on the level of disease, but this appears less important compared with the effect of Si. Thus, there was a very clear increase in Si content in the leaves where the pathogen was inhibited (Shetty et al., 2011). Furthermore, preliminary experiments showed that the content of other nutrients in rose leaves, including potassium, was not significantly different between plants receiving 0 or 3.6 mm Si (data not shown).
All phenylpropanoids are derived from cinnamic acid, which is formed from Phe by the action of PAL. PAL is the branch-point enzyme between primary metabolism and the branch of secondary metabolism leading to the phenylpropanoid pathway, which is considered to be one of the most important metabolic pathways due to its responsibility for the synthesis of a large range of secondary metabolites, including phenolic acids and flavonoids (Dixon and Paiva, 1995). CAD catalyzes the final step in a branch of phenylpropanoid synthesis specific for the production of lignin monomers, and an increased expression of this enzyme could indicate increased lignification (Walter et al., 1988), which, however, was not observed in the rose-P. pannosa interaction. A large number of stress-induced phenylpropanoids are derived from the C15 flavonoid skeleton, which is biosynthesized via CHS, the key enzyme in the flavonoid branch of the phenylpropanoid pathway, catalyzing the production of tetrahydroxychalcone, the precursor of all flavonoids (Dixon and Paiva, 1995; Winkel-Shirley, 2001).
Many plant phenolics can function as passive or inducible barriers against pathogens, and it is well known that, for example, the content of flavonoids can increase or the flavonoid composition can change in response to pathogen attack. However, the involvement of flavonoids in plant defense depends on the species (Dixon and Paiva, 1995; Carlsen et al., 2008). Initial screening of methanol extracts by HPLC and LC-MS from Si-treated rose leaves both with and without powdery mildew inoculation revealed that the contents of phenolic acids and flavonol glycosides were clearly affected, whereas the contents of flavan-3-ols (proanthocyanidins), which are known to play a role in defense in some plants (Miranda et al., 2007; Koskimäki et al., 2009), were not significantly affected. Consequently, the focus in this investigation was on the changes in the contents of phenolic acids and flavonol glycosides. Chlorogenic acid, neochlorogenic acid, and an unknown phenolic acid were detected in rose leaves. The two identified phenolic acids are well-known constituents in aerial parts of many plant species (Christensen et al., 2008; Grevsen et al., 2008; Schmitzer et al., 2009). However, neochlorogenic acid has, to the best of our knowledge, not previously been reported as a constituent in the aerial parts of roses. Chlorogenic acid was the phenolic acid present in the highest concentration, with an increase of more than 80% in Si+ inoculated compared with the Si− uninoculated leaves.
Flavonol glycosides like quercetin and kaempferol are well-known constituents of rose species, and the flavonoids identified in this investigation (Table I) have all previously been detected in rose species (Biolley et al., 1994a, 1994b; Helsper et al., 2003; Kumar et al., 2009; Schmitzer et al., 2009), except for quercetin-3-O-gentiobioside. Among the 11 quantified flavonoids, rutin and quercitrin occurred in the highest concentrations, with rutin increasing more than 20-fold in Si+ inoculated compared with Si− uninoculated plants (Fig. 3C; Supplemental Table S2).
