Abstract
Systemic acquired resistance (SAR) is a state of heightened defense to a broad spectrum of pathogens that is activated throughout a plant following local infection. Development of SAR requires the translocation of one or more mobile signals from the site of infection through the vascular system to distal (systemic) tissues. The first such signal identified was methyl salicylate (MeSA) in tobacco (Nicotiana tabacum). Subsequent studies demonstrated that MeSA also serves as a SAR signal in Arabidopsis (Arabidopsis thaliana) and potato (Solanum tuberosum). By contrast, another study suggested that MeSA is not required for SAR in Arabidopsis and raised questions regarding its signaling role in tobacco. Differences in experimental design, including the developmental age of the plants, the light intensity, and/or the strain of bacterial pathogen, were proposed to explain these conflicting results. Here, we demonstrate that the length of light exposure that plants receive after the primary infection determines the extent to which MeSA is required for SAR signaling. When the primary infection occurred late in the day and as a result infected plants received very little light exposure before entering the night/dark period, MeSA and its metabolizing enzymes were essential for SAR development. In contrast, when infection was done in the morning followed by 3.5 h or more of exposure to light, SAR developed in the absence of MeSA. However, MeSA was generally required for optimal SAR development. In addition to resolving the conflicting results concerning MeSA and SAR, this study underscores the importance of environmental factors on the plant’s response to infection.
Despite their sessile lifestyle, plants actively regulate growth, development, and physiological processes that allow them to survive in a constantly changing environment. Plants possess chemical and mechanical defenses to reduce the damage caused by pathogenic microbes, which are constantly present in their environment. However, constitutive activation of these defenses has a negative impact on plant growth and development (van Hulten et al., 2006). To minimize this cost, many defenses are activated or amplified only in the presence of a pathogen. One such inducible defense response is systemic acquired resistance (SAR), which provides enhanced immunity to a secondary (2°) pathogen infection in tissues distal to the site of primary (1°) infection (Ross, 1961). Induction of various defense-related genes, including PATHOGENESIS-RELATED1 (PR-1), also occurs in the distal tissues. Since systemic PR-1 expression tightly correlates with SAR development, the expression of this gene is often used as a molecular marker for SAR.
The last 50 years of research have revealed several molecular and genetic players relevant to the development of SAR. Recent efforts have focused on identifying the mobile signal responsible for alerting the distal/systemic tissues after the 1° infection. Since SAR development requires salicylic acid (SA), it was initially suggested that SA serves as the long-distance signal. However, grafting experiments with tobacco (Nicotiana tabacum) plants expressing the bacterial NahG gene, which encodes the SA-degrading enzyme salicylate hydroxylase, showed that SA is not the mobile signal (Vernooij et al., 1994). Subsequent studies revealed that methyl salicylate (MeSA), a derivative of SA, is an important signal for SAR. Using grafted tobacco plants silenced for the expression of either SA-Binding Protein2 (SABP2), with its SA-inhibitable MeSA esterase activity, or SA Methyl Transferase1 (SAMT1), which synthesizes MeSA, Park et al. (2007) demonstrated that SAR development requires SAMT1 in the 1° infected tissue to produce MeSA. Concurrently, SA-mediated inhibition of SABP2 must occur in this infected tissue to enable sufficient levels of MeSA to accumulate. MeSA is then translocated through the phloem to the systemic leaves, where it is converted back to active SA by SABP2 (Park et al., 2007). Additional genetic, biochemical, and pharmacological studies have confirmed MeSA’s role as a SAR signal in tobacco and have expanded this finding to several other plant species (Park et al., 2007, 2009; Vlot et al., 2008; Liu et al., 2010b; Manosalva et al., 2010). For example, the Arabidopsis (Arabidopsis thaliana) bsmt1-3 mutant, which contains a knockout mutation in the SAMT1 ortholog BENZOIC ACID/SA METHYL TRANSFERASE1 (BSMT1), produces very little MeSA in response to pathogen infection as compared with wild-type plants (Liu et al., 2010b). These plants also accumulate reduced levels of SA and its storage form, SA O-β-glucoside (SAG), in the distal tissues, and they are defective for SAR, although resistance in the inoculated tissue is unaffected.
In addition to MeSA, several candidate SAR signals that are linked to lipid metabolism and translocation have been described (Maldonado et al., 2002; Truman et al., 2007; Chaturvedi et al., 2008; Jung et al., 2009; Chanda et al., 2011). Analysis of petiole exudates has identified two specific lipids, azelaic acid and jasmonic acid, that accumulate in the vascular sap of plants responding to a SAR-inducing 1° infection (Truman et al., 2007; Jung et al., 2009). While the role of jasmonic acid in SAR remains controversial (Shah, 2009), infiltration of azelaic acid induces SA-dependent disease resistance in both the treated leaf and distal tissue (Jung et al., 2009). Analysis of SAR-defective Arabidopsis mutants also has implicated DEFECTIVE IN INDUCED RESISTANCE1 (DIR1), which encodes a putative apoplastic protein with homology to family 2 lipid transfer proteins (Maldonado et al., 2002), and SUPPRESSOR OF FATTY ACID DESATURASE1 (SFD1), which encodes dihydroxyacetone phosphate reductase, an enzyme involved in glycerol-3-phosphate (G3P) metabolism (Chanda et al., 2011). Petiole exudates from pathogen-infected dir1-1 and sfd1 mutants combined, but not singly, elicited SAR when infiltrated into wild-type plants, arguing that the DIR1 protein is the carrier of an SFD1-derived metabolite (Chaturvedi et al., 2008). Subsequent studies have suggested that G3P also plays a role in SAR signaling, since (1) G3P levels are reduced in pathogen-inoculated gly1/sfd1 mutants, (2) G3P enhances SAR in wild-type plants and restores SAR in mutants defective for G3P synthesis, and (3) G3P and DIR1 are mutually interdependent for translocation to the systemic tissues (Chanda et al., 2011). However, radiotracer studies indicated that G3P is modified in the infiltrated leaf and that an unidentified derivative, rather than G3P, is translocated to the systemic leaves.
A link among DIR1, G3P, and MeSA-dependent SAR signaling was recently revealed with the discovery that pathogen-infected dir1-1 mutants contain elevated levels of MeSA, display heightened expression of BSMT1, and accumulate reduced levels of SA and SAG in systemic leaves (Liu et al., 2011). Additionally, infiltrating G3P into lower leaves of wild-type Arabidopsis induced the expression of an SABP2 homolog in the systemic leaves and repressed the systemic expression of BSMT1 (Chanda et al., 2011). Together, these findings argue that DIR1, probably in complex with a G3P-derived signal molecule, activates SAR in the distal tissues by down-regulating the expression of BSMT1, thereby facilitating the accumulation of free SA for the activation or potentiation of defense responses (Liu et al., 2011).
