Abstract
A controlled flow of porphyrin metabolites is critical for organisms, but little is known about the control of porphyrin biosynthesis under environmental stress. We monitored transgenic rice (Oryza sativa) plants expressing Myxococcus xanthus protoporphyrinogen oxidase (PPO) for their response to drought stress. Transgenic plants showed significantly improved drought tolerance, as indicated by a higher shoot water potential, less oxidative damage, and a more favorable redox balance compared with wild-type plants. Both transgenic and wild-type plants responded to the onset of drought stress, even prior to changes in shoot water potential and oxidative metabolism, by drastically scavenging porphyrin intermediates in leaves, which was crucial for alleviating reactive oxygen species-induced stress. Protoporphyrin IX, protochlorophyllide, magnesium-protoporphyrin IX, and its methyl ester were absent or hardly detected with the intensification of water stress (–3.1 MPa) in the wild type, whereas transgenic plants retained these intermediates to some extent. Additionally, the expression and activity of most enzymes involved in porphyrin biosynthesis, particularly in the chlorophyll branch, were primarily down-regulated under dehydrating conditions, with stronger repression in the wild type than in transgenic plants. There was up-regulation of Glutamate 1-Semialdehyde Aminotransferase, PPO1, and Fe Chelatase2 transcripts in drought-stressed transgenic plants, enabling the transgenic plants to make larger pools of 5-aminolevulinic acid and protoporphyrin IX available for subsequent steps in the heme branch. Overexpression of PPO ultimately protected the transgenic plants from drought-induced cytotoxicity, demonstrating clearly that manipulation of porphyrin biosynthesis can produce drought-tolerant plants. Our results support a possible role for tetrapyrroles in signaling their metabolic state and in plant protection under drought stress conditions.
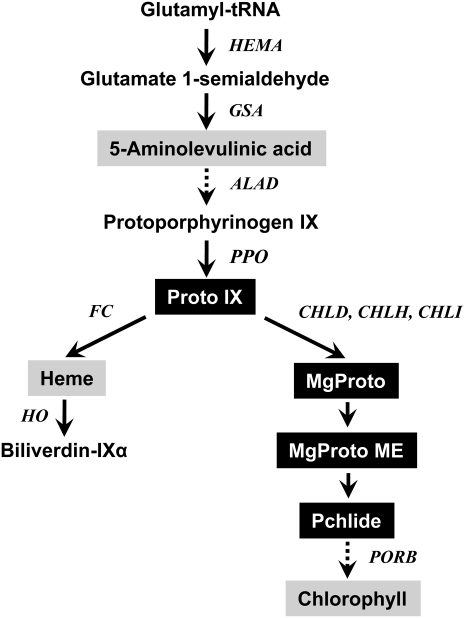
Different forms of tetrapyrroles function in plants as electron carriers, signaling factors, and catalysts for redox reactions. Tetrapyrrole biosynthesis starts at glutamyl-tRNAGlu, and the subsequently formed 5-aminolevulinic acid (ALA) is metabolized to form tetrapyrroles through a variety of reactions (Beale and Weinstein, 1990; Fig. 1). Protoporphyrinogen oxidase (PPO) is the last enzyme before the branch in the tetrapyrrole biosynthetic pathway, and its product, protoporphyrin IX (Proto IX), is directed to the magnesium (Mg) and iron (Fe) branches for chlorophyll and heme biosynthesis, respectively. Many intermediates in the porphyrin biosynthetic pathway, such as Proto IX and its various Mg2+ derivatives, including protochlorophyllide (Pchlide), interact with reactive oxygen species (ROS) such as singlet oxygen, which is harmful to cells and causes the peroxidation of membrane lipids (Valenzeno, 1987; Dolphin, 1994; Reinbothe et al., 1996). All living organisms face the danger of uncontrolled chemical reactions involving tetrapyrroles, due to their remarkable reactivity (Jung et al., 2004, 2008; Kariola et al., 2005; van Lis et al., 2005; Yao and Greenberg, 2006).
Figure 1.
The porphyrin pathway in plants showing intermediates and genes analyzed in this study. Intermediates quantified in this study are highlighted. CHLD, D-subunit of Mg-chelatase; CHLH, H-subunit of Mg-chelatase; CHLI, I-subunit of Mg-chelatase.
Each enzymatic step involved in porphyrin synthesis is tightly regulated to avoid the phytotoxic accumulation of intermediates while ensuring sufficient enzymatic capacity and continuous cofactor supply to the cognate apoproteins (Tanaka and Tanaka, 2007). The latter is exemplified by the coordination of chlorophyll synthesis with the production of light-harvesting chlorophyll proteins (Lhcs) encoded by the nucleus, which is in part mediated by retrograde signals from the chloroplast to the nucleus (Strand et al., 2003; Nott et al., 2006; Ankele et al., 2007). In addition, tetrapyrrole synthesis and degradation are carefully adjusted to the cellular requirements, reflecting the different needs under varying environmental conditions (Reinbothe and Reinbothe, 1996; Tewari and Tripathy, 1998; Wilson et al., 2003). There is a certain concentration threshold for photosensitizing porphyrin(ogen)s per light interval, below which plastids with their highly efficient antioxidative defense system can keep these metabolites in their reduced, and therefore nontoxic, state as well as sufficiently detoxify basal levels of photosensitization products (Keetman et al., 2002). Once this limit is exceeded, excited porphyrin(ogen)s tend to spread into other cellular compartments that are less well protected against their photodynamic action.