Antimicrobial Activity of Major Phenolics in Rose
The substantial increase in the contents of phenolic acids and flavonoids in Si+ inoculated leaves correlated with a 46% reduction in disease severity compared with Si− inoculated leaves (Supplemental Fig. S3). In order to elucidate whether the identified phenolics could help explain the Si-mediated protection, we tested the ability of chlorogenic acid and rutin to reduce powdery mildew development in roses, as these secondary metabolites were among the phenolics that were detected in the highest amounts in Si+ inoculated leaves. Both rutin and chlorogenic acid had an antimicrobial effect on P. pannosa when applied to leaves, reducing disease severity by 40% to 50% in planta. Interestingly, both spray application and leaf infiltration gave comparable disease reductions (Fig. 4). Since germination of conidia as well as the ability of conidia to form appressoria were reduced to the same extent by chlorogenic acid and rutin following both application methods, it appears that these two phenolics and perhaps other phenolics as well can be transported from the cell lumen to the epidermis to act as antimicrobial compounds against P. pannosa. In accordance with this, von Röpenack et al. (1998) also suggested that the phenolic conjugate p-coumaroyl-hydroxyagmatine was transported in vesicles in barley (Hordeum vulgare) leaves to the sites of attempted penetration by Blumeria graminis f. sp. hordei. An antimicrobial effect of phenolics is also in accordance with the increased amounts of chlorogenic acid and rutin as well as other phenolics observed in Si− inoculated compared with Si− uninoculated rose leaves (Figs. 2 and 3; Supplemental Tables S1 and S2). Phenolic acids and flavonol glycosides have also been shown to play an important role in the defense strategy of other plant species against pathogens. For example, in apple (Malus domestica) leaves and fruits infected with Venturia inaequalis, it has been shown that the content of phenolic acids (e.g. chlorogenic acid), flavonol glycosides (e.g. rutin, quercitrin, and isoquercitrin), and flavan-3-ols increased significantly in infected leaves compared with healthy tissues (Petkovšek et al., 2008, 2009). Chlorogenic acid has also been shown to play a major role in relation to scab resistance in potato (Solanum tuberosum) caused by Streptomyces scabies (Johnson and Schaal, 1952), and rutin and other flavonols showed significant antifungal activity against the fungi Cylindrocarpon destructans, Phytophthora megasperma, and Verticillium dahliae attacking olive trees (Olea europaea); therefore, rutin and other flavonols are believed to play a major role in plant defense of olive plants (Báidez et al., 2006, 2007). A characteristic for the most widespread phenolic acids and flavonols is that they are not induced following infection (i.e. they do not act as phytoalexins but are considered phytoanticipins, which are preformed antifungal compounds, present in different amounts, that may undergo postinfection increases following infection; Harborne, 1999). In accordance with this, application of the compounds reduced prepenetration growth of P. pannosa but not the frequencies of fungal developmental stages or host defense responses after penetration.
Expression of Key Phenylpropanoid Pathway Genes Is Altered by Si Application and by Powdery Mildew Infection
Like the transcriptomic analysis of the wheat-B. graminis and the Arabidopsis-Golovinomyces cichoracearum pathosystems (Fauteux et al., 2005; Chain et al., 2009), we found an up-regulation of genes involved in secondary metabolism in the rose-P. pannosa pathosystem. However, neither Chain et al. (2009) nor Fauteux et al. (2005) were able to identify the key secondary metabolite genes or suggest a function of secondary metabolite genes in their pathosystems. Transcriptomic analysis of Si effects in wheat, Arabidopsis, rice, and tomato additionally indicated that Si had a limited role on the transcriptome in the absence of stress induced by pathogen inoculation (Watanabe et al., 2004; Fauteux et al., 2005; Chain et al., 2009; Ghareeb et al., 2011). In contrast, by using quantitative real-time reverse transcription (RT)-PCR, we demonstrated that the transcript levels for PAL, CAD, and CHS were often elevated by Si application compared with Si− uninoculated plants (Table III). Furthermore, in several cases, the Si+ uninoculated plants had an increased content of phenolics compared with the uninoculated controls, thus substantiating the gene expression results that Si primarily induces resistance in rose plants against powdery mildew. The discrepancy between our findings and those from the Arabidopsis and wheat transcriptomic analyses could reflect differences in experimental design. Thus, in the investigations of Arabidopsis and wheat (Fauteux et al., 2005; Chain et al., 2009), leaves of different physiological age were pooled for RNA extraction, whereas we only analyzed the fifth developed leaves.
