Abstract
The Arabidopsis (Arabidopsis thaliana) phytoalexin-deficient mutant pad2-1 displays enhanced susceptibility to a broad range of pathogens and herbivorous insects that correlates with deficiencies in the production of camalexin, indole glucosinolates, and salicylic acid (SA). The pad2-1 mutation is localized in the GLUTAMATE-CYSTEINE LIGASE (GCL) gene encoding the first enzyme of glutathione biosynthesis. While pad2-1 glutathione deficiency is not caused by a decrease in GCL transcripts, analysis of GCL protein level revealed that pad2-1 plants contained only 48% of the wild-type protein amount. In contrast to the wild type, the oxidized form of GCL was dominant in pad2-1, suggesting a distinct redox environment. This finding was corroborated by the expression of GRX1-roGFP2, showing that the cytosolic glutathione redox potential was significantly less negative in pad2-1. Analysis of oxidative stress-related gene expression showed a higher transcript accumulation in pad2-1 of GLUTATHIONE REDUCTASE, GLUTATHIONE-S-TRANSFERASE, and RESPIRATORY BURST OXIDASE HOMOLOG D in response to the oomycete Phytophthora brassicae. Interestingly, oligogalacturonide elicitation in pad2-1 revealed a lower plasma membrane depolarization that was found to act upstream of an impaired hydrogen peroxide production. This impaired hydrogen peroxide production was also observed during pathogen infection and correlated with a reduced hypersensitive response in pad2-1. In addition, a lack of pathogen-triggered expression of the ISOCHORISMATE SYNTHASE1 gene, coding for the SA-biosynthetic enzyme isochorismate synthase, was identified as the cause of the SA deficiency in pad2-1. Together, our results indicate that the pad2-1 mutation is related to a decrease in GCL protein and that the resulting glutathione deficiency negatively affects important processes of disease resistance.
Interactions between plants and microbes are the result of a finely tuned coevolution. To counter microbial attacks, plants have developed perception systems that activate various defense mechanisms. Two main defense pathways are described in plant innate immunity (Boller and Felix, 2009). The first, named pathogen-associated molecular pattern (PAMP)-triggered immunity, is based on the recognition of pathogen-, microbe-, or damage-associated molecular patterns by pattern recognition receptors (Jones and Dangl, 2006). The second and more specialized effector-triggered immunity is activated when plant disease resistance gene products detect the presence of pathogen effectors.
The perception of an invader’s molecular tag is followed by several changes in plants (Tsuda and Katagiri, 2010). At the cellular level, many signaling events are rapidly detected, such as ion fluxes (Ca2+, K+, NO3−Cl−) and enhanced production of reactive oxygen species (ROS), mainly catalyzed by plasma membrane NADPH oxidases, encoded by Respiratory Burst Oxidase Homolog (Rboh) genes (Simon-Plas et al., 2002; Torres et al., 2002, 2006). This oxidative burst contributes, together with nitric oxide (NO) production, redox state changes, and mitogen-activated protein kinase cascade activation, to a transcriptional reprogramming leading to the activation of defense responses (Asai et al., 2002; Wendehenne et al., 2002; Torres et al., 2006; Besson-Bard et al., 2008; Foyer et al., 2009). Changes in hormonal balance, particularly salicylic acid (SA), jasmonic acid (JA), and ethylene (ET), have been shown to be key events in the activation and fine-tuning of plant immunity upon pathogen attack. Finally, the production of antimicrobial compounds such as phytoalexins (Hammerschmidt, 1999), pathogenesis-related (PR) proteins (van Loon et al., 2006), and cell wall strengthening arise from this defense activation to restrict pathogen growth.
In Arabidopsis (Arabidopsis thaliana), it is now well accepted that SA signaling is generally important for immunity against biotrophs, whereas JA/ET signaling is important for immunity against necrotrophs (Glazebrook, 2005). However, it has been shown that disease resistance of Arabidopsis against the oomycete pathogen Phytophthora brassicae is SA, JA, and ET independent but needs the indole glucosinolate/camalexin pathways (Roetschi et al., 2001; Schlaeppi et al., 2010). In the case of biotrophic pathogens, like P. brassicae, a form of plant programmed cell death, named the hypersensitive response (HR), can be detected during the incompatible interaction that may restrict pathogen growth (Roetschi et al., 2001; Hofius et al., 2007). On the whole, this complex defense network allows plants to resist most of their invaders.
Studies with plant mutants have provided essential information to understand the molecular mechanisms underlying plant disease resistance. The Arabidopsis phytoalexin-deficient mutant pad2-1 displays susceptibility to a broad range of pathogens and pests, including necrotrophs (Botrytis cinerea, Alternaria brassicicola), hemibiotrophs and biotrophs (P. brassicae, Pseudomonas syringae), and insect herbivores (Spodoptera littoralis; Glazebrook and Ausubel, 1994; Glazebrook et al., 1997; Reuber et al., 1998; Roetschi et al., 2001; Ferrari et al., 2003; van Wees et al., 2003; Bohman et al., 2004; Parisy et al., 2007; Schlaeppi et al., 2008). The pad2-1 mutation is localized in the single-copy gene At4g23100, which encodes Glutamate-Cysteine Ligase (GCL), the first enzyme involved in the biosynthesis of the tripeptide glutathione. Due to mutation S298N in GCL, pad2-1 contains only approximately 20% of wild-type glutathione (Parisy et al., 2007). Three other allelic mutations have been found in GCL: the root-meristemless1 mutant (rml1; D258N) possesses only approximately 3% of wild-type glutathione, which leads to the frequent abortion of plant development (Vernoux et al., 2000); the cadmium-sensitive2-1 mutant (cad2-1; ΔP237, ΔK238, and V239L) has approximately 30% of wild-type glutathione and is sensitive to heavy metals (Cobbett et al., 1998) but is moderately susceptible to P. brassicae (Parisy et al., 2007); the regulator of APX2 1-1 mutant (rax1-1; R228K) has approximately 40% of wild-type glutathione and is sensitive to high light (Ball et al., 2004) but shows a wild-type resistance to P. brassicae (Parisy et al., 2007). Thus, these results highlighted that glutathione plays a major role in many cellular processes such as development and responses to biotic and abiotic stresses (Potters et al., 2002; Noctor, 2006; Foyer and Noctor, 2011). Complementary results have shown that glutathione biosynthesis is controlled by transcriptional and posttranslational regulation of GCL and not by its second step catalyzed by glutathione synthase (GS; May et al., 1998; Jez et al., 2004; Hicks et al., 2007).
Many studies have shown that pad2-1 displays a pleiotropic phenotype. Concerning defense responses, the production of camalexin, the main phytoalexin of Arabidopsis, is strongly affected in pad2-1 in response to P. syringae pv maculicola (approximately 15% of the wild type; Glazebrook and Ausubel, 1994), B. cinerea (approximately 25% of the wild type; Ferrari et al., 2003), A. brassicicola (approximately 35% of the wild type; van Wees et al., 2003), or P. brassicae (approximately 40% of the wild type; Roetschi et al., 2001; Parisy et al., 2007). This camalexin deficiency is directly due to glutathione depletion, since glutathione is required for glutathione S-transferase (GSTF6)-catalyzed synthesis of glutathione-indole-3-acetonitrile, a precursor of camalexin biosynthesis (Su et al., 2011). Besides, the impaired camalexin biosynthesis and resistance to P. brassicae in pad2-1 can be restored using either glutathione feeding or transformation with wild-type GCL cDNA (Parisy et al., 2007).
Glucosinolates are another class of sulfur-containing compounds of Brassicaceae whose degradation products are well known to be toxic against insects and pathogens (Halkier and Gershenzon, 2006). Interestingly, Schlaeppi and colleagues recently showed that pad2-1 is impaired in the production of indole glucosinolates in response to insects (approximately 55% of the wild type; Schlaeppi et al., 2008) or P. brassicae infection (approximately 40% of the wild type; Schlaeppi et al., 2010). Moreover, stress-induced SA accumulation and PR-1 expression are also very low in pad2-1 in response to P. brassicae (Roetschi et al., 2001). The SA deficiency of pad2-1 is possibly linked to the requirement of glutathione to modulate the redox state needed for the oligomer/monomer transition of Nonexpressor of Pathogenesis-Related1 (NPR1) genes preceding NPR1 translocation into the nucleus to activate PR-1 gene expression (Després et al., 2003; Mou et al., 2003; Pieterse and Van Loon, 2004). However, it remains unexplained how glutathione modulates SA acting upstream of NPR1.