In contrast to the above results, Attaran et al. (2009) reported that MeSA is not a critical signal for SAR. Analysis of two bsmt1 mutants revealed that both were impaired for MeSA accumulation following pathogen infection; however, they developed SAR and exhibited systemic accumulation of SA and PR-1 transcripts to comparable levels as wild-type plants. In an effort to explain why the bsmt1 mutants analyzed by Zeier and coworkers (Attaran et al., 2009) developed SAR, while the bsmt1 mutant reported by Klessig and coworkers (Liu et al., 2010b) did not, it was suggested that differences in experimental design, such as the developmental age of the plants at the time of infection, the light intensity, and/or the virulence of the pathogen, influenced SAR development and thus the requirement for MeSA (Liu et al., 2011).
In many plant species, resistance to pathogen infection increases as a function of age; this phenomenon is often termed age-related resistance (Develey-Rivière and Galiana, 2007). Thus, older plants often exhibit at least some level of resistance against pathogens that are virulent on younger plants. In addition, age-related resistance further enhances resistance to infection by avirulent pathogens, as manifested by decreased levels of pathogen replication and/or spread in older plants, as compared with younger plants (Rusterucci et al., 2005; Develey-Rivière and Galiana, 2007). Another factor that influences SAR is the pathogen used for the 1° inoculation. Traditionally, SAR was considered to be a form of resistance activated in response to infection with necrotizing pathogens (i.e. those that induce necrotic lesions at the infection site). However, virulent bacterial pathogens also induce SAR (Mishina and Zeier, 2007). Light is yet another factor that plays an important role in plant-pathogen interactions (Roden and Ingle, 2009). Both the intensity and the duration of light after infection have been shown to influence the development of resistance-associated necrotic lesions (known as the hypersensitive response [HR]) and the accumulation of PR-1 transcripts and SA as well as the development of local resistance and/or SAR (Lozano and Sequeira, 1970; Guo et al., 1993; Genoud et al., 2002; Zeier et al., 2004; Chandra-Shekara et al., 2006; Griebel and Zeier, 2008).
In this paper, we have manipulated each of these factors to assess whether they influence the ability of BSMT1-defective Arabidopsis and tobacco to develop SAR. Here, we report that the length of light exposure that plants receive after the 1° infection plays a critical role in determining the extent to which SAR development depends on MeSA. When pathogen infection was performed in the morning followed by an extended period of light, SAR development was not obligately dependent on MeSA. However, if infection occurred in the late afternoon followed by little or no light prior to the night/dark period, MeSA was essential for SAR development. This finding not only provides a resolution to the conflicting evidence concerning MeSA’s role as a SAR signal in Arabidopsis and tobacco but also underscores the influence of environmental factors on the plant’s response to infection and the need to consider these when comparing and contrasting results from different experiments.
RESULTS
The Time of Inoculation Influences SAR Development in bsmt1 Mutants
Previous studies generated conflicting results regarding the ability of Arabidopsis bsmt1 mutants to develop SAR and thus raised questions concerning MeSA’s role in signaling this response (Attaran et al., 2009; Liu et al., 2010b). Several factors might account for these conflicting results, including (1) Zeier’s group used 6-week-old plants while the plants used by Klessig and coworkers were 3 to 4 weeks old; (2) the light intensity used by Zeier’s group was roughly half as intense as that used by the Klessig’s laboratory; and (3) Zeier’s laboratory used virulent Pseudomonas syringae pv maculicola (Psm) for the 1° infection to induce SAR, while Klessig’s group used avirulent coronatine-deficient Psm carrying AvrRpt2 (Psm AvrRpt2 cor−). To determine whether any of these factors affect SAR development in the bsmt1 mutant background, they were altered independently or in combination. For most experiments, SAR was then assessed using both the bsmt1-1 T-DNA knockout mutant employed by Zeier and coworkers (Attaran et al., 2009) as well as the bsmt1-3 mutant used by Liu et al. (2010b).
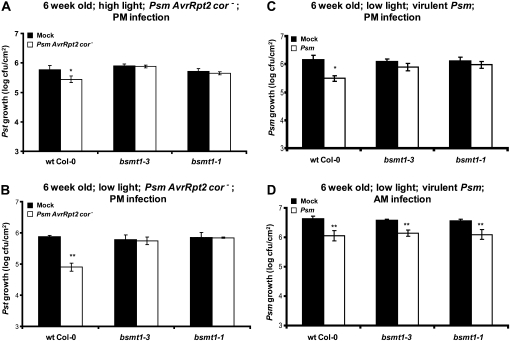
The first factor tested was whether the age of the plants at the time of infection influences SAR development. Six-week-old wild-type plants and bsmt1 mutants were inoculated on three leaves with either buffer or Psm AvrRpt2 cor− to induce SAR. The uninoculated leaves of these plants were challenged with virulent Pseudomonas syringae pv tomato DC3000 (Pst), and bacterial growth was monitored. In wild-type plants, a 1° infection with Psm AvrRpt2 cor− induced SAR, based on the approximately 5-fold reduction in the growth of virulent Pst as compared with mock-inoculated control plants (Fig. 1A). In contrast, SAR was not observed in bsmt1-3 or bsmt1-1, since Pst growth was comparable regardless of whether the 1° inoculum was buffer or Psm AvrRpt2 cor−. Since increasing the age of the plants at the time of inoculation did not confer SAR in the mutants, the combined effects of plant maturity and the lower light intensity used by Zeier’s group (photon flux density of 70 μE m−2 s−1) were assessed. SAR was not observed in bsmt1 mutants grown under these conditions (Fig. 1B). Thus, in an effort to fully replicate the conditions used by Attaran et al. (2009), SAR was assessed in 6-week-old plants that were grown under the lower intensity light and received a 1° inoculation with virulent Psm. In response to a 1° infection with virulent Psm, wild-type plants developed SAR while the bsmt1 mutants did not (Fig. 1C).
Figure 1.
Effects of different conditions on SAR development in wild-type and bsmt1 mutant plants. A and B, Growth of virulent Pst at 2 to 3 d post 2° inoculation on 6-week-old wild-type (wt) and bsmt1 mutant plants that were previously mock inoculated with MgCl2 (black bars) or inoculated with Psm AvrRpt2 cor− (SAR induction; white bars). C and D, Growth of virulent Psm strain ES4326 at 2 to 3 d post 2° inoculation on 6-week-old wild-type and bsmt1 mutant plants previously mock inoculated or Psm infected. Photon flux density of 140 μE m−2 s−1 (high light) was used in A, while 70 μE m−2 s−1 (low light) was used in B through D. The 1° and 2° infections in D were done in the morning (9:00–9:30 am) versus in the late afternoon for A through C (5:30–6:00 pm). All experiments were done at least twice with similar results; means of three replicates ± sd are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (* P < 0.05, ** P < 0.01, Student’s t test) between Pst (or Psm) growth in plants induced for SAR by Psm AvrRpt2 cor− (or Psm) versus growth on mock-inoculated controls for each genotype. Pst growth levels among the four panels cannot be compared, since the experiments were done separately over several weeks to months and basal resistance varies somewhat from experiment to experiment; thus, each experiment has an internal control. Note that the stronger SAR observed with low light (B) compared with high light (A) was not reproducible.