PAL and CHS Gene Expression: Possible Correlations to the Biosynthesis of Phenolic Acids and Flavonoids in Rose
We found a clear correlation between the elevated PAL and CHS transcript levels and an increased biosynthesis of phenolics in rose leaves, especially for both the Si+ treatments. However, when comparing the up-regulation of PAL and CHS at specific time points after inoculation (24, 72, and 120 hai) and the amounts of phenolics produced, some discrepancies were revealed. In particular, the down-regulation of PAL at 120 hai compared with 72 hai for the Si+ inoculated treatment (Table III) is not reflected in a decreased production of phenolic acids from 72 to 120 hai. In fact, the total production of phenolic acids increased from 1.38 mg g−1 sample at 72 hai to 1.58 mg g−1 sample at 120 hai (Fig. 2A; Supplemental Table S1). Therefore, a decrease in the concentration of phenolic acids at 72 to 120 hai would have been expected to result from the down-regulation of PAL during this time interval. A possible explanation for this could be that the down-regulation of PAL only occurs at 72 to 120 hai. Thus, the genes coding for the production of specific enzymes involved in the biosynthesis (e.g. cinnamate 4-hydroxylase, p-coumarate:CoA ligase) are not affected or are down-regulated after 72 hai. Alternatively, the levels of PAL are kept high (i.e. there is no need to keep transcribing the gene if the enzyme is still present). Simple phenolic acid derivatives such as p-coumaroyl CoA is the shikimic acid-derived starting unit in the biosynthesis of flavonoids (Dixon and Paiva, 1995; Winkel-Shirley, 2001). A change in the biosynthesis of flavonoids, therefore, may affect the pool of phenolic acids and hence the content of phenolic acids.
The up-regulation of CHS at 24 to 120 hai compared with 0 hai in the Si+ inoculated treatment is consistent with an increase in the amounts of most flavonol glycosides in rose leaves at these time points (Fig. 3; Supplemental Table S2). However, in the Si− inoculated rose leaves, there appeared to be a trend, although not significant, to a decrease in the amounts of flavonol glycosides at 24 to 120 hai (Fig. 3A; Supplemental Table S2), which is also in accordance with the down-regulation of CHS observed at 24 to 120 hai (Table III). Furthermore, the up-regulation of CHS in the Si+ uninoculated plants resulted in an increase in the content of individual flavonoids, such as avicularin and quercitrin, but not in the total amounts of flavonol glycosides (Fig. 3, D and E; Supplemental Table S2). Finally, it is interesting that rutin, isoquercitrin, avicularin, and quercitrin have almost the same concentration profiles after P. pannosa infection and in Si+ and Si− plants at the different time points (Fig. 3, C and D; Supplemental Table S2). This clearly indicates some correlation between the biosynthesis of these closely related flavonoids in response to P. pannosa infection of rose leaves. Our results here also demonstrate that specific genes that encode flavonoid enzymes involved in the biosynthesis of specific flavonoids (Dixon and Paiva, 1995; Winkel-Shirley, 2001) are expressed differently after Si application and infection with powdery mildew, which explains why some flavonoids are produced in much higher amounts compared with others in the different treatments (Fig. 3; Supplemental Table S2). Therefore, it would be interesting to investigate the expression of specific genes involved in the biosynthesis of antimicrobial phenolics in more detail in order to understand the fundamental mechanisms of disease resistance of rose plants against powdery mildew and antimicrobial defense mechanisms in general. Hodson et al. (2005) found Si uptake in a number of different plants, and the Si concentration varied among these species. The relationship between function and level of Si uptake in the investigated species is not fully understood. Based on the results of this study, it could be interesting to investigate whether Si also plays a role in the induced resistance of other horticultural species, but also wild-type plants in natural settings, and whether there is a correlation between Si uptake and defense against pathogens.
In conclusion, this study has demonstrated that the accumulation of fungitoxic phenolic compounds, in particular chlorogenic acid and rutin, was stimulated by Si application. Exogenous application of these phenolic compounds to rose plants enhanced resistance against powdery mildew. Thus, Si plays an important and active role in stimulating the antimicrobial defense of roses.
MATERIALS AND METHODS
Plants and Treatment with Si
The miniature potted rose (Rosa hybrida ‘Smart’), which is highly susceptible to powdery mildew, was obtained from Aarhus University, Department of Horticulture. Roses were propagated, maintained, and treated with Si as described by Shetty et al. (2011).
Soluble Si was supplied in the nutrient solution at a concentration of 3.6 mm Si from SiKal (9.1% Si and 25.5% potassium as potassium metasilicate [K2SiO3]; Yara Industries), as described by Shetty et al. (2011). After the propagation period (4 weeks), plants were moved to the growth chamber and watered for the first time with the Si+ or Si− solution. Plants were subsequently watered with the two nutrient solutions every 72 h until disease scoring, a total of 10 times. After 3 weeks in the growth chamber, half of the plants in each of the two groups (Si+ and Si−) were inoculated with the pathogen and denoted Si+ inoculated and Si− inoculated, respectively. The remaining plants from each group were not inoculated and denoted Si+ uninoculated and Si− uninoculated, respectively.