Many studies reported that the total glutathione concentration, as well as the ratio of reduced to oxidized forms of glutathione, affect cellular redox homeostasis during plant development or environmental stress (May et al., 1998; Mullineaux and Rausch, 2005; Foyer and Noctor, 2011). These cellular redox changes influence the genome-wide expression profile (Ball et al., 2004) and protein activity. For example, GCL is redox activated by the formation of two disulfide bonds (Jez et al., 2004; Hothorn et al., 2006; Hicks et al., 2007). Despite the apparent impact of glutathione depletion on defense signaling events and responses such as HR, these underlying mechanisms have not yet been studied using a genetic approach.
The aim of this study was to identify the molecular origin of the glutathione depletion in pad2-1 and to investigate the putative links between glutathione, the cellular redox potential, ROS production, defense gene expression, and HR. For this purpose and to better understand the disease susceptibility of pad2-1, we compared early signaling events occurring in wild-type Columbia-0 (Col-0) and pad2-1 cells treated with oligogalacturonides (OG), a damage-associated molecular pattern that elicits typical defense responses, and during plant infection with P. brassicae. Our results indicate that the glutathione deficiency in pad2-1 is related to a decreased GCL protein content. They also highlight a role of glutathione in modulating the cellular redox environment, membrane depolarization, ROS and NO production, expression of key defense-related genes, and HR development. These data highlight the crucial role of glutathione in signaling processes underlying essential defense responses in plants.
RESULTS
The GCL Protein Amount Is Lower in pad2-1 and Its Redox State Is More Oxidized
The pad2-1 mutation S298N of GCL causes a low glutathione content as compared with the wild type (approximately 20% of the wild type; Parisy et al., 2007). Since the glutathione deficiency of pad2-1 is not related to a reduced expression of GCL or GS genes, which encode the two enzymes involved in glutathione synthesis (Parisy et al., 2007), we analyzed if the S298N mutation affects the GCL protein amount. While the cad2-1, rax1-1, and rml1-1 mutations have been proposed to directly affect amino acid residues involved in the binding of one of the GCL substrates (Glu, Cys, or ATP; Hothorn et al., 2006), nothing was known about the pad2-1 mutation. Using PyMOL software (PyMOL Molecular Graphics System; Schrödinger), in silico prediction indicated that the S298N exchange in pad2-1 did not affect the ternary structure of GCL, contrary to cad2-1 and rax1-1 mutations, which displayed a minor structure modification in one β-sheet (data not shown). Moreover Ser-298 has not been described to be involved in the catalytic site of GCL (involving 12 residues in Arabidopsis: Glu-115, Arg-228, Tyr-229, Met-232, Met-247, Thr-250, Asp-258, Arg-300, Trp-304, Tyr-338, Phe-383, and Tyr-391) or in its thiol-based redox regulation sites (Hothorn et al., 2006; Hicks et al., 2007; Gromes et al., 2008). To test the hypothesis that the pad2-1 mutation could affect GCL protein folding or stability, we first compared the GCL protein level between pad2-1 and wild-type plants by western-blot analysis using an antibody raised against purified recombinant AtGCL (Hicks et al., 2007). As GCL occurs in oxidized and reduced forms, tissue extracts were reduced prior to western blotting. Interestingly, pad2-1 leaf tissue contained only 48% of the wild-type amount of GCL. However, in the allelic cad2-1 and rax1-1 mutants, the GCL levels were almost the same as in the wild type (Fig. 1A). These results show that pad2-1 is the only one to display a very low GCL protein level, which could explain its low glutathione content.
Figure 1.
Analysis of the total content and redox state of GCL. Total proteins were extracted under reducing (+0.1 m DTT) or nonreducing (−DTT) conditions. Oxidized (ox) and reduced (red) forms of GCL were analyzed by western blotting (WB) using an antibody raised against recombinant AtGCL. A, Total amount of GCL in Col-0 and glutathione-deficient mutants pad2-1, cad2-1, and rax1-1. B, Changes of the redox state of GCL after H2O2 treatment in Col-0 and pad2-1 plants. Leaf discs were treated with 5 mm H2O2. Results are representative of three independent experiments. Equal protein loading was checked by staining with Coomassie blue. The percentage values for total GCL are means ± se of three independent experiments. [See online article for color version of this figure.]
GCL activity is regulated by cellular redox state (Jez et al., 2004; Hothorn et al., 2006; Hicks et al., 2007). In Arabidopsis, the oxidation of the inactive reduced form switches the protein into an active form via the formation of intramolecular disulfide bonds (Hicks et al., 2007). Therefore, we investigated the oxidized and reduced forms of GCL after oxidative stress (Fig. 1B). For this purpose, leaf discs were treated with 5 mm hydrogen peroxide (H2O2) and total proteins were extracted in reducing (+dithiothreitol [DTT]) or nonreducing (−DTT) conditions and examined by western blotting. Under reducing conditions, only one band was detected, with a molecular mass of 50 kD in both wild-type and pad2-1 plants (Fig. 1). In nonreducing conditions, two bands were detected in wild-type plants, corresponding to the oxidized and reduced forms of GCL, with apparent molecular masses of 56 and 50 kD, respectively. In accordance with Hicks et al. (2007), the reduced form was found to be dominant in wild-type plants. Oxidative stress induced by H2O2 shifted GCL to the oxidized active form. Under nonreducing conditions, both the reduced and oxidized forms of GCL were detected at a very low level in pad2-1. However, in contrast to wild-type plants, the oxidized form of GCL was already dominant at 0 min, and no obvious change of the redox state of the enzyme was observed within 60 min after treatment with H2O2. Thus, the redox regulation of the GCL enzyme is impaired in pad2-1 plants. These data also suggest that pad2-1 might display a distinct redox environment, known to be essentially maintained by the glutathione pool (Foyer and Noctor, 2011).
The Glutathione Redox Potential Is Less Reducing in pad2-1 Than in the Wild Type
To investigate whether the cytosolic glutathione redox potential (EGSH) differs between pad2-1 and the wild type, EGSH was measured with the glutathione-specific redox-sensitive GFP (roGFP) variant GRX1-roGFP2. It was previously demonstrated that this fusion sensor of the human Glutaredoxin1 (GRX1) and roGFP2 equilibrates with glutathione and thus reports the local EGSH (Gutscher et al., 2008).
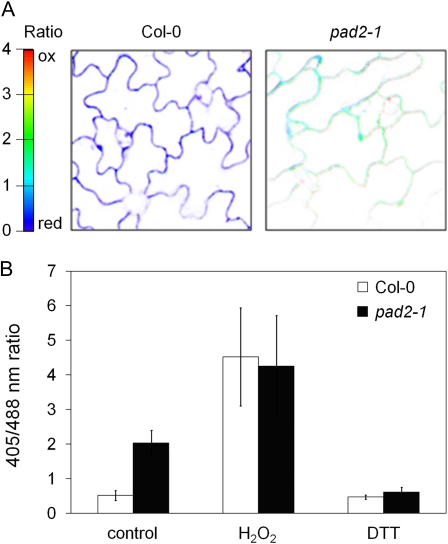
Wild-type and pad2-1 seedlings expressing GRX1-roGFP2 in the cytosol were subjected to ratiometric analysis of GRX1-roGFP2 fluorescence through confocal imaging with excitation at 405 and 488 nm (Fig. 2). In basal conditions, the 405/488 fluorescence ratio of GRX1-roGFP2 was higher in pad2-1 (2.03 ± 0.36) than in the wild type (0.51 ± 0.14), indicating that GRX1-roGFP2 is more oxidized in pad2-1 plants (Fig. 2).
Figure 2.