These unexpected results prompted us to search for other differences between the experimental protocols used for SAR analysis. Both Zeier’s and Klessig’s groups used a short-day (9 h of light) growth condition, which has been shown by several laboratories to stimulate a more robust and reproducible SAR response. However, Zeier’s group inoculated plants in the morning (am), while Klessig’s group generally infected plants in the late afternoon (between 5:30 and 6:00 pm). Thus, our plants received little to no light after infection before the long night period began (15 h; 6:00 pm–9:00 am). When the SAR analyses were repeated with the added factor of performing am 1° and 2° infections (9:00–9:30 am), the bsmt1 mutants were SAR proficient, restricting growth of the challenging Psm to a comparable extent as wild-type plants (Fig. 1D). Based on this result, the time at which the 1° and 2° infections are performed appears to be a critical factor in determining whether BSMT1 and its product, MeSA, are required for SAR development.
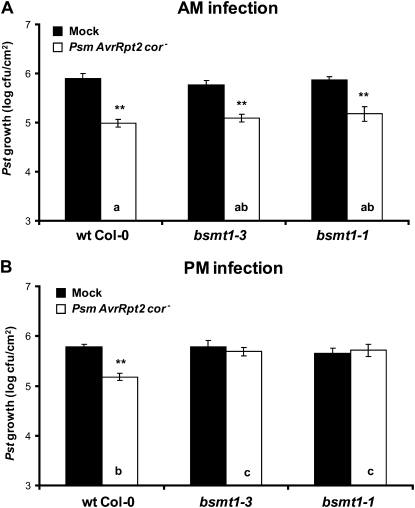
To assess whether am inoculations alone were sufficient to restore SAR in the bsmt1 mutants, or whether the combined effects of am infections, plant age, light intensity, and the virulence of the SAR-inducing pathogen were required, SAR was monitored after am or pm infections of plants grown under standard Klessig laboratory conditions (3- to 4-week-old plants grown under higher light intensity [140 μE m−2 s−1] and inoculated with avirulent Psm AvrRpt2 cor− for SAR induction). As reported previously (Liu et al., 2010b), pm-inoculated bsmt1 mutants were compromised for SAR development (Fig. 2B). In stark contrast, these mutants were SAR proficient when the inoculations were performed in the am (Fig. 2A). Together, these results suggested that the time of inoculation is a major factor influencing SAR development in the bsmt1 mutants.
Figure 2.
Time of infection during the day affects the requirement of MeSA for SAR development. The 1° and 2° infections of 3- to 4-week-old wild-type (wt) and bsmt1 mutant plants were done in the morning (A; 9:00–9:30 am) or late afternoon (B; 5:30–6:00 pm). Growth of Pst at 2 to 3 d post 2° inoculation from plants previously mock inoculated with MgCl2 (black bars) or infected with Psm AvrRpt2 cor− (SAR induction; white bars) is presented. All experiments were done at least twice with similar results; means of three replicates ± sd are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (** P < 0.01, Student’s t test) between Pst growth in plants induced for SAR by Psm AvrRpt2 cor− versus growth in mock-inoculated controls. Letters within the white bars indicate whether there was a statistically significant difference (P < 0.05) in Pst growth in Psm AvrRpt2 cor−-inoculated wild-type and bsmt1-3 plants that were subjected to different times (am and pm) of inoculation, using ANOVA and posthoc tests. Bars with the same letter indicate no significant difference, while bars with different letters indicate significantly different levels of Pst growth.
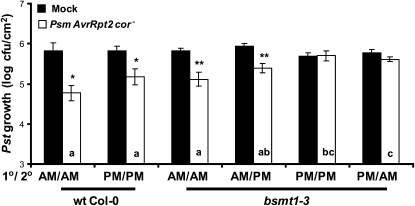
Timing of the 1°, But Not the 2°, Infection Is Critical for SAR in the bsmt1-3 Mutant
To assess whether the 1° infection, the 2° infection, or both must be performed in the am to induce SAR in bsmt1 mutants, wild-type and bsmt1-3 plants grown under standard Klessig laboratory conditions were subjected to 1° and/or 2° infections in either the am or pm using Psm AvrRpt2 cor− to induce SAR. bsmt1-3 as well as wild-type plants, which received the 1° inoculation in the am, developed SAR regardless of whether the 2° infection was in the am or pm (Fig. 3). Subjecting bsmt1-3 to a 1° inoculation in the pm failed to induce SAR, regardless of the time when the 2° inoculations were performed. Based on these results, the time of the 1° infection was primarily responsible for the strength of the SAR displayed by bsmt1-3.
Figure 3.
MeSA-independent induction of SAR requires extended light exposure after the inducing 1° infection but not after the challenging 2° infection. Three- to four-week-old wild-type (wt) and bsmt1 mutant plants were subjected to 1° or 2° infection in either the morning (9:00–9:30 am) or late afternoon (5:30–6:00 pm). Growth of the virulent Pst at 2 d post 2° inoculation on wild-type and bsmt1 mutant plants previously mock inoculated with MgCl2 (black bars) or infected with Psm AvrRpt2 cor− (SAR induction; white bars) is presented. The experiment was done twice with similar results; means of three replicates ± sd are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (* P < 0.05, ** P < 0.01, Student’s t test) between Pst growth in plants induced for SAR by Psm AvrRpt2 cor− versus growth on mock-inoculated controls. Letters within the white bars indicate whether there was a statistically significant difference (P < 0.05) in Pst growth in Psm AvrRpt2 cor−-inoculated wild-type and bsmt1-3 plants that were subjected to different times (am and pm) of inoculation, using ANOVA and posthoc tests. Bars with the same letter indicate no significant difference, while bars with different letters indicate significantly different levels of Pst growth.
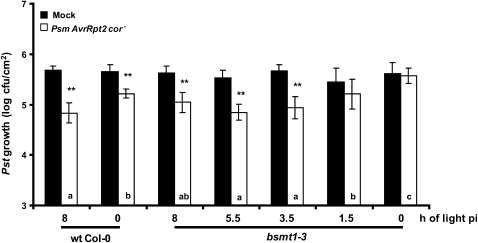
To determine the duration of light exposure required for MeSA-independent SAR development, wild-type and bsmt1-3 plants were subjected to increasing hours of light exposure after 1° and 2° infections. Consistent with prior results (Liu et al., 2010b), SAR development was compromised in bsmt1-3 when no light was provided after infection (Fig. 4). One and a half hours of light exposure after infection was not enough to induce SAR in bsmt1-3. Light exposure of 3.5 to 8 h resulted in SAR development. Interestingly, wild-type plants generally developed a stronger SAR when inoculation was done in the am versus the pm, as seen in Figures 2, 4, and 6 but not in Figure 3. Note that in each of the four sets of experiments whose results are presented in Figures 2, 3, 4, and 6, the same set of wild-type and bsmt1-3 plants were grown together and then divided for am versus pm infection (Figs. 2 and 3), for different lengths of light exposure before the dark/night period (Fig. 4), or for am versus pm versus 2,2,2,2′-tetra-fluoroacetophenone (tetraFA) treatment (Fig. 6), so that the only variable was the length of light exposure (except in Fig. 6, where treatment with tetraFA was also variable).