Inoculation with Podosphaera pannosa
Inoculum of P. pannosa was produced and inoculation took place as described by Shetty et al. (2011). The fifth developed leaves of 7-week-old plants were inoculated and denoted Si+ inoculated and Si− inoculated. Fifth leaves of Si+ uninoculated and Si− uninoculated plants were similarly marked at the same time point.
For all investigations of metabolites and gene expression, two independent experiments were carried out, each comprising a total of 168 plants. In each experiment, 88 plants were inoculated (44 Si+ and 44 Si−) and 80 plants were left uninoculated (40 Si+ and 40 Si−). Leaves from 160 plants (80 inoculated and 80 uninoculated) were sampled for further analyses as described below, and eight plants were used for disease assessment 9 dai as described below.
Sampling of Plant Material for Extraction of Metabolites and RNA
From each of the two experiments, leaves of Si+ and Si− plants, inoculated with P. pannosa or left uninoculated, were sampled for extraction of polyphenols (phenolic acids and flavonoids). Marked leaves from 20 plants of each of the four treatments were harvested at 0, 24, 48, and 120 h, ground in liquid nitrogen, and split into two portions. Approximately 0.2 g of the ground material was immediately stored at −80°C for RNA extraction. Another 2 g of ground plant material was freeze dried and stored at −80°C for extraction of polyphenols.
Extraction of Plant Material for Metabolite Analyses
Ground samples of freeze-dried rose leaves were extracted with 8 mL of aqueous 80% methanol in a centrifuge tube with lid and placed in an orbital shaker (200 rpm). From each of the two independent experiments, three independent extractions were carried out (0.4 g; 0.1 mm or less particle size) in darkness for 90 min at room temperature (22°C). After the extraction, the samples were centrifuged for 10 min using a Sorvall SA-600 head (maximum centrifugal force = 20.845; Buch & Holm), and the supernatant was collected and stored at −20°C until analysis. The samples were filtered through a nylon 0.45-μm Cameo 25P syringe filter (Bie & Berntsen) before analysis by HPLC and LC-electrospray ionization-MS/MS for phenolic acids and flavonoids. The efficiency and reproducibility of the extraction procedure described above were determined by duplicate extractions (2 × 8 mL of 80% methanol or 2 × 8 mL of 90% methanol). This showed that extraction by 1 × 8 mL of 80% methanol was reproducible (coefficient of variation < 5%) and ensured the extraction of more than 95% of the total flavonoids and phenolic acids in the samples. For determination of the efficiency of the extraction method, the extract samples were centrifuged between each extraction and the supernatant was collected and analyzed.
Flavonoid and Phenolic Acid Standards
Quercetin-3-O-galactoside (hyperoside), quercetin-3-O-glucoside (isoquercitrin), quercetin-3-O-rhamnoside (quercitrin), kaempferol-3-O-rhamnoside (afzelin), and 3-O-caffeoylquinic acid were purchased from Extrasynthese, and quercetin-3-O-arabinoside (avicularin) was purchased from Phytolab. Quercetin-3-O-rutinoside (rutin) and 5-O-caffeoylquinic acid (chlorogenic acid) were purchased from Sigma-Aldrich.
Identification and Quantification of Phenolic Acids and Flavonoids in Extracts
Phenolic constituents in extracts of rose leaves of cv Smart were determined by HPLC combined with photodiode array (PDA) detection and LC-electrospray ionization-MS/MS (Table I). LC-MS data were obtained using an LTQ XL Linear Ion Trap Mass Spectrometer (Thermo Scientific) equipped with a PDA detector and an evaporative light-scattering detector (elsd; Sedex 80LT; SEDERE). Settings for the Elsd were 50°C for temperature and 3.7 bar for nitrogen pressure. Settings for the mass spectrometer fitted with the atmospheric pressure chemical ionization source operated in negative mode were 40, 10, and 0 (arbitrary units) for sheath, auxiliary, and sweep gas flow rates, respectively. Discharge voltage and current were 1.13 kV and 5.38 mA, respectively. Vaporizer temperature was 400°C, capillary temperature was 250°C, capillary voltage was −16.3 V, tube lens was 100 V, and automatic gain control target settings were 3 × 104 for full MS. Separations were performed on a Zorbax Eclipse XDB-C18 column (5 μm, 150 × 4.6 mm; Agilent) with the following solvents: solvent A = 0.1% formic acid (HPLC grade, purity of 99%; Sigma-Aldrich) in water, solvent B = 0.1% formic acid in acetonitrile (HPLC grade; Fisher Scientific). The solvent gradient was 0 to 5 min, isocratic 5% B; 5 to 65 min, linear gradient from 5% to 20% B; 65 to 75 min, linear gradient from 20% to 100% B; 75 to 80 min, isocratic 100% B; 80 to 85 min, linear gradient from 100% to 5% B; 85 to 90 min, isocratic 5% B. Flow was 0.8 L min−1, column temperature was 35°C, and injection volume was 10 μL. The flow was split 50:50 (Elsd detector:MS detector) at exit of the PDA detector.