Comparison of the glutathione redox potential of Col-0 and pad2-1 epidermal cells expressing GRX1-roGFP2 in the cytosol. A, Images were taken by confocal laser scanning microscopy. GRX1-roGFP2 was excited with 405- and 488-nm wavelengths. The color scale of the fluorescence ratio represents the redox state of roGFP2 oscillating between an oxidized (ox) state (red) and a reduced (red) state (blue). B, Quantitative ratio values for images taken on Col-0 and pad2-1 mutant tissue. Water-treated (control), fully oxidized (10 mm H2O2), or fully reduced (10 mm DTT) samples for Col-0 and the pad2-1 mutant were compared (mean ± se; n = 6). Results are representative of three independent experiments.
For probe calibration, wild-type and pad2-1 tissues were treated with 10 mm H2O2 or 10 mm DTT to fully oxidize or reduce the GRX1-roGFP2 protein (Fig. 2B). After 10 mm H2O2 treatment, the GRX1-roGFP2 ratio of the wild type (4.52 ± 1.41) and pad2-1 (4.24 ± 1.46) increased to almost the same value. Similarly, the ratio of the DTT-treated tissues was comparable in the wild type (0.46 ± 0.06) and pad2-1 (0.61 ± 0.13). These results showed that the ratiometric behavior of the fluorescent probe, including its dynamic range, was the same in the mutant and the wild-type plants. Based on these calibration values, the ratio values measured in leaves under nonstress conditions were converted to EGSH according to Meyer et al. (2007). While EGSH was −312 ± 10 mV in the wild type, it was only −275 ± 5 mV in pad2-1. Together, these data indicated that the glutathione redox buffer in pad2-1 has less reducing power than that in the wild type.
Expression of Oxidative Stress-Related Genes Is Higher in pad2-1 during P. brassicae Infection
Since EGSH is less reducing in pad2-1 compared with the wild type, we evaluated whether pad2-1 perceived a stronger oxidative stress during pathogen infection. Thus, we chose to analyze the expression of oxidative stress-related genes by real-time quantitative PCR at 24 h post inoculation (hpi) with P. brassicae (Fig. 3).
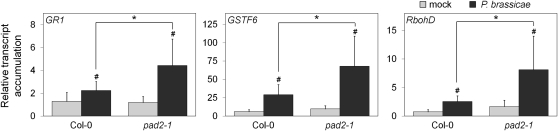
Figure 3.
Expression of oxidative stress-related genes in response to P. brassicae at 24 hpi. Plant leaves were infected by agar plug inoculation of 8-d-old mycelium. Mycelium-free plugs were used for mock treatment. The transcript accumulation of genes encoding GR1, GSTF6, and RbohD was analyzed by real-time qPCR. After normalization with UBQ10, results were expressed as fold change in transcript level compared with the time zero sample (mean ± se; n = 3) from three independent biological experiments. Hash marks indicate statistically significant differences between mock and infected samples (P < 0.05), and asterisks indicate statistically significant differences between infected Col-0 and infected pad2-1 samples (P < 0.05), using unpaired heteroscedastic Student’s t test.
NADPH-dependent Glutathione Reductase1 (GR1) is involved in the glutathione-ascorbate cycle by catalyzing the regeneration of oxidized to reduced glutathione under oxidative stress (Foyer and Noctor, 2011). Indeed, the gr1 mutant of Arabidopsis showed a low capacity to maintain a reduced glutathione pool after H2O2 treatment and displayed a similar EGSH to pad2-1 (Marty et al., 2009). During infection, P. brassicae triggered a significant expression of GR1 transcripts that was more pronounced in pad2-1 than in the wild type (Fig. 3). No significant difference was found between unchallenged pad2-1 and wild-type plants.
Among plant GST genes, which are induced by a variety of stimuli, AtGSTF6 (also called AtGST1) was reported to be induced upon infection or in response to H2O2 treatment (Marrs, 1996; Nutricati et al., 2006; Belhaj et al., 2009). In this study, we show that the transcript level of GSTF6 was significantly up-regulated in response to P. brassicae both in pad2-1 and the wild type (Fig. 3). However, the increase in transcript accumulation in pad2-1 was more than 2-fold higher than in the wild type.
In addition to GR1 and GSTF6 genes, we chose to analyze the expression of the RbohD gene, because Rboh genes encode NADPH oxidase enzymes that catalyze the production of the ROS O2.− (Levine et al., 1994; Torres et al., 2002; Sagi and Fluhr, 2006). Moreover, RbohD has been shown to be involved in the enhanced production of H2O2 detected after OG elicitation (Galletti et al., 2008) or in response to P. brassicae (Belhaj et al., 2009). Figure 3 shows that the transcript level of the RbohD gene was 2.5 times higher in pad2-1 compared with wild-type plants in response to P. brassicae.
pad2-1 Is Impaired in H2O2 and NO Production during Biotic Stresses
As the expression of the oxidative stress-related marker genes GR1, GSTF6, and RbohD was significantly higher in pad2-1 than in the wild type, we checked H2O2 production in response to two biotic stress conditions: elicitation by OG and inoculation with P. brassicae (Fig. 4).
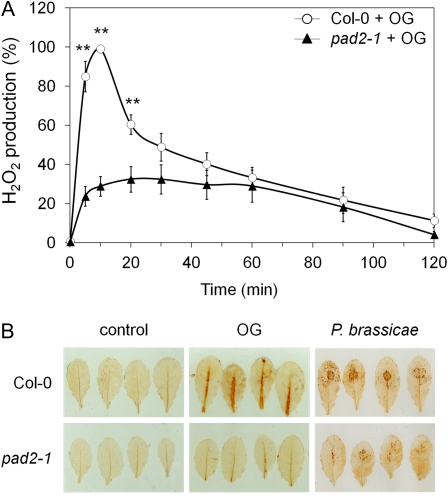
Figure 4.
H2O2 production in response to OG or P. brassicae in Col-0 and pad2-1. A, H2O2 was measured using the chemiluminescence of luminol after OG treatment (0.5 g L−1) in Col-0 and pad2-1 cell suspensions. Data are expressed as percentage of the maximum H2O2 production in OG-treated Col-0 cells. Values are means ± se of three independent experiments. Asterisks indicate statistically significant differences between the wild type and pad2-1, using Student’s t test (P < 0.01). B, In planta H2O2 detection. Leaves were treated with 2.5 g L−1 OG during 20 min or inoculated for 1 d with P. brassicae agar plugs before DAB staining. H2O2 production was detected as brown precipitates. Results are representative of three independent experiments.
H2O2 production was first determined in cell suspensions using the chemiluminescence of luminol. In wild-type cells, OG treatment elicited a transient increase in H2O2 accumulation, which peaked at 10 min and decreased slowly until 120 min (Fig. 4A). In pad2-1 cells, the transient increase in H2O2 production was delayed and represented only 30% of the maximal production detected in wild-type cells. This low content of H2O2 in pad2-1 was due to an impairment of its production rather than to an increase in its degradation, as H2O2 half-life and global peroxidase or catalase activities were similar in the two genotypes (Supplemental Fig. S1).
The unexpected loss of OG-induced H2O2 accumulation was also observed in pad2-1 leaves using a modified diaminobenzidine (DAB) staining method (Fig. 4B). In presence of peroxidases, which are ubiquitous in plant tissue, H2O2 reacts with DAB to produce brown precipitates (Thordal-Christensen et al., 1997). In order to allow a higher sensitivity of the approach, an excess of horseradish peroxidase was added to the DAB solution prior to infiltration into leaves. This modification allowed improved visualization of OG-induced H2O2 accumulation. No precipitate was detected in control leaves of wild-type and pad2-1 plants. While a strong brown precipitate was observed in wild-type leaves treated during 20 min with OG, H2O2 production was only weakly detected in pad2-1 leaves under the same conditions. Similar results were obtained with leaves infected with P. brassicae for 1 d. Wild-type leaves showed an intense brown precipitate at the infection site, whereas the response was much weaker in pad2-1 leaves (Fig. 4B). H2O2 accumulation in response to OG treatment or P. brassicae infection was completely abolished in the rbohD mutant (data not shown; Belhaj et al., 2009), demonstrating that the H2O2 accumulation in response to P. brassicae or OG treatment depends on RbohD.