Figure 4.
The duration of light exposure after infection affects the requirement for MeSA during SAR development. Immediately following 1° and 2° inoculations, 3- to 4-week-old wild-type (wt) and bsmt1-3 plants were subjected to 8, 5.5, 3.5, 1.5, and 0 h of light. The growth of virulent Pst in uninoculated, distal tissues was monitored at 2 d post 2° inoculation in plants that previously received a mock inoculation with MgCl2 (black bars) or were infected with Psm AvrRpt2 cor− (SAR induction; white bars). Means of six replicates ± sd of the combined results from two experiments are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (** P < 0.01, Student’s t test) between Pst growth in plants induced for SAR by Psm AvrRpt2 cor− versus growth on mock-inoculated control plants for each genotype and duration of light exposure. Letters within the white bars indicate whether there was a statistically significant differences (P < 0.05) in Pst growth in Psm AvrRpt2 cor−-inoculated wild-type and bsmt1-3 plants that were subjected to different times (am and pm) of inoculation, using ANOVA and posthoc tests. Bars with the same letter indicate no significant difference, while bars with different letters indicate significantly different levels of Pst growth.
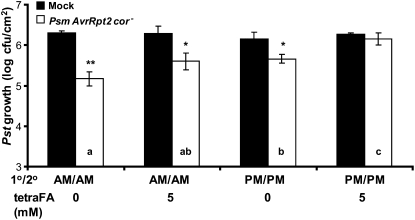
Figure 6.
Suppression of SAR development by inhibition of MeSA esterase activity with the synthetic SA analog tetraFA is light dependent. Ten millimolar HEPES buffer (pH 7.0) with or without 5 mm tetraFA was applied to uninoculated, distal leaves of 3- to 4-week-old wild-type Col-0 at 24 and 48 h post 1° infection, which was done either in the morning (9:00–9:30 am) or late afternoon (5:30–6:00 pm). Growth of the virulent Pst at 3 d post 2° inoculation on plants previously mock inoculated with MgCl2 (black bars) or infected with Psm AvrRpt2 cor− (SAR induction; white bars) is presented. The experiment was done twice with similar results; means of three replicates ± sd are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (* P < 0.05, ** P < 0.01, Student’s t test) between Pst growth in plants induced for SAR by Psm AvrRpt2 cor− versus growth on mock-inoculated controls. Letters within the white bars indicate whether there was a statistically significant difference (P < 0.05) in Pst growth in Psm AvrRpt2 cor−-inoculated plants that were subjected to different tetraFA treatments, using ANOVA and posthoc tests. Bars with the same letter indicate no significant difference, while bars with different letters indicate significantly different levels of Pst growth.
SAR Is Also Partially Restored by am Inoculations in MeSA Esterase-, DIR1-, and SFD1/GLY1-Deficient Arabidopsis but Not in FMO1-Defective Plants
We then tested whether SAR could be restored in other SAR-deficient mutants by subjecting them to 1° and 2° inoculations in the am. Our previous studies in Arabidopsis, tobacco, and potato (Solanum tuberosum; Park et al., 2007; Vlot et al., 2008; Manosalva et al., 2010) showed that MeSA esterase activity is required in the distal tissue for SAR development when inoculation was done in the afternoon. Consistent with these results, SAR did not develop in the med4-1 mutant, which is MeSA esterase deficient due to RNA interference (RNAi)-mediated silencing of multiple MeSA esterase-encoding genes, when inoculations were performed in the pm (Fig. 5; Vlot et al., 2008). However, when infections were performed in the am, SAR was at least partially restored. A similar result was observed when MeSA esterase activity was inhibited in the distal leaves by tetraFA, a synthetic analog of SA that inhibits MeSA esterases (Park et al., 2007; Manosalva et al., 2010). When both 1° and 2° infections were performed in the pm, the tetraFA-treated systemic leaves failed to develop SAR (Fig. 6). By contrast, am infections at least partially restored SAR in tetraFA-treated leaves.
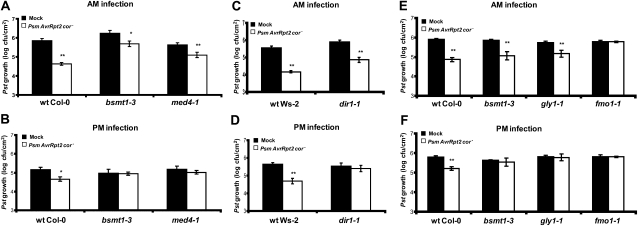
Figure 5.
Characterization of light-dependent induction of SAR in wild-type plants and SAR-deficient mutants bsmt1-3, med4-1, dir1-1, gly1-1, and fmo1-1. The 1° and 2° infections were done in the morning (9:00–9:30 am) or late afternoon (5:30–6:00 pm) in 3- to 4-week-old wild-type (wt), bsmt1-3, and med4-1 in which multiple MeSA esterases were silenced (A and B), dir1-1 (C and D), and gly1-1 and fmo1-1 (E and F). The bsmt1-3 mutant served as a control. Growth of the virulent Pst was determined at 2 to 3 d post 2° inoculation on wild-type and mutant plants previously mock inoculated with MgCl2 (black bars) or infected with Psm AvrRpt2 cor− (SAR induction; white bars). All experiments were done twice with similar results; means of three replicates ± sd are presented. Asterisks directly above each set of white/black bars indicate statistically significant differences (* P < 0.05, ** P < 0.01, Student’s t test) between Pst growth in plants induced for SAR by Psm AvrRpt2 cor− versus growth on mock-inoculated control plants for each genotype. Pst growth levels among the six panels cannot be compared, since experiments were done separately over several weeks to months and basal resistance varies somewhat from experiment to experiment; thus, each experiment has an internal control.
Since recent studies have linked DIR1 and SFD1/GLY1 to MeSA-dependent SAR development (Chanda et al., 2011; Liu et al., 2011), we examined whether the time of inoculation also influences SAR in the dir1-1 and gly1-1 mutants. As was observed for bsmt1-, med4-1-, and tetraFA-treated plants, SAR was restored in dir1-1 and gly1-1 that received am inoculations (Fig. 5, C and E).