Phenolic acids and flavonoids were quantified in extracts by HPLC-PDA on an Agilent 1100 HPLC system (Agilent Technologies). The phenolic acids and flavonoids were monitored at 320 and 360 nm, and UV spectra were recorded from 210 to 600 nm. Separations were performed under the same HPLC conditions as used for LC-MS analyses, thus making comparison of chromatograms and spectra completely reliable. Flavonoids and phenolic acids were determined in extracts from external calibration curves of rutin and chlorogenic acid, respectively. Mr correction factors were taken into account in the quantification of the individual polyphenols. Mean recovery rates (approximate accuracy) for chlorogenic acid and rutin were more than 98%, with a relative sd of less than 5%, and were determined by spiking a known amount of authentic standards of chlorogenic acid and rutin, respectively, to rose leaf extract samples. The precision of the HPLC method was determined by four injections of a rose leaf extract sample on the same day (intraday variation) and on four different days (interday variation). The overall intraday and interday variations were found to be less than 5% for both flavonoids and phenolic acids.
RNA Extraction and Quantitative Real-Time RT-PCR Analysis
Total RNA was extracted from 150 mg of homogenized plant tissue using the Ambion RNAqueous kit with plant RNA Isolation Aid added (Applied Biosystems) following the manufacturer’s protocol. Removal of genomic DNA and cDNA synthesis were carried out as described by Shetty et al. (2009). The 18S rRNA gene was used as a reference gene (Shimada et al., 2003). Primer design and testing as well as quantitative RT-PCR were carried out as described by Bedini et al. (2005). The following primers were used: for 18S rRNA, forward, 5′-CGGCTACCACATCCAAGGAA-3′, and reverse, 5′-GCTGGAATTACCGCGGCT-3′; for PAL, forward, 5′-TCCTGACTGGCGAAAAGTTC-3′, and reverse, 5′-GAAGAGGTTCACCGTTCCAA-3′; for CHS, forward, 5′-ACAGCAACTCCTCCCAACTG-3′, and reverse, 5′-CGCTGGAATTTCTCCTTGAG-3′; for CAD, forward, 5′-AGGACGGAGGAGGCTAGGTTA-3′, and reverse, 5′-ATGGCATGGGTTACTTCAGC-3′.
Effects of Chlorogenic Acid and Rutin on Disease Severity and Infection Biology
In two separate, independent experiments, the inhibitory activities of chlorogenic acid and rutin on P. pannosa were tested in rose leaves following either spray application or leaf infiltration of the compounds. Each compound was dissolved in water (1 mg mL−1) using a sonicator (Barson Sonifier 250; Buch & Holm). Each experiment comprised a total of 36 plants. The fifth developed leaves (7-week-old plants) were labeled and sprayed with one of the compounds until runoff (six plants for each compound), whereas another set of plants were infiltrated with the compounds (six plants for each compound) using a Hagborg device (Hagborg, 1970). Water-sprayed or infiltrated plants served as controls (six plants for each compound). Plants were inoculated with P. pannosa and incubated as described above. Disease severity (percentage coverage with powdery mildew) was determined using a stereomicroscope at 9 dai. For each treatment, 22 leaflets were used to calculate the mean disease severity.