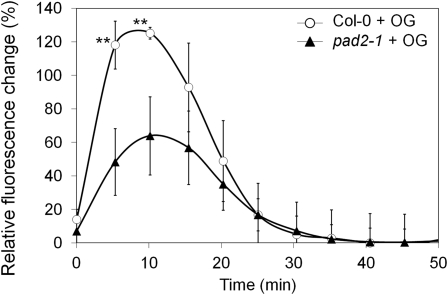
Another second messenger rapidly produced after elicitation is NO (Foissner et al., 2000; Besson-Bard et al., 2008). Using the NO-sensitive probe 4,5-diaminofluorescein diacetate (DAF-2DA), intracellular NO production was monitored during 12 h in leaf discs infiltrated with OG (8-h data shown in Supplemental Fig. S2). In water-infiltrated leaf discs, a basal fluorescence was observed in the wild type and pad2-1, probably reflecting constitutive NO production and/or the wounding response caused by the preparation of leaf discs. After OG treatment, an increase in fluorescence was observed in the wild type and pad2-1. However, this increase was significantly less pronounced in pad2-1 at 8 h after treatment. The use of the specific NO scavenger carboxy-PTIO confirmed the NO specificity of the probe by suppressing the fluorescence triggered by OG in wild-type and pad2-1 cells.
The Lower Plasma Membrane Depolarization in pad2-1 Cells Acts Upstream of ROS Production
Ion fluxes have been described to act upstream of the oxidative burst, known to be involved in HR and notably observed during the resistance of wild-type Arabidopsis to P. brassicae (Heath, 2000; Delledonne et al., 2001; Roetschi et al., 2001; Wendehenne et al., 2002; Greenberg and Yao, 2004). Having established that the production of H2O2 was altered in pad2-1, we checked whether a perturbation of ion fluxes at the plasma membrane level could be the source of this impairment in pad2-1. To test the hypothesis, wild-type and pad2-1 cell suspensions were treated with OG and changes in the plasma membrane potential were monitored by using the voltage-sensitive fluorescent probe bis-(1,3-dibutylbarbituric acid)-trimethine oxonol [DiBAC4(3); Fig. 5; Lamotte et al., 2006; Konrad and Hedrich, 2008].
Figure 5.
Plasma membrane potential changes in response to OG treatment in Col-0 and pad2-1 cell suspensions. Cells were incubated with the voltage-sensitive fluorescent probe DiBAC4(3) (10 μm) in the dark before OG treatment (0.5 g L−1). Plasma membrane depolarization was monitored by following the variation of DiBAC4(3) fluorescence and was expressed as a relative percentage: [fluorescence (OG) – fluorescence (water)]/fluorescence (water). The chart shows mean values ± se out of five independent experiments. Asterisks indicate statistically significant differences between the wild type and pad2-1, using Student’s t test (P < 0.01).
In wild-type and pad2-1 cells responding to OG treatment, a transient increase in fluorescence, reflecting a plasma membrane depolarization, occurred within the first 5 min and then peaked at 10 min. Within 30 min, DiBAC4(3) fluorescence returned to the basal level (Fig. 5). Interestingly, in pad2-1 cells, the maximal fluorescence at 10 min was two times lower than in wild-type cells. This result shows that pad2-1 is partly impaired in OG-dependent plasma membrane depolarization. Addition of the anionic channel inhibitor niflumic acid to wild-type cells 10 min before treatment totally inhibited the increase in DiBAC4(3) fluorescence, indicating the involvement of anion channels in this process (Supplemental Fig. S3A). Moreover, the total inhibition of OG-dependent H2O2 production by niflumic acid treatment provided evidence that ROS production occurs downstream of anion channel activation and plasma membrane depolarization (Supplemental Fig. S3B).
These results indicate that pad2-1 is impaired in plasma membrane depolarization and H2O2 and NO production during elicitation.
pad2-1 Is Impaired in HR Development in Response to P. brassicae
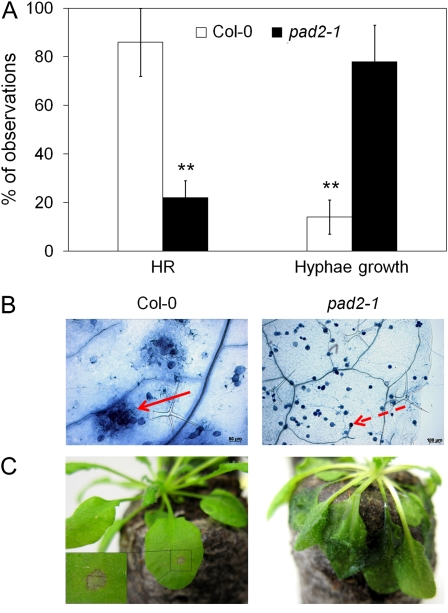
In many plant-pathogen interactions, one of the mechanisms stopping the pathogen invasion into leaf tissue is the HR. Since pad2-1 is impaired in key defense-related signaling events in response to biotic stresses and because it is susceptible to many pathogens, we compared HR formation in the wild type and pad2-1 after inoculation with P. brassicae (Fig. 6). Plants were inoculated by spraying a zoospore suspension, and the formation of HR and pathogen growth were analyzed by trypan blue staining at 1 d post inoculation (dpi). No significant difference in zoospore germination and pathogen penetration efficiency was found between the wild type and pad2-1 (data not shown). However, in wild-type plants, HR occurred in 86% of attempted infections, whereas this percentage was reduced to 22% in pad2-1 (Fig. 6A). The reduced rate of HR formation correlated with the enhanced rate of pathogen growth. Encysted zoospores producing hyphae were seen only in 14% of the observations in the wild type compared with 78% in pad2-1 (Fig. 6A). The different responses of the two genotypes to P. brassicae were also illustrated at 7 dpi. The wild type showed HR at both microscopic and macroscopic levels (Fig. 6, B and C), whereas in pad2-1, the pathogen developed extensively, as demonstrated by the massive occurrence of oospores (Fig. 6B) and the wilting of leaves (Fig. 6C).
Figure 6.
HR and P. brassicae development in Col-0 and pad2-1 plants. Leaves were inoculated by spraying a zoospore suspension of P. brassicae (105 zoospores mL−1) and harvested at the time indicated below. HR and pathogen structures were observed with lactophenol trypan blue staining. A, Percentages of the microscopic observations of three biological replicates at 1 dpi. Asterisks indicate statistically significant differences between the wild type and pad2-1, using a χ2 test (P < 0.01). B, Microscopic observation of HR (arrow) and pathogen development (dotted arrow; oospore) at 7 dpi. C, HR and water-soaked disease symptoms at 7 dpi with P. brassicae agar plugs in Col-0 and pad2-1 plants, respectively.
Expression of SA-Related Genes Is Impaired during P. brassicae Infection of pad2-1
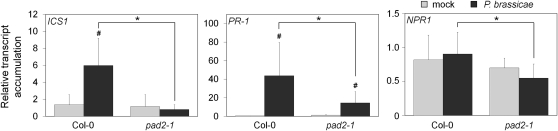
One important phenotypic aspect observed in pad2-1 is the impairment of SA accumulation in response to P. brassicae (Roetschi et al., 2001). In order to find the putative origin of this SA deregulation, we first analyzed the expression of the ISOCHORISMATE SYNTHASE1 (ICS1) gene, which encodes an important enzyme involved in SA biosynthesis (Nawrath and Métraux, 1999; Wildermuth et al., 2001). ICS1 transcript accumulation was 6-fold increased in the wild type in response to P. brassicae, but no increase in transcript level was detected in pad2-1 (Fig. 7). In parallel, the regulation of PR-1 and NPR1 gene expression was checked at 24 hpi with P. brassicae (Fig. 7). In accordance with the low ICS1 transcript levels and the previously reported reduction of SA accumulation in pad2-1, transcript accumulation of the SA marker gene PR-1 was three times lower in pad2-1 compared with the wild type. NPR1 gene expression was not induced in either genotype.
Figure 7.