We also tested whether am inoculations would restore SAR in the FLAVIN-DEPENDENT MONOOXYGENASE1(FMO1)-defective mutant. In addition to loss of SAR, fmo1 fails to accumulate SA or PR-1 transcripts in the systemic leaves but exhibits wild-type levels of resistance in the inoculated leaves (Mishina and Zeier, 2006). Due to the close correlation between systemic FMO1 expression and SAR development, FMO1 has been used as a marker for SAR (Mishina and Zeier, 2006, 2007). Unlike the other SAR-defective mutants tested in our study, am inoculations did not restore SAR in fmo1-1 (Fig. 5, E and F).
Systemic SA Accumulation Is Restored in am-Inoculated bsmt1-3
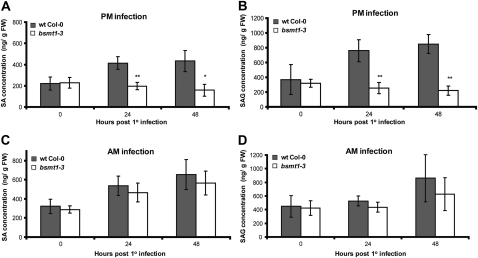
To assess whether SAR in am-inoculated bsmt1-3 correlates with the restoration of systemic SA and SAG accumulation, the levels of both compounds were quantified in the distal leaves of Psm AvrRpt2 cor−-infected bsmt1-3 and wild-type plants. Consistent with our previous results (Liu et al., 2010b), pm-inoculated wild-type plants exhibited a substantial increase in systemic SA and SAG levels by 48 h post 1° inoculation, whereas no increase was detected in bsmt1-3 plants (Fig. 7, A and B). In contrast, both wild-type and bsmt1-3 plants exhibited comparable increases in systemic SA and SAG levels following am inoculations (Fig. 7, C and D). Presumably, this increase is due primarily to de novo SA synthesis in the distal tissue, since bsmt1-3 is unable to produce elevated levels of MeSA in the 1° infected leaves for translocation to the distal tissue (Liu et al., 2010b).
Figure 7.
The effects of time of 1° infection on SA and SAG levels in the distal tissue. SA (A and C) and SAG (B and D) contents were quantified in uninoculated distal leaves of 3- to 4-week-old wild-type (wt) and bsmt1-3 plants infected in the morning (9:00–9:30 am) versus late afternoon (5:30–6:00 pm) with Psm AvrRpt2 cor−. The experiments in A and B were done twice with similar results; means of three replicates ± sd are presented. The data presented in C and D (means of six replicates ± sd) are the combined results from two independent experiments, each of which gave similar results. Asterisks directly above each set of white/black bars indicate statistically significant differences (* P < 0.05, ** P < 0.01, Student’s t test) between levels in bsmt1-3 versus wild-type Col-0 for each time point. The levels of SA and SAG cannot be compared between am (A and B) and pm (C and D) infection, since the experiments were done several months apart and SA and SAG levels vary between experiments, thus requiring internal controls in each experiment. FW, Fresh weight.
am Inoculations Partially Restore SAR in MeSA Esterase- and SA Methyl Transferase-Deficient Tobacco
Since the requirement for both MeSA and its metabolizing enzymes to signal SAR was initially identified in pm-inoculated tobacco (Park et al., 2007), we tested whether SAR in SABP2-silenced or SAMT1-silenced plants would be restored by am inoculations. Three lower leaves from 6-week-old wild-type tobacco and the silenced lines RNAi::NtSABP2 #1-2 and RNAi::NtSAMT1 #28 (Park et al., 2007) were inoculated with buffer or Tobacco mosaic virus (TMV). Five days post 1° infection, all plants were infected with TMV on the upper leaves, and the size of TMV-induced 2° lesions was measured 5 d later. In tobacco, SAR is manifested by a reduction in the size of 2° versus 1° necrotic lesions following infection with TMV; this reduction in 2° lesion size occurs because the plant is better able to restrict TMV replication and spread the second time it encounters the virus. When the 1° and 2° infections were done in the pm, the 2° lesions formed on wild-type tobacco previously given a 1° TMV infection were approximately 30% smaller than those on plants that received a mock 1° infection, indicating the development of SAR (Table I). In contrast, pm-inoculated RNAi::NtSABP2 #1-2 and RNAi::NtSAMT1 #28 transgenic lines did not develop SAR, as the 2° lesions were of similar size, regardless of whether the plants received a 1° infection with buffer or TMV. When TMV inoculations were performed in the am, the RNAi::NtSABP2 #1-2 and RNAi::NtSAMT1 #28 transgenic lines developed SAR. However, since the silenced lines displayed a 23% to 33% reduction in lesion size, while comparably inoculated wild-type plants exhibited a 43% reduction, MeSA appears to be required for maximal SAR induction in tobacco, as was generally the case in Arabidopsis.
Table I. Requirement of MeSA and its modulating esterases and methyl transferase for induction of SAR in tobacco is dependent on light.
Six-week-old wild-type, RNAi::NtSABP2 #1-2, and RNAi::NtSAMT1 #28 plants were subjected to 1° and 2° inoculations either in the morning (9:00–9:30 am) or late afternoon (5:30–6:00 pm). SAR development was assessed by monitoring the reduction in the size of 2° TMV lesions on plants that received a 1° inoculation of TMV versus that on plants that received a 1° inoculation of HEPES buffer (Mock). The sizes of lesions are presented as means ± sd. The experiment was done at least twice with similar results. N/A, Not applicable.
| am Infection | pm Infection | |||||||||||
| Parameter | Wild Type | RNAi::NtSABP2 #1-2 | RNAi::NtSAMT1 #28 | Wild Type | RNAi::NSABP2 #1-2 | RNAi::NtSAMT1 #28 | ||||||
| 1° Infection | Mock | TMV | Mock | TMV | Mock | TMV | Mock | TMV | Mock | TMV | Mock | TMV |
| 2° TMV lesion size (mm ± sd) | 1.58 ± 0.56 | 0.91 ± 0.33 | 1.99 ± 0.57 | 1.34 ± 0.53 | 1.67 ± 0.54 | 1.28 ± 0.44 | 1.69 ± 0.52 | 1.2 ± 0.56 | 1.66 ± 0.49 | 1.52 ± 0.52 | 1.48 ± 0.49 | 1.46 ± 0.48 |
| Reduction (%) in 2° lesion size to Mock | N/A | 42.8 | N/A | 32.8 | N/A | 23.2 | N/A | 29.1 | N/A | 8.4 | N/A | 1.3 |
| SAR | N/A | + | N/A | + | N/A | + | N/A | + | N/A | − | N/A | − |
Similar results were obtained when SABP2 activity was inhibited in the distal leaves by treating them with tetraFA. Consistent with previous results (Park et al., 2009), SAR was suppressed in tetraFA-treated leaves if the inoculations were performed in the pm (Table II). However, SAR was at least partially restored in tetraFA-treated plants if they were inoculated in the am.
Table II. Suppression of SAR development by inhibition of MeSA esterase activity with tetraFA in TMV-infected wild-type tobacco is dependent on light.