Infection biology and defense responses were compared between chlorogenic acid- and rutin-treated leaves (spray and infiltration) and their respective water-treated controls. Three leaves from each treatment were collected at 72 hai, cleared, and examined using bright-field and epifluorescence microscopy as described by Shetty et al. (2003). The number of nongerminated conidia was recorded on each leaf; subsequently, the development of 50 randomly chosen germinated conidia was studied on each leaf (a total of 150 conidia per treatment and time point). For each conidium, it was recorded whether it formed a germ tube, formed appressoria, caused penetration, caused the formation of single or multiple FEC and papillae at penetration sites, and whether ESH formed. Penetration was considered to occur when a haustorium or a FEC developed from a conidium, or rather an appressorium. FEC and papillae were considered to stop infection when no ESH developed from germlings where these responses occurred.
Statistical Analyses
Data from metabolite quantification activity assays and studies of disease severity represent continuous variables and were analyzed by ANOVA assuming a normal distribution. Variances were stabilized by appropriate transformation of data if necessary.
For gene expression studies, statistical evaluations of the relative expression levels of the target genes were performed for Si− inoculated, Si+ uninoculated, and Si+ inoculated plants compared with Si− uninoculated plants at each time point and normalized to the 18S rRNA expression level. The analyses were performed using the relative expression software tool REST as described by Pfaffl et al. (2002).
Data from studies of infection biology represent discrete variables, since it was recorded whether a certain event took place (e.g. whether a conidium germinated or not, whether a germinated conidium formed appressoria, and whether appressoria with successful penetration formed haustoria or not). Consequently, these data were analyzed by logistic regression assuming a binomial distribution (corrected for overdispersion when present; Collett, 1991). For comparison of variables (percentages), odds ratios (Collett, 1991) were calculated using control (Si−) plants as a reference (odds ratio = 1.00).
All data were analyzed by PC-SAS (release 9.2; SAS Institute), and hypotheses were rejected at P < 0.05. All experiments were performed twice. Statistical tests were performed to ensure that the individual experiments gave the overall same conclusions. Because similar, but not identical, results were obtained, only results from one of the experiments are presented. Throughout the article, all differences are significant unless specifically mentioned.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Chemical structures of identified phenolic acids and flavonoids in leaves of rose in this study.
Supplemental Figure S2. Typical HPLC-PDA chromatograms of aqueous 80% methanol extracts of leaves of rose at 320 nm for the treatments Si− uninoculated, Si+ uninoculated, Si− inoculated, and Si+ inoculated.
Supplemental Figure S3. Comparison of powdery mildew severity at 9 dai in the fifth developed leaf (of every branch) of cv Smart (highly susceptible) either treated with 3.6 mm Si (Si+) or 0 mm (Si−) Si.
Supplemental Table S1. Contents of total phenolic acids and selected phenolic acids in leaves of rose for the treatments Si− uninoculated, Si+ uninoculated, Si− inoculated, and Si+ inoculated.
Supplemental Table S2. Contents of total flavonoids and selected flavonoids in leaves of rose for the treatments Si− uninoculated, Si+ uninoculated, Si− inoculated, and Si+ inoculated.
Acknowledgments
We thank Yara Industries for providing the Si product SiKal, Kurt Dahl and Theo Bølsterli for help in propagating and maintaining the rose plants, and Kim Guldberg Vitten for technical assistance.