Expression of SA-related genes in response to P. brassicae at 24 hpi. Plant leaves were agar plug inoculated with 8-d-old mycelium. Mycelium-free plugs were used for mock treatment. The transcript accumulation of ICS1, PR-1, and NPR1 was analyzed by real-time qPCR. After normalization with UBQ10, results were expressed as fold change in transcript level compared with the time zero sample (mean ± se; n = 3) from three independent biological experiments. Hash marks indicate statistically significant differences between mock and infected samples (P < 0.05), and asterisks indicate statistically significant differences between infected Col-0 and infected pad2-1 samples (P < 0.05), using unpaired heteroscedastic Student’s t test.
Transcript Levels of Genes Involved in Camalexin and Indole Glucosinolate Biosynthesis Are Similar in pad2-1 and the Wild Type
Together with camalexin, glucosinolates have recently been shown to play a major role in the resistance to P. brassicae (Schlaeppi et al., 2010). The pad2-1 mutant displayed a reduced accumulation of camalexin and glucosinolates (Roetschi et al., 2001; Parisy et al., 2007; Schlaeppi et al., 2010). To understand whether this defense pathway was down-regulated at the transcriptional level, gene expression was analyzed for CYP79B2 and CYP79B3, which encode two enzymes involved in the conversion of Trp to indole-3-aldoxime, a common precursor of camalexin and indole glucosinolates (Zhao et al., 2002; Bednarek et al., 2009). Furthermore, the expression of the indole glucosinolate-specific CYP81F2 gene and the camalexin-specific PAD3 gene (CYP71B15) were analyzed (Böttcher et al., 2009; Pfalz et al., 2009). All four genes were similarly up-regulated in response to P. brassicae in pad2-1 and wild-type plants (Supplemental Fig. S4). Together, these results indicate that the impairment in the indole glucosinolate/camalexin pathway, revealed by the lower metabolite content in pad2-1, is not due to the different transcript accumulation of the analyzed biosynthetic genes.
DISCUSSION
The Glutathione Depletion in pad2-1 Is Related to a Lower Amount of GCL Protein
GCL is described as the major control point of glutathione synthesis in plants, and the amount of glutathione seems to depend on the regulation of GCL at both transcriptional and posttranslational levels (May et al., 1998; Jez et al., 2004; Hicks et al., 2007). Under normal physiological conditions, GCL and GS transcript levels are similar in pad2-1 and wild-type plants (Parisy et al., 2007). Moreover, during infection with P. brassicae, GCL and GS transcript accumulation is higher in pad2-1 as compared with the wild type, while the glutathione level remains lower in the mutant (approximately 20% of the wild-type amount; Parisy et al., 2007).
To establish a functional relationship between glutathione and GCL in pad2-1, we compared the amount and posttranslational regulation of the GCL enzyme in pad2-1 and wild-type plants. GCL activity is modulated by redox control through the formation of two intramolecular disulfide bonds (C186-C406 and C349-C364), which enable a switch of the inactive reduced form to the active oxidized one under oxidative conditions (Jez et al., 2004; Hothorn et al., 2006; Hicks et al., 2007). Under reducing conditions, our results showed that the total amount of GCL was drastically lower in pad2-1 compared with the wild type or the two allelic mutants cad2-1 and rax1-1 (Fig. 1A). Studying the crystal structure of GCL, Hothorn et al. (2006) showed that cad2-1 and rax1-1 mutations are located in regions critical for binding of the substrates Glu and/or Cys, thus explaining the glutathione depletion in cad2-1 and rax1-1. However, all the critical residues involved in the catalytic activity or the redox regulation of GCL are not affected by the pad2-1 mutation S298N. Our results indicate that the glutathione deficiency of pad2-1 is likely caused by a drastic decrease in GCL protein content. Nevertheless, we cannot fully exclude that the proximity of the pad2-1 mutation to Arg-300 might affect Glu binding of the GCL enzyme (Hothorn et al., 2006), leading to a change in enzyme activity and thus a decrease in glutathione content.
pad2-1 Plants Sense a Permanent Oxidative Stress
Glutathione is known to play a central role in antioxidant processes as an efficient scavenger of ROS through its cysteinyl thiol group (Noctor, 2006), although its direct role is still disputed (Winterbourn, 2008). The balance between the reduced and oxidized forms of glutathione is involved in cellular redox homeostasis and influences, directly or indirectly, the regulation of many cellular processes at the gene and/or protein levels (Cobbett et al., 1998; Mou et al., 2003; Ball et al., 2004; Jez et al., 2004). To check whether the susceptibility of pad2-1 to pathogens could be related to a deregulation of the glutathione-dependent control of the cellular redox state, we analyzed, on the one hand, the reduced and oxidized forms of GCL and, on the other hand, the EGSH with the GRX1-roGFP2 fluorescent probe in vivo (Meyer et al., 2007; Gutscher et al., 2008; Schwarzländer et al., 2008).
Our study confirms that the oxidative stress triggered by H2O2 switches the reduced to the oxidized form of the GCL enzyme in the wild type (Fig. 1B), as demonstrated previously by Hicks et al. (2007). The more oxidized GCL protein observed in pad2-1 (Fig. 1B) is most probably due to a less reducing EGSH caused by the diminished glutathione levels in the mutant, as revealed by the GRX1-roGFP2 measurements.
In the absence of any treatment, GRX1-roGFP2 was more oxidized in pad2-1 than in the wild type, indicating higher EGSH in pad2-1 (EGSH of approximately −275 mV compared with −312 mV in the wild type). The gr1 mutant of Arabidopsis showed a low capacity to maintain a reduced glutathione pool after H2O2 treatment and displayed a similar EGSH to pad2-1 (−270 mV; Marty et al., 2009). Similarly, in the glutathione-deficient cad2-1 mutant, containing 30% of wild-type glutathione, an increased fraction of GRX1-roGFP2 is found in the oxidized state (EGSH of approximately −300 mV; Meyer et al., 2007). The gr1 and cad2-1 mutants are susceptible to P. syringae pv tomato and pv maculicola, respectively (Parisy et al., 2007; Mhamdi et al., 2010). Moreover, cad2-1 is moderately susceptible to P. brassicae, but not to such an extent as pad2-1 (Parisy et al., 2007). All the above-mentioned mutants, having higher EGSH, are more sensitive to pathogen attack (Meyer et al., 2007; Parisy et al., 2007; this work). These data collectively indicate that a shift in the EGSH in the cytosol is sufficient to reduce plant disease resistance, as recently shown by Maughan et al. (2010). Modulation of the cellular redox state is known to be one of the components acting on the regulation of gene expression through oxidation or a reduction of transcription factors (Apel and Hirt, 2004; Mittler et al., 2004). Interestingly, during P. brassicae infection, the expression of marker genes of oxidative stress, such as GR1, GSTF6, and RbohD, was significantly higher in pad2-1, suggesting the perception of a more oxidized environment during biotic stresses (Fig. 3). However, our results show that H2O2 production was drastically decreased in pad2-1 after OG elicitation or P. brassicae infection (Fig. 4; Supplemental Fig. S2). As there was no difference in the ability to degrade H2O2 between pad2-1 and the wild type (Supplemental Fig. S1), this striking discrepancy highlights a central role of glutathione in the regulation of ROS production. As RbohD has been shown to be the main source of ROS detected after OG treatment (Galletti et al., 2008) or P. brassicae inoculation (Belhaj et al., 2009), these results indicate that RbohD gene expression is not sufficient to trigger enhanced NADPH oxidase activity but that posttranslational modifications and upstream signaling events, like plasma membrane depolarization, are also needed (Gauthier et al., 2007, Jeworutzki et al., 2010). As NADPH oxidase activity is directly regulated by cytosolic Ca2+ partly released from internal pools such as the endoplasmic reticulum (Lecourieux et al., 2006; Vandelle et al., 2006), it is interesting that S-nitrosoglutathione influences cardiac reticulum Ca2+ release by activating the ryanodine receptor via S-nitrosylation (Sun et al., 2008). As pad2-1 produces less NO and glutathione, one hypothesis would suggest that cytosolic Ca2+ variations might be impaired in pad2-1, thus explaining its impaired H2O2 production.