The 1° and 2° inoculations in 6-week-old wild-type tobacco were done either in the morning (9:00–9:30 am) or late afternoon (5:30–6:00 pm). Ten millimolar HEPES buffer (pH 7.0) with or without 10 mm tetraFA was applied to the uninoculated distal leaves 48 and 72 h post 1° infection with either TMV or HEPES buffer (Mock). The sizes of lesions are presented as means ± sd. The experiment was done twice with similar results. N/A, Not applicable.
| Parameter | am Infection | pm Infection | ||||||
| 1° Infection | Mock | TMV | Mock | TMV | Mock | TMV | Mock | TMV |
| 2° Treatment (tetraFA; mm) | 0 | 0 | 10 | 10 | 0 | 0 | 10 | 10 |
| 2° TMV lesion size (mm ± sd) | 1.72 ± 0.60 | 1.09 ± 0.62 | 1.59 ± 0.55 | 1.29 ± 0.48 | 1.76 ± 0.54 | 1.25 ± 0.38 | 1.63 ± 0.53 | 1.51 ± 0.55 |
| Reduction (%) in 2° lesion size to Mock | N/A | 36.6 | N/A | 18.9 | N/A | 29.0 | N/A | 7.4 |
| SAR | N/A | + | N/A | + | N/A | + | N/A | − |
DISCUSSION
Role(s) of Light in SAR and Other Immune Responses
The results presented here resolve the discrepancy between our previous studies and those of Zeier and coworkers. As was reported previously by Zeier’s group (Attaran et al., 2009), we found that if Arabidopsis bsmt1 mutants are inoculated in the am, MeSA is not obligately required for SAR. In contrast, when the 1° infection occurs late in the day, MeSA is required for SAR, just as we reported previously (Park et al., 2007; Vlot et al., 2008; Liu et al., 2010b, 2011; Manosalva et al., 2010). am inoculations also partially restored SAR in Arabidopsis and tobacco plants lacking MeSA esterase activity due to mutations, gene silencing, or treatment with tetraFA. Interestingly, am inoculations of wild-type plants generally induced an even stronger SAR response than was detected in either pm-inoculated wild-type plants or am-inoculated MeSA metabolism-defective Arabidopsis and tobacco. Based on these results, it appears that MeSA and its metabolizing enzymes are required not only for SAR in pm-inoculated plants but also for maximal SAR development in am-inoculated plants.
This work extends previous studies showing the importance of light for the plant’s immune response (for review, see Roden and Ingle, 2009). In a variety of plant species, defense responses in the inoculated leaf, such as HR formation, PR-1 expression, SA accumulation, and/or restriction of pathogen growth, are suppressed when plants are placed in continuous darkness after pathogen infection rather than left in the light (Lozano and Sequeira, 1970; Guo et al., 1993; Genoud et al., 2002; Zeier et al., 2004; Chandra-Shekara et al., 2006). Continuous darkness also blocked SAR development and the systemic accumulation of SA, SAG, and PR-1 transcripts in Arabidopsis inoculated with avirulent Psm avrRpm1 (Zeier et al., 2004). Here, we demonstrate that pm-inoculated Arabidopsis and tobacco, which are subjected to darkness before returning to the light, are still able to develop SAR, but this response is generally weaker than that displayed by plants inoculated in the am.
In addition to a qualitative requirement for light, analyses of pathogen-infected tobacco, rice (Oryza sativa), and Arabidopsis have revealed a quantitative aspect, as the strength of defense responses in the inoculated leaf directly correlates with the number of hours of light received after infection (Lozano and Sequeira, 1970; Zeier et al., 2004; Chandra-Shekara et al., 2006; Griebel and Zeier, 2008). In particular, detailed analyses of Arabidopsis inoculated with Psm avrRpm1 at different times of day revealed that the levels of SA accumulation, PR-1 expression, HR development, and restriction of pathogen growth in the inoculated leaf correlated with the length of light exposure received before entering a dark period: the longer the period of exposure, the stronger the SAR (Griebel and Zeier, 2008). Consistent with these results, we observed that SAR in pathogen-inoculated wild-type Arabidopsis and tobacco generally was stronger when the inoculations were performed in the morning rather than late afternoon. Further analyses revealed that performing the 1° inoculation in the am was critical for restoring SAR in the bsmt1-3 mutant, whereas the time of the 2° infection was less critical. The correlation between am 1° inoculations and heightened resistance could be due to the greater number of light hours after infection or to the circadian rhythm. Although we have not assessed these possibilities directly, the finding that midafternoon inoculations, which provided 3.5 h of light before the evening dark period, were sufficient to induce the maximum level of SAR achievable in the bsmt1-3 mutant suggests that it is the duration of light, rather than the circadian rhythm, that influences resistance levels. Griebel and Zeier (2008) came to a similar conclusion based on their observation that SA levels in Psm avrRpm1-inoculated Arabidopsis did not vary in accordance with the circadian rhythm.
The mechanism through which light regulates resistance is currently unclear. Recent studies have suggested that light and pathogen resistance are linked via chloroplasts, which are not only the light-harvesting centers of the cell but also the site of SA biosynthesis and reactive oxygen species production (Karpinski et al., 2003). Supporting this possibility, functional chloroplasts are required for pathogen-induced HR formation (Genoud et al., 2002). Furthermore, high-intensity light conditions, which induce photooxidative stress due to the excess excitation energy, induce a variety of responses associated with resistance, including increased levels of reactive oxygen species, programmed cell death, induction of PR-1 expression, and enhanced resistance to a virulent bacterial pathogen in both the treated and systemic tissue (Mühlenbock et al., 2008). Light and disease resistance also may be linked via cross-talk between photoreceptor signaling and components of the resistance pathway. Light-dependent resistance to Turnip crinkle virus was shown to require cryptochrome 2 and phototropin 2 (Jeong et al., 2010). By contrast, HR development and local resistance to infection by Pst avrRpt2 were found to be dependent on phytochromes A and B (Genoud et al., 2002). Analysis of phyAphyB inoculated with Psm avrRpm1 failed to detect a similar requirement for phytochromes during local resistance responses (Griebel and Zeier, 2008). However, phytochromes A and B were required for SAR, as Psm-inoculated phyAphyB plants failed to restrict the growth of the challenging bacteria, did not exhibit systemic SA accumulation, and failed to express PR-1 or FMO1 in the distal leaves. Consistent with FMO1 functioning downstream of a light-dependent step in SAR induction, we demonstrated that am inoculations fail to restore SAR in the fmo1-1 mutant. By contrast, MeSA and the lipid-associated signals appear to function either upstream of this light-dependent step or in a separate pathway, as defects in MeSA metabolism or lipid metabolism/transport did not block the ability of am inoculations to induce SAR.