References
- Báidez AG, Gómez P, Del Río JA, Ortuño A. (2006) Antifungal capacity of major phenolic compounds of Olea europaea L. against Phytophthora megasperma Drechsler and Cylindrocarpon destructans (Zinssm.) Scholten. Physiol Mol Plant Pathol 69: 224–229 [Google Scholar]
- Báidez AG, Gómez P, Del Río JA, Ortuño A. (2007) Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb.: role of phenolic compounds in plant defense mechanism. J Agric Food Chem 55: 3373–3377 [DOI] [PubMed] [Google Scholar]
- Barz W, Bless W, Borger-Papendorf G, Gunia G, Mackenbock V, Meier D, Otto C, Super E. (1990) Phytoalexins as part of induced defense mechanisms in plants: their elicitation, function and metabolism. Chadwick DJ, Marsh J, , Bioactive Compounds from Plants. John Wiley & Sons, New York, pp 140–156 [DOI] [PubMed] [Google Scholar]
- Bedini E, De Castro C, Erbs G, Mangoni L, Dow JM, Newman M-A, Parrilli M, Unverzagt C. (2005) Structure-dependent modulation of a pathogen response in plants by synthetic O-antigen polysaccharides. J Am Chem Soc 127: 2414–2416 [DOI] [PubMed] [Google Scholar]
- Bélanger RR, Benhamou N, Menzies JG. (2003) Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f.sp. tritici). Phytopathology 93: 402–412 [DOI] [PubMed] [Google Scholar]
- Biolley J-P, Jay M, Viricel M-R. (1994a) Flavonoid diversity and metabolism in 100 Rosa × hybrida cultivars. Phytochemistry 35: 413–419 [Google Scholar]
- Biolley J-P, Jay M, Viricel M-R. (1994b) Pigmentation patterns of modern rose mutants throw light on the flavonoid pathway in Rosa × hybrida. Phytochemistry 36: 1189–1196 [Google Scholar]
- Carlsen SCK, Understrup A, Fomsgaard IS, Mortensen AG, Ravnskov S. (2008) Flavonoids in roots of white clover: interaction of arbuscular mycorrhizal fungi and a pathogenic fungus. Plant Soil 302: 33–43 [Google Scholar]
- Chain F, Côté-Beaulieu C, Belzile F, Menzies JG, Bélanger RR. (2009) A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol Plant Microbe Interact 22: 1323–1330 [DOI] [PubMed] [Google Scholar]
- Christensen LP, Kaack K, Fretté XC. (2008) Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur Food Res Technol 227: 293–305 [Google Scholar]
- Collett D. (1991) Modelling Binary Data. Chapman & Hall, London [Google Scholar]
- de Ascensao ARFDC, Dubery IA. (2003) Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 63: 679–686 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Achnine L, Kota P, Liu C-J, Reddy MSS, Wang L. (2002) The phenylpropanoid pathway and plant defence: a genomics perspective. Mol Plant Pathol 3: 371–390 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7: 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken C. (2005) A review of biological control of rose powdery mildew (Spaerotheca pannosa var. rosae) by fungal antagonists. Acta Hortic 690: 193–196 [Google Scholar]
- Epstein E. (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci USA 91: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR. (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett 249: 1–6 [DOI] [PubMed] [Google Scholar]
- Fawe A, Abou-Zaid M, Menzies JG, Bélanger RR. (1998) Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 88: 396–401 [DOI] [PubMed] [Google Scholar]
- Ghareeb H, Bozsó Z, Ott GP, Repenning C, Stahl F, Wydra K. (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75: 83–89 [Google Scholar]
- Grevsen K, Fretté XC, Christensen LP. (2008) Concentration and composition of flavonol glycosides and phenolic acids in aerial parts of stinging nettle (Urtica dioica L.) are affected by high nitrogen fertilization and by harvest time. Eur J Hortic Sci 73: 20–27 [Google Scholar]
- Hagborg WAF. (1970) A device for injecting solutions and suspensions into thin leaves of plants. Can J Bot 48: 1135–1136 [Google Scholar]
- Harborne JB. (1999) The comparative biochemistry of phytoalexin induction in plants. Biochem Syst Ecol 27: 335–367 [Google Scholar]
- Helsper JPFG, de Vos CHR, Maas FM, Jonker HH, Van Den Broeck HC, Jordi W, Pot CS, Keizer LCP, Schapendonk AHCM. (2003) Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol Plant 117: 171–178 [Google Scholar]
- Hodson MJ, White PJ, Mead A, Broadley MR. (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot (Lond) 96: 1027–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst RK. (1983) Compendium of Rose Diseases. American Phytopathology Press, St. Paul [Google Scholar]
- Johnson G, Schaal LA. (1952) Relation of chlorogenic acid to scab resistance in potatoes. Science 115: 627–629 [DOI] [PubMed] [Google Scholar]