pad2-1 Is Impaired in Early Signaling Events Critical for HR Induction
Ion fluxes across membranes were shown to act upstream of PAMP-triggered early signaling events, including changes in plasma membrane potential, as well as H2O2 and NO synthesis (Garcia-Brugger et al., 2006; Gauthier et al., 2007; Jeworutzki et al., 2010). Our data show a strong decrease in plasma membrane depolarization in pad2-1 cells responding to OG elicitation (Fig. 5). In the wild type, the use of the anionic channel inhibitor niflumic acid has shown that channel activity is involved in plasma membrane depolarization and acts upstream of ROS production during OG elicitation (Supplemental Fig. S3). In accordance with its lower plasma membrane depolarization, pad2-1 also displayed reduced NO and H2O2 production in response to elicitor treatment or P. brassicae infection. These data are consistent with those of Belhaj et al. (2009), who found that the Arabidopsis resistance to Phytophthora1 mutant, which is highly susceptible to P. brassicae, is also impaired in H2O2 production. More generally, the impairment of early signaling events observed in pad2-1 might explain, at least partly, the strong reduction of HR in response to P. brassicae (Fig. 6). Indeed, plasma membrane depolarization and ROS and NO production have been reported to mediate pathogen- or PAMP-triggered defense gene expression and cell death (Wendehenne et al., 2002; Zago et al., 2006; Zaninotto et al., 2006; Hofius et al., 2007; Mur et al., 2008). In mammals, recent studies indicate that some channels could be regulated via S-glutathionylation (Yang et al., 2011). Thus, glutathione depletion could disturb ion fluxes across the plasma membrane, impairing its normal depolarization.
SA-Dependent Defense Responses, But Not the Indole Glucosinolate/Camalexin Pathway, Are Blocked at the Transcriptional Level in pad2-1
Glutathione has been shown to be important for inducing the SA-dependent defense pathway, notably by modifying the redox state of NPR1 (Després et al., 2003; Mou et al., 2003). Moreover, glutathione accumulation has been shown to coincide with SA production and the concomitant activation of the PR-1 marker gene (Koornneef et al., 2008). Previous experiments have also demonstrated that pad2-1 is impaired in the synthesis of SA and in PR-1 gene expression (Roetschi et al., 2001; Parisy et al., 2007). Our results show that, in contrast to the wild type, PR-1 gene expression remains very low during P. brassicae infection on pad2-1 plants (Fig. 7). As NPR1 gene expression was not affected in pad2-1 (Fig. 7), it provides further evidence that redox regulation of the NPR1 protein is mandatory to trigger PR-1 gene expression (Després et al., 2003; Mou et al., 2003; Pieterse and Van Loon, 2004). Using the glutathione-deficient cad2-1 and rax1-1 mutants, Ball et al. (2004) also showed that the content of glutathione influences the expression of other defense-related genes. However, redox regulation of NPR1 cannot fully explain how glutathione can modulate the level of SA. Because the ICS1 enzyme has been shown to participate in the biosynthesis of SA (Wildermuth et al., 2001), ICS1 expression was followed in response to P. brassicae. Interestingly, we found that the normal up-regulation of ICS1 gene expression after pathogen infection is totally abolished in pad2-1 (Fig. 7), thus providing a possible explanation for the low SA levels observed in this mutant. As the two transcription factors SAR Deficient1 (SARD1) and CBP60g have been shown to be key regulators for ICS1 expression induction via binding to its promoter (Zhang et al., 2010), further investigation will be needed to fully understand exactly how glutathione depletion and redox potential modifications affect SA biosynthesis.
Production of the secondary metabolites camalexin and indole glucosinolates is strongly affected in pad2-1 in response to pathogens or insects (Glazebrook and Ausubel, 1994; Roetschi et al., 2001; Ferrari et al., 2003; van Wees et al., 2003; Parisy et al., 2007; Schlaeppi et al., 2008, 2010). Here, we show that these deficiencies are not caused by the down-regulation of genes encoding enzymes of the biosynthetic pathway, because CYP79B2, CYP79B3, CYP81F2, and CYP71B15 are normally expressed in pad2-1 in response to P. brassicae (Supplemental Fig. S4). The camalexin and indole glucosinolate deficiencies are more likely to be caused by the lack of glutathione as a biosynthetic substrate. Glutathione was recently identified as the sulfur donor of camalexin and indole glucosinolate biosynthesis (Geu-Flores et al., 2009; Su et al., 2011). Furthermore, we cannot exclude that many heme-containing enzymes, such as cytochrome P450, could also be disturbed in their catalytic process by the distinct redox environment existing in pad2-1 (Fig. 2). Indeed, it is interesting that the iron (III) protoporphyrin IX, important for P450 oxidoreductase activity, is maintained in the catalytic site by a thiol from a cysteinyl residue. This heme-Cys bond could probably be modified by the glutathione depletion, thus disturbing the normal electron transfer flow. In mammals, recent results have defined how thiol/disulfide redox switches control heme binding to regulate the activities of different enzymes (Ragsdale and Yi, 2011).
CONCLUSION
In summary, we report that the glutathione depletion in pad2-1 is related to an alteration of GCL content associated with redox state modifications that disturb biological responses under normal and stress conditions. Our results highlight that glutathione modulates early signaling events (plasma membrane depolarization and ROS and NO production), governs oxidative stress-related processes (redox state and GR1, GSTF6, and RbohD gene expression), defense gene expression (ICS1 and PR-1), and programmed cell death (HR) that normally enhance disease resistance. These deficiencies, in addition to the previously described deficiency in the accumulation of indole-derived secondary metabolites, are likely to contribute to the enhanced susceptibility of pad2-1 to pathogens and pests.
MATERIALS AND METHODS
Cell Culture Conditions and Treatment
Arabidopsis (Arabidopsis thaliana) ecotype Col-0 wild-type and pad2-1 cell suspensions were cultivated in Gamborg B5 medium (Duchefa; Gamborg et al., 1968) with 30 g L−1 Suc and 0.2 mg L−1 1-naphthalene acetic acid (Duchefa) on a rotary shaker (120 rpm) at 24°C under continuous light (25 μmol m−2 s−1). Cell suspension was routinely subcultured once per week. For elicitor treatment, cells were collected 1 d after subculturing, washed with M10 buffer (10 mm MES, 175 mm mannitol, 0.5 mm K2SO4, and 0.5 mm CaCl2, pH 6.2), and resuspended at 0.1 g fresh weight of cells mL−1 M10 buffer. After 1 h of equilibration (125 rpm at 24°C), cells were treated with water or 0.5 g L−1 OG (Goëmar Laboratories), and measurements of plasma membrane depolarization or H2O2 production were performed.
Plant Growth Conditions and Treatment
Wild-type and pad2-1, cad2-1, and rax1-1 mutant Arabidopsis plants were grown in Jiffy-7 peat pellets (Jiffy) in a controlled growth chamber under a 10/14-h day/night cycle at 20°C/18°C (70% relative humidity) with a light intensity of 175 μmol m−2 s−1 provided by fluorescent tubes.
The transformation of wild-type and pad2-1 plants with GRX1-roGFP2 was done by floral dip, according to Clough and Bent (1998).
For elicitation, leaf discs (7 mm diameter) from 8-week-old plants were treated with water or 2.5 g L−1 OG in 50 mm Tris-HCl (pH 7.5) under vacuum conditions for 2 min and then collected at the indicated times. For NO production measurement, the NO scavenger carboxy-PTIO (0.5 mm; Sigma-Aldrich) and the fluorescent probe DAF-2DA (20 μm; Sigma-Aldrich) were added in the Tris buffer at the same time as OG. Precisely eight leaf discs from eight plants were used for each treatment. For GCL redox state analysis, 12 discs were cut and treated from three plants per kinetic point. For H2O2 detection, leaves were cut and infiltrated with 20 mm MES buffer (pH 6.2) containing 2.5 g L−1 OG, 10 units mL−1 peroxidase, and 1 g L−1 DAB (Sigma-Aldrich).