Multiple Mobile SAR Signals
We (Vlot et al., 2009; Liu et al., 2011) and others (Zeier et al., 2004) have postulated that there are multiple mobile SAR signals that are produced at the site of 1° infection. In addition to MeSA, the GLY1 and DIR1 genes, whose products are involved in lipid metabolism and translocation, are required for SAR development (Maldonado et al., 2002; Chanda et al., 2011). A link between these signaling components was recently established with the discovery that pathogen-infected dir1-1 plants contain elevated levels of BSMT1 mRNA and MeSA but accumulate reduced levels of SA in the systemic leaves (Liu et al., 2011). In addition, local G3P treatment was shown to modulate the expression of BSMT1 and an SABP2 homolog in distal leaves (Chanda et al., 2011). The ability of am inoculations to restore SAR in the dir1-1 and gly1-1 mutants, as well as in the MeSA metabolism mutants bsmt1-3 and med4-1, and in tetraFA-treated plants provides further evidence for a linkage between these SAR signals.
An SA-independent pathway leading to SAR also has been proposed, based on the observation that very high light intensity (500 μmol photons m−2 s−1) conditions induced SAR in the absence of systemic SA accumulation or PR-1 expression (Zeier et al., 2004). One possible component of this light-dependent, SA-independent pathway is FMO1, as its expression in the inoculated leaf is mediated by an SA-independent pathway, and its expression in the systemic leaves is phytochrome dependent (Mishina and Zeier, 2006; Griebel and Zeier, 2008). The inability of am inoculations to restore SAR in plants defective for FMO1, in comparison with the MeSA metabolism and lipid metabolism/translocation mutants, also is consistent with the possibility that these SAR signaling components belong to distinct pathways that are regulated via different mechanisms. Since fmo1 plants display wild-type levels of SA in the Psm-inoculated leaves but no accumulation of SA in the systemic leaves, it was previously proposed that systemic SA accumulation in wild-type plants is due to de novo SA synthesis, rather than to transport from the inoculated leaf (Mishina and Zeier, 2006). Our analysis of the bsmt1-3 mutant similarly suggests that systemic SA accumulation is at least partially due to de novo synthesis. In response to an am inoculation, these plants accumulated SA in the systemic leaves despite their inability to generate MeSA and thereby translocate MeSA from the inoculated leaves. However, since pm inoculations did not induce SA accumulation in the bsmt1-3 mutant, de novo SA synthesis in the systemic leaves appears to be activated only under certain SAR-inducing conditions.
Taken together, we postulate that there are at least two pathways that work synergistically to induce SAR. In am-inoculated plants, the extended light exposure would activate a light-dependent pathway that requires FMO1 but not MeSA to induce SAR via SA generated de novo in the systemic leaves. A second, MeSA-dependent but light- and FMO1-independent, pathway also would be activated, and its contribution would be required for maximal SAR activation. In contrast, plants that receive a pm inoculation, followed shortly thereafter by a dark period, cannot activate the light-dependent pathway effectively. In this situation, the MeSA-dependent pathway, possibly combined with delayed and/or suboptimal activation of the light-dependent pathway, would be responsible for SAR induction.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) mutants used in this study, bsmt1-1 (SALK_140496), bsmt1-3 (SAIL_776_B10), med4-1, dir1-1, gly1-1, and fmo1-1, and the control plant (wild-type Columbia [Col-0] or Wassilewskija-2) have been described previously (Maldonado et al., 2002; Bartsch et al., 2006; Vlot et al., 2008; Attaran et al., 2009; Liu et al., 2010b, 2011; Chanda et al., 2011). Seeds for soil-grown plants were sown on autoclaved soil. After 3 d of prechilling at 4°C, the seeds were germinated and grown under a 9-h-light (9:00 am–6:00 pm)/15-h-dark photoperiod with photon flux densities of 140 or 70 μE m−2 s−1 at 22°C and 70% relative humidity. The 3- to 4-week-old or 6-week-old plants were used in the SAR assay as described in the figure legends. The T2 generation of transgenic SABP2-silenced line 1-2 (RNAi::SABP2 #1-2) and SAMT1-silenced line 28 (RNAi::SAMT1 #28) in the tobacco (Nicotiana tabacum ‘Xanthi nc’) background, which carries the N gene for resistance to TMV (Park et al., 2007), was grown in a growth chamber with a 14-h-light (8:00 am–10:00 pm)/10-h-dark photoperiod with photon flux density of 110 μE m−2 s−1 at 22°C and 70% relative humidity.
Pathogen Experiments
The induction of SAR by avirulent or virulent pathogens in Arabidopsis has been described previously (Attaran et al., 2009; Liu et al., 2010b, 2011). In brief, coronatine-deficient Pseudomonas syringae pv maculicola carrying AvrRpt2 (Psm AvrRpt2 cor−) or virulent Psm strain ES4326 at a concentration of 1 × 106 colony-forming units (cfu) mL−1 or 5 × 106 cfu mL−1, respectively, in 10 mm MgCl2 was infiltrated into abaxial surfaces of three leaves per plant with a 1-mL syringe to induce SAR in 3- to 4-week-old or 6-week-old plants grown under a 9-h-light/15-h-dark photoperiod. Plants infiltrated with 10 mm MgCl2 served as mock controls. At 2 to 3 d post 1° infection, two or three uninoculated distal systemic leaves were challenged with virulent Pseudomonas syringae pv tomato DC3000 (Pst) or Psm at a density of 1 × 105 or 1 × 106 cfu mL−1, respectively, in 10 mm MgCl2. Pst and Psm growth was determined by shaking 4-mm leaf discs taken at 2 to 3 d post 2° infection in 10 mm MgCl2 supplemented with 0.1 m Suc at room temperature for 4 h. The resulting bacterial suspensions were serially diluted, and spots of 20 μL per dilution were grown on Kings’ B medium with 20 μg mL−1 rifampicin, 50 μg mL−1 kanamycin, and 1.7% agar at 28°C for 48 h, after which time the number of colonies formed was counted. Each data point contains three replicates, and each replicate contains three leaf discs. The exception is Figure 4, in which we combined the data from two independent experiments.
The protocol for SAR experiments in tobacco was described previously (Park et al., 2007; Liu et al., 2010a). In brief, TMV at a concentration of 1 μg mL−1 in 10 mm HEPES buffer, pH 7.0, and mock (10 mm HEPES buffer, pH 7.0) inoculations were rubbed onto the lower three leaves with cheesecloth and carborundum, which was previously applied to the surface of leaves, of approximately 6-week-old tobacco plants. Five days post 1° infection with TMV or mock, three uninoculated systemic leaves of all plants were challenged with TMV. The size of 2° TMV lesions were measured at 5 d post 2° infection with a Vernier caliper as described previously (Liu et al., 2010a). Percentage reduction in size of the 2° lesions on plants previously receiving the 1° TMV infection compared with 2° lesions on 1° mock-infected tobacco served as a measure of SAR.
The infections/inoculations in Arabidopsis and tobacco during the day were done in the morning between 9:00 and 9:30 am or in late afternoon between 5:30 and 6:00 pm. The growth conditions in the growth chamber are described above. The 1° and 2° infections/inoculations in Arabidopsis or tobacco were both performed at either am or pm in all experiments in this study except in Figure 3, with the alternation of the time of 1° and 2° infections/inoculations during the day.