- Koskimäki JJ, Hokkanen J, Jaakola L, Suorsa M, Tolonen A, Mattila S, Pirttilä AM, Hohtola A. (2009) Flavonoid biosynthesis and degradation play a role in early defence responses of bilberry (Vaccinium myrtillus) against biotic stress. Eur J Plant Pathol 125: 629–640 [Google Scholar]
- Kumar N, Bhandari P, Singh B, Bari SS. (2009) Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem Toxicol 47: 361–367 [DOI] [PubMed] [Google Scholar]
- Ma JF. (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50: 11–18 [Google Scholar]
- Ma JF, Takahashi E. (2002) Soil, Fertilizer, and Plant Silicon Research in Japan. Elsevier Science, Amsterdam [Google Scholar]
- Ma JF, Yamaji N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11: 392–397 [DOI] [PubMed] [Google Scholar]
- Masada S, Terasaka K, Oguchi Y, Okazaki S, Mizushima T, Mizukami H. (2009) Functional and structural characterization of a flavonoid glucoside 1,6-glucosyltransferase from Catharanthus roseus. Plant Cell Physiol 50: 1401–1415 [DOI] [PubMed] [Google Scholar]
- Miranda M, Ralph SG, Mellway R, White R, Heath MC, Bohlmann J, Constabel CP. (2007) The transcriptional response of hybrid poplar (Populus trichocarpa × P. deltoides) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol Plant Microbe Interact 20: 816–831 [DOI] [PubMed] [Google Scholar]
- Osbourn AE. (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8: 1821–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton HB, Kelly JW, Ferare J. (2003) Pot rose production. Roberts A, Debener T, Gudin S, , Encyclopedia of Rose Science. Elsevier Science, Oxford, pp 587–593 [Google Scholar]
- Petkovšek MM, Štampar F, Veberič R. (2008) Increased phenolic content in apple leaves infected with the apple scab pathogen. J Plant Pathol 90: 49–55 [Google Scholar]
- Petkovšek MM, Štampar F, Veberič R. (2009) Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol Mol Plant Pathol 74: 60–67 [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. (2002) Relative Expression Software Tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rémus-Borel W, Menzies JG, Bélanger RR. (2005) Silicon induces antifungal compounds in powdery mildew-infected wheat. Physiol Mol Plant Pathol 66: 108–115 [Google Scholar]
- Rodrigues FA, Datnoff LE, Korndorfer GH, Seebold KW, Rush MC. (2001) Effect of silicon and host resistance on sheath blight development in rice. Plant Dis 85: 827–832 [DOI] [PubMed] [Google Scholar]
- Rodrigues FA, McNally DJ, Datnoff LE, Jones JB, Labbé C, Benhamou N, Menzies JG, Bélanger RR. (2004) Silicon enhances the accumulation of diterpenoid phytoalexins in rice: a potential mechanism for blast resistance. Phytopathology 94: 177–183 [DOI] [PubMed] [Google Scholar]
- Schmitzer V, Veberic R, Osterc G, Stampar F. (2009) Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J Am Soc Hortic Sci 134: 491–496 [Google Scholar]
- Shetty NP, Jensen JD, Knudsen A, Finnie C, Geshi N, Blennow A, Collinge DB, Jørgensen HJL. (2009) Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat. J Exp Bot 60: 4287–4300 [DOI] [PubMed] [Google Scholar]
- Shetty NP, Kristensen BK, Newman M-A, Møller K, Gregersen PL, Jørgensen HJL. (2003) Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol Mol Plant Pathol 62: 333–346 [Google Scholar]
- Shetty R, Jensen B, Shetty NP, Hansen M, Hansen CW, Starkey KR, Jørgensen HJL. (July 3, 2011) Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa Plant Pathol http://dx.doi.org/10.1111/j.1365-3059.2011.02493.x
- Shimada Y, Goda H, Nakamura A, Takatsuto S, Fujioka S, Yoshida S. (2003) Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol 131: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Röpenack E, Parr A, Schulze-Lefert P. (1998) Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley. J Biol Chem 273: 9013–9022 [DOI] [PubMed] [Google Scholar]
- Walter MH, Grima-Pettenati J, Grand C, Boudet AM, Lamb CJ. (1988) Cinnamyl-alcohol dehydrogenase, a molecular marker specific for lignin synthesis: cDNA cloning and mRNA induction by fungal elicitor. Proc Natl Acad Sci USA 85: 5546–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Shimoi E, Ohkama N, Hayashi H, Yoneyama T, Yazaki J, Fujii F, Shinbo K, Yamamoto K, Sakata K, et al. (2004) Identification of several rice genes regulated by Si nutrition. Soil Sci Plant Nutr 50: 1273–1276 [Google Scholar]
- Winkel-Shirley B. (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]