Pathogen Growth and Plant Inoculation
Phytophthora brassicae isolate D was routinely grown on 20% V8 agar medium (Campbell Foods) supplemented with 3.5 g L−1 CaCO3 (pH 5) in a controlled chamber in the dark at 19°C.
For H2O2 detection and gene expression analysis, 4-week-old plants were inoculated by putting agar plugs of 8-d-old mycelium upside down on the leaf surface. Mycelium-free plugs were used for mock treatments. Twelve leaves from three plants per time point were used, and susceptibility was checked at 7 dpi by estimating symptom development as described previously (Parisy et al., 2007). For HR and susceptibility assays, plants were inoculated by spraying a suspension of zoospores at a concentration of 105 zoospores mL−1. Inoculated plants were put under the same conditions as pathogen growth conditions at 100% relative humidity with a dark period for the first 14 h.
Measurement of Plasma Membrane Depolarization
Plasma membrane depolarization was monitored using the voltage-sensitive fluorescent probe DiBAC4(3) (Sigma-Aldrich; Konrad and Hedrich, 2008). After 1 h of equilibration, cells were incubated for 30 min with 10 μm DiBAC4(3) in M10 buffer in the dark. Then, 0.5 mL of cells was transferred onto 24-well plates (Costar) and treated with water or OG. DiBAC4(3) fluorescence was recorded at 1-min intervals using a fluorimeter (Fluoroskan Ascent Fluorometer [Labsystems]; λexcitation = 485 nm and λemission = 535 nm) and expressed as relative fluorescence units. Cells were maintained under shaking during the course of the experiment.
H2O2 Production Measurement and Leaf Tissue Localization
In cell suspensions, H2O2 production was determined using the chemiluminescence of luminol. An aliquot of cells (250 μL) was added to 300 μL of H50 buffer (50 mm HEPES, 175 mm mannitol, 0.5 mm K2SO4, and 5 mm CaCl2, pH 8.5) and 50 μL of 0.3 mm luminol. Chemiluminescence was measured with a luminometer (Lumat LB9507; Berthold). The content of H2O2 (nmol g−1 fresh weight of cells) was calculated using a calibration based on the addition of H2O2 to the aliquot of cells.
In plants, a modified procedure described by Thordal-Christensen et al. (1997) was applied. Leaves were vacuum infiltrated with 20 mm MES buffer (pH 6.2) containing 2.5 g L−1 OG, 1 g L−1 DAB, and 10 units mL−1 horseradish peroxidase, then washed with MES buffer. The same solution without OG was used as a control. Infiltrated leaves were incubated in the dark at room temperature for 1 to 8 h. Then, leaves were destained with a solution of glycerol:lactic acid:ethanol (1:1:3, v/v/v) at 85°C for 5 min. H2O2 production was visualized macroscopically as brown precipitates in plant tissues.
Measurement of H2O2 Half-Life, Total Peroxidase Activity, and Total Catalase Activity
H2O2 half-life and catalase activity were determined spectrophotometrically by measuring the absorbance of H2O2 at 240 nm, and peroxidase activity was determined by measuring the absorbance of oxidized guaiacol at 470 nm (for details, see Supplemental Protocol S1).
NO Production Measurement
Intracellular NO accumulation was determined using the specific fluorophore DAF-2DA (Sigma-Aldrich) as described by Besson-Bard et al. (2009). Briefly, after infiltration and treatment with fluorophore, leaf discs were incubated for 1 h in the dark, washed three times with 50 mm Tris buffer, pH 7.5, to wash off excessive fluorophore, and transferred onto 96-well plates (Costar) containing 100 μL of the treatment solution. NO production was measured using a fluorimeter (Fluoroskan Ascent Fluorometer [Labsystems]; λex = 485 nm and λem = 510 nm). Fluorescence was expressed as relative fluorescence units.
Protein Extraction and Western-Blot Analyses
Totals proteins were extracted from 12 leaf discs in 350 μL of extraction buffer (50 mm HEPES, 10 mm EGTA, 10 mm EDTA, 1 mm Na3VO4, 50 mm β-glycerolphosphate, 10 mm NaF, 5 mg L−1 leupeptin, 5 mg L−1 antipain, and 1 mm phenylmethylsulfonyl fluoride, pH 7.5) with or without 0.1 m DTT. After centrifugation (15 min, 10,000g), 15 μg of soluble proteins was treated with Laemmli buffer (4 min at 100°C) and subjected to 12% SDS-PAGE before transfer to nitrocellulose membranes (Hybond ECL; Amersham Biosciences) for western-blot analysis. GCL protein (At4g23100) was detected with an antibody raised against recombinant AtGCL (antibody provided by J. Jez, Donald Danforth Plant Science Center). Primary antibody detection was performed as described for the LumiGlo detection kit (Cell Signaling Technology) with a horseradish peroxidase-coupled secondary antibody (Sigma-Aldrich).
RNA Extraction and Real-Time Quantitative PCR Analysis
Total RNA was extracted using the total RNA isolation kit (Promega), and 1 μg of total RNA was reverse transcribed using the SuperScript III reverse transcriptase kit (Invitrogen). Real-time quantitative (q)PCR was performed using 2 μL of 80-fold diluted cDNA, ABsolute qPCR SYBR Green ROX mix (containing Taq polymerase, deoxyribonucleotide triphosphate, and SYBR Green dye; ABgene), and 500 nm primers (primer sequences are shown in Supplemental Table S1) in a 5-μL volume. Triplicate quantitative assays per biological experiment were performed by using the LightCycler480 detection system (Roche). The activation factor of gene expression was determined with the comparative cycle threshold (Ct) method (Livak and Schmittgen, 2001): 2−ΔΔCt ; with ΔΔCt = ΔCt(t = 24 h) − ΔCt(t = 0) and ΔCt = Ct(target gene) – Ct(reference gene) for both inoculated and mock samples. UBQ10 was used as the reference gene (Supplemental Table S1).
HR and Pathogen Development
The infected leaves were harvested at the appropriate time points and stained with an alcoholic lactophenol trypan blue solution (Keogh et al., 1980) by boiling for 1 min and keeping them overnight in the staining solution. Leaves were clarified in 70% (w/v) chloral hydrate solution in water. HR and pathogen structures were examined with a Leitz DM RB microscope (Leica).
Confocal Laser Scanning Microscopy Imaging and Ratiometric Analysis
Confocal laser scanning microscopy imaging and ratiometric analysis in wild-type and pad2-1 plants expressing GRX1-roGFP2 were carried out as described by Schwarzländer et al. (2008). Briefly, the fluorescence of GRX1-roGFP2 was analyzed in leaf tissue of 7-d-old in vitro-grown seedlings. Images were taken with a 25× lens, in multitrack mode, with line switching and averaging of two frames. The probe was excited with a 405-nm laser in track 1 and with a 488-nm laser in track 2. GRX1-roGFP2 fluorescence was collected with a band-pass filter of 505 to 530 nm.
Ratiometric image analysis was done using a custom Matlab analysis suite as described by Schwarzländer et al. (2008).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Measurement of H2O2 half-life and total peroxidase and catalase activities in Col-0 and pad2-1 plants.
Supplemental Figure S2. Intracellular NO production at 8 h after treatment with OG in Col-0 and pad2-1 plants.
Supplemental Figure S3. Effect of the anionic channel inhibitor niflumic acid on OG-induced changes of plasma membrane potential and H2O2 production in Col-0 cell suspensions.
Supplemental Figure S4. Expression of genes involved in camalexin and glucosinolate biosynthesis in response to P. brassicae at 24 hpi.
Supplemental Table S1. Primers used for qPCR.
Supplemental Protocol S1. Measurement of H2O2 half-life, total peroxidase activity, and total catalase activity.
Acknowledgments
We thank Agnès Klinguer and Delphine Desqué for excellent technical assistance and Joseph Jez for providing the anti-GCL antibody. We also thank François Bouteau for helpful discussion.