In Planta TetraFA Assay
The tetraFA was obtained from Rieke Metals. The use of tetraFA for inhibition of the MeSA esterase activities in Arabidopsis and tobacco SAR has been described previously (Park et al., 2009). In brief, three lower leaves of 3- to 4-week-old Arabidopsis or 6-week-old tobacco were infected/inoculated with Psm AvrRpt2 cor− at a concentration of 1 × 106 cfu mL−1 in 10 mm MgCl2 or TMV at a concentration of 1 μg mL−1 in 10 mm HEPES buffer, pH 7.0, respectively. Ten millimolar HEPES buffer (pH 7.0) with or without tetraFA (5 mm tetraFA with Arabidopsis and 10 mm tetraFA with tobacco) was applied to the uninfected/uninoculated leaves by using a fine glass chromatography sprayer at 24 and 48 h post 1° infection in Arabidopsis or at 48 and 72 h post 1° infection in tobacco. After 24 or 48 h post tetraFA treatment in Arabidopsis or tobacco, respectively, the tetraFA-treated leaves were challenged with the virulent Pst or TMV. The SAR measurements in Arabidopsis and tobacco are described above.
SA and SAG Quantification
SA and SAG were extracted and quantified from approximately 250 mg of tissue per sample using HPLC analysis on an ARH-601 organic acids column (100 mm × 6.5 mm; Transgenomic, Inc.) run at 55°C in 0.01 n H2SO4 with a flow rate of 0.6 mL min−1, as described previously (Liu et al., 2010b). Three to four leaves per plant from approximately 40 plants grown under short-day conditions were used for each genotype and time point.
Acknowledgments
We thank D’Maris Dempsey for critically reading the manuscript; Drs. Robin K. Cameron, Jane E. Parker, and Pradeep Kachroo for providing seeds of the Arabidopsis dir1-1 mutant and its control plant (wild-type Wassilewskija-2), the fmo1-1 mutant, and the gly1-1 mutant, respectively; Dr. Seth Davis for asking the critical question (when were we infecting the plants?); Drs. Gerold Beckers and Patricia Manosalva for providing unpublished results; Drs. Zhangjun Fei and Shan Gao for assistance with statistical analyses; and Drs. Corina Vlot, Dhirendra Kumar, and Sang-Wook Park for helpful discussions.
References
- Attaran E, Zeier TE, Griebel T, Zeier J. (2009) Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21: 954–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, et al. (2011) Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43: 421–427 [DOI] [PubMed] [Google Scholar]
- Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. (2006) Light-dependent hypersensitive response and resistance signaling against Turnip crinkle virus in Arabidopsis. Plant J 45: 320–334 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R, Shah J. (2008) Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J 54: 106–117 [DOI] [PubMed] [Google Scholar]
- Develey-Rivière M-P, Galiana E. (2007) Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol 175: 405–416 [DOI] [PubMed] [Google Scholar]
- Genoud T, Buchala AJ, Chua NH, Métraux JP. (2002) Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J 31: 87–95 [DOI] [PubMed] [Google Scholar]
- Griebel T, Zeier J. (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Reimers PJ, Leach JE. (1993) Effect of light on incompatible interactions between Xanthomonas oryzae and rice. Physiol Mol Plant Pathol 42: 413–425 [Google Scholar]
- Jeong R-D, Chandra-Shekara AC, Barman SR, Navarre D, Klessig DF, Kachroo A, Kachroo P. (2010) Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc Natl Acad Sci USA 107: 13538–13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. (2009) Priming in systemic plant immunity. Science 324: 89–91 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. (2003) Light perception in plant disease defence signalling. Curr Opin Plant Biol 6: 390–396 [DOI] [PubMed] [Google Scholar]
- Liu P-P, Bhattacharjee S, Klessig DF, Moffett P. (2010a) Systemic acquired resistance is induced by R gene-mediated responses independent of cell death. Mol Plant Pathol 11: 155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-P, von Dahl CC, Park S-W, Klessig DF. (2011) Interconnection between methyl salicylate and lipid-based long-distance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiol 155: 1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-P, Yang Y, Pichersky E, Klessig DF. (2010b) Altering expression of benzoic acid/salicylic acid carboxyl methyltransferase 1 compromises systemic acquired resistance and PAMP-triggered immunity in Arabidopsis. Mol Plant Microbe Interact 23: 82–90 [DOI] [PubMed] [Google Scholar]
- Lozano JC, Sequeira L. (1970) Differentiation of races of Pseudomonas solanacearum by leaf infiltration technique. Phytopathology 60: 833–838 [Google Scholar]
- Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419: 399–403 [DOI] [PubMed] [Google Scholar]
- Manosalva PM, Park SW, Forouhar F, Tong LA, Fry WE, Klessig DF. (2010) Methyl esterase 1 (StMES1) is required for systemic acquired resistance in potato. Mol Plant Microbe Interact 23: 1151–1163 [DOI] [PubMed] [Google Scholar]
- Mishina TE, Zeier J. (2006) The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141: 1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina TE, Zeier J. (2007) Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J 50: 500–513 [DOI] [PubMed] [Google Scholar]
- Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20: 2339–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116 [DOI] [PubMed] [Google Scholar]
- Park SW, Liu P-P, Forouhar F, Vlot AC, Tong L, Tietjen K, Klessig DF. (2009) Use of a synthetic salicylic acid analog to investigate the roles of methyl salicylate and its esterases in plant disease resistance. J Biol Chem 284: 7307–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden LC, Ingle RA. (2009) Lights, rhythms, infection: the role of light and the circadian clock in determining the outcome of plant-pathogen interactions. Plant Cell 21: 2546–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF. (1961) Systemic acquired resistance induced by localized virus infections in plants. Virology 14: 340–358 [DOI] [PubMed] [Google Scholar]
- Rusterucci C, Zhao Z, Haines K, Mellersh D, Neumann A, Cameron RK. (2005) Age-related resistance to Pseudomonas syringae pv. tomato is associated with the transition to flowering in Arabidopsis and is effective against Peronospora parasitica. Physiol Mol Plant Pathol 66: 222–231 [Google Scholar]
- Shah J. (2009) Plants under attack: systemic signals in defence. Curr Opin Plant Biol 12: 459–464 [DOI] [PubMed] [Google Scholar]
- Truman W, Bennett MH, Kubigsteltig I, Turnbull C, Grant M. (2007) Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci USA 104: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J. (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA 103: 5602–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J. (1994) Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vlot AC, Liu P-P, Cameron RK, Park SW, Yang Y, Kumar D, Zhou FS, Padukkavidana T, Gustafsson C, Pichersky E, et al. (2008) Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J 56: 445–456 [DOI] [PubMed] [Google Scholar]
- Zeier J, Pink B, Mueller MJ, Berger S. (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219: 673–683 [DOI] [PubMed] [Google Scholar]