References
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, Creissen G, Funck D, Jimenez A, Kular B, Leyland N, Mejia-Carranza J, Reynolds H, et al. (2004) Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. Plant Cell 16: 2448–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106 [DOI] [PubMed] [Google Scholar]
- Belhaj K, Lin BQ, Mauch F. (2009) The chloroplast protein RPH1 plays a role in the immune response of Arabidopsis to Phytophthora brassicae. Plant J 58: 287–298 [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D. (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59: 21–39 [DOI] [PubMed] [Google Scholar]
- Bohman S, Staal J, Thomma BP, Wang ML, Dixelius C. (2004) Characterisation of an Arabidopsis-Leptosphaeria maculans pathosystem: resistance partially requires camalexin biosynthesis and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J 37: 9–20 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Böttcher C, Westphal L, Schmotz C, Prade E, Scheel D, Glawischnig E. (2009) The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21: 1830–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR. (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23: 817–824 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Bloom AJ, Queval G, Noctor G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60: 455–484 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155: 2–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S. (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K. (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A. (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact 19: 711–724 [DOI] [PubMed] [Google Scholar]
- Gauthier A, Lamotte O, Reboutier D, Bouteau F, Pugin A, Wendehenne D. (2007) Cryptogein-induced anion effluxes: electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell death. Plant Signal Behav 2: 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geu-Flores F, Nielsen MT, Nafisi M, Møldrup ME, Olsen CE, Motawia MS, Halkier BA. (2009) Glucosinolate engineering identifies a gamma-glutamyl peptidase. Nat Chem Biol 5: 575–577 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM. (1994) Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA 91: 8955–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM. (1997) Use of Arabidopsis for genetic dissection of plant defense responses. Annu Rev Genet 31: 547–569 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Yao N. (2004) The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6: 201–211 [DOI] [PubMed] [Google Scholar]
- Gromes R, Hothorn M, Lenherr ED, Rybin V, Scheffzek K, Rausch T. (2008) The redox switch of gamma-glutamylcysteine ligase via a reversible monomer-dimer transition is a mechanism unique to plants. Plant J 54: 1063–1075 [DOI] [PubMed] [Google Scholar]
- Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. (2008) Real-time imaging of the intracellular glutathione redox potential. Nat Methods 5: 553–559 [DOI] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57: 303–333 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R. (1999) Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol 37: 285–306 [DOI] [PubMed] [Google Scholar]
- Heath MC. (2000) Hypersensitive response-related death. Plant Mol Biol 44: 321–334 [DOI] [PubMed] [Google Scholar]
- Hicks LM, Cahoon RE, Bonner ER, Rivard RS, Sheffield J, Jez JM. (2007) Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana. Plant Cell 19: 2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofius D, Tsitsigiannis DI, Jones JDG, Mundy J. (2007) Inducible cell death in plant immunity. Semin Cancer Biol 17: 166–187 [DOI] [PubMed] [Google Scholar]
- Hothorn M, Wachter A, Gromes R, Stuwe T, Rausch T, Scheffzek K. (2006) Structural basis for the redox control of plant glutamate cysteine ligase. J Biol Chem 281: 27557–27565 [DOI] [PubMed] [Google Scholar]
- Jeworutzki E, Roelfsema MR, Anschütz U, Krol E, Elzenga JT, Felix G, Boller T, Hedrich R, Becker D. (2010) Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca-associated opening of plasma membrane anion channels. Plant J 62: 367–378 [DOI] [PubMed] [Google Scholar]
- Jez JM, Cahoon RE, Chen SX. (2004) Arabidopsis thaliana glutamate-cysteine ligase: functional properties, kinetic mechanism, and regulation of activity. J Biol Chem 279: 33463–33470 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Keogh RC, Deverall BJ, McLeod S. (1980) Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Br Mycol Soc 74: 329–333 [Google Scholar]
- Konrad KR, Hedrich R. (2008) The use of voltage-sensitive dyes to monitor signal-induced changes in membrane potential-ABA triggered membrane depolarization in guard cells. Plant J 55: 161–173 [DOI] [PubMed] [Google Scholar]
- Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CM. (2008) Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol 147: 1358–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. (2006) Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic Biol Med 40: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. (2006) Calcium in plant defence-signalling pathways. New Phytol 171: 249–269 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Marrs KA. (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Marty L, Siala W, Schwarzländer M, Fricker MD, Wirtz M, Sweetlove LJ, Meyer Y, Meyer AJ, Reichheld JP, Hell R. (2009) The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc Natl Acad Sci USA 106: 9109–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan SC, Pasternak M, Cairns N, Kiddle G, Brach T, Jarvis R, Haas F, Nieuwland J, Lim B, Müller C, et al. (2010) Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci USA 107: 2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Sánchez-Fernández R, Van Montagu M, Inzé D. (1998) Evidence for posttranscriptional activation of gamma-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA 95: 12049–12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. (2007) Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J 52: 973–986 [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP, et al. (2010) Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan WH, Dong XN. (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T. (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res 86: 459–474 [DOI] [PubMed] [Google Scholar]
- Mur LAJ, Kenton P, Lloyd AJ, Ougham H, Prats E. (2008) The hypersensitive response: the centenary is upon us but how much do we know? J Exp Bot 59: 501–520 [DOI] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP. (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. (2006) Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29: 409–425 [DOI] [PubMed] [Google Scholar]
- Nutricati E, Miceli A, Blando F, De Bellis L. (2006) Characterization of two Arabidopsis thaliana glutathione S-transferases. Plant Cell Rep 25: 997–1005 [DOI] [PubMed] [Google Scholar]
- Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. (2007) Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J 49: 159–172 [DOI] [PubMed] [Google Scholar]
- Pfalz M, Vogel H, Kroymann J. (2009) The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 21: 985–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Van Loon LC. (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7: 456–464 [DOI] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40: 537–548 [Google Scholar]
- Ragsdale SW, Yi L. (2011) Thiol/disulfide redox switches in the regulation of heme binding to proteins. Antioxid Redox Signal 14: 1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W, Ausubel FM. (1998) Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J 16: 473–485 [DOI] [PubMed] [Google Scholar]
- Roetschi A, Si-Ammour A, Belbahri L, Mauch F, Mauch-Mani B. (2001) Characterization of an Arabidopsis-Phytophthora pathosystem: resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J 28: 293–305 [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi K, Abou-Mansour E, Buchala A, Mauch F. (2010) Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J 62: 840–851 [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. (2008) The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J 55: 774–786 [DOI] [PubMed] [Google Scholar]
- Schwarzländer M, Fricker MD, Müller C, Marty L, Brach T, Novak J, Sweetlove LJ, Hell R, Meyer AJ. (2008) Confocal imaging of glutathione redox potential in living plant cells. J Microsc 231: 299–316 [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein JP. (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31: 137–147 [DOI] [PubMed] [Google Scholar]
- Su T, Xu J, Li Y, Lei L, Zhao L, Yang H, Feng J, Liu G, Ren D. (2011) Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana. Plant Cell 23: 364–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. (2008) Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry 47: 13985–13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB. (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. (2010) Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Vandelle E, Poinssot B, Wendehenne D, Bentéjac M, Alain P. (2006) Integrated signaling network involving calcium, nitric oxide, and active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses. Mol Plant Microbe Interact 19: 429–440 [DOI] [PubMed] [Google Scholar]
- van Loon LC, Rep M, Pieterse CMJ. (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44: 135–162 [DOI] [PubMed] [Google Scholar]
- van Wees SCM, Chang HS, Zhu T, Glazebrook J. (2003) Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol 132: 606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Lamotte O, Frachisse JM, Barbier-Brygoo H, Pugin A. (2002) Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell 14: 1937–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286 [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. (2011) Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem 286: 9298–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago E, Morsa S, Dat JF, Alard P, Ferrarini A, Inzé D, Delledonne M, Van Breusegem F. (2006) Nitric oxide- and hydrogen peroxide-responsive gene regulation during cell death induction in tobacco. Plant Physiol 141: 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto F, La Camera S, Polverari A, Delledonne M. (2006) Cross talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiol 141: 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu S, Ding P, Wang D, Cheng YT, He J, Gao M, Xu F, Li Y, Zhu Z, et al. (2010) Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc Natl Acad Sci USA 107: 18220–18225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. (2002) Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16: 3100–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]