Aside from photosensitizing porphyrins, drought stress can also lead to a substantial accumulation of superoxide, hydrogen peroxide (H2O2), hydroxyl radicals, and singlet oxygen that need to be detoxified in order for plants to achieve drought tolerance (Chaves and Oliveira, 2004; Wang et al., 2005). The ability of CO2 fixation to use reducing power is decreased under drought conditions. In other words, absorbed energy exceeds the demands of CO2 fixation, and as a result, ROS are produced. Cellular water deficit can result in solute concentration, changes in cell volume and membrane shape, disruption of the water potential gradient, turgor loss, membrane integrity disruption, and protein denaturation, thereby reducing stem and root elongation, leaf expansion, and stomatal movement (Hsiao, 1973; Bray, 1997). Accumulation of a compatible solute such as Pro may play a role not only in dehydration avoidance, by increasing the cellular solute content and thus maintaining a higher water content, but also in dehydration tolerance, by protecting protein and membrane structure, regulating redox status, or acting as a scavenger of ROS (Smirnoff and Cumbes, 1989; Hare et al., 1998). The extent of stress-induced damage can also be attenuated by the action of the cell’s antioxidant systems, including ascorbate (Asc), glutathione (GSH), and enzymes capable of scavenging ROS (Asada, 1999; Mittler, 2002). During stress, the pools of cellular Asc and GSH shift toward their oxidized forms and the redox state of Asc and GSH is decreased (Hendry et al., 1992; Tommasi et al., 1999). The major events in plant responses to dehydration stress are perception and transduction of the stress signals through signaling components along with the resulting activation of a large number of stress-related genes to counteract the detrimental conditions (Bray, 1997). Water deficit is probably the most important stress factor determining plant growth and productivity, and many approaches have been sought for improving the performance of crops grown under periodic drought conditions (Capell et al., 2004; Gutterson and Zhang, 2004; Nelson et al., 2007).
Here, we report that drought treatment has a dramatic effect on the metabolic regulation of the porphyrin biosynthetic pathway. The sustained level of porphyrin intermediates during drought resulted in enhancing tolerance of transgenic rice (Oryza sativa) plants overexpressing Myxococcus xanthus PPO. We found that drought stress controls metabolite levels in the porphyrin biosynthetic pathway, providing novel information on how porphyrin levels are regulated to overcome drought-caused stress conditions. Finally, we propose a model for the mechanism linking porphyrin metabolism to plant drought responses and possibly stress tolerance, in which certain tetrapyrrole intermediates may have roles as indicators of metabolic imbalance in the plastids under stress conditions.
RESULTS
Effects of Drought on Water Relations in Wild-Type and Transgenic Rice Plants Overexpressing PPO
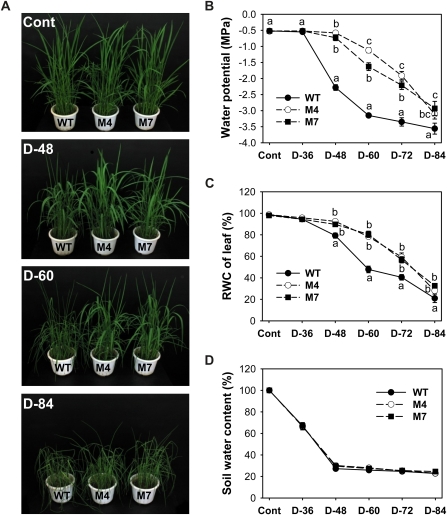
Drought tolerance was obtained with rice plants expressing M. xanthus PPO, demonstrating the potential of this strategy for improving drought tolerance in crop plants (Fig. 2). Our previous work showed that these transgenic plants increased chloroplastic and mitochondrial PPO activity, which was sufficient to promote the peroxidizing herbicide tolerance (Jung et al., 2004). To study the role of tetrapyrroles in drought tolerance, plants were subjected to drought treatment that consisted of withholding watering for 84 h. The transgenic plants expressing M. xanthus PPO typically exhibited much less wilting than wild-type plants after a severe drought period. During growth under optimal conditions, transgenic plants did not obviously differ in appearance from wild-type plants. During the drought period, wild-type plants wilted progressively, whereas transgenic plants did not show any drought symptoms until 48 h after drought and then partially wilted after 60 h of drought (Fig. 2A). The decreased water availability can be quantified as a decrease in shoot water potential (ΨW). Compared with transgenic plants, wild-type plants wilted faster after 60 h without watering, reaching their lowest value of –3.1 MPa shoot ΨW (Fig. 2B). The relative water content (RWC) of wild-type plants was reduced during 60 h of drought stress, reaching 48% of control values (Fig. 2C). However, the RWC of transgenic plants was merely reduced 17% to 21% during drought, showing that transgenic plants were more resistant than wild-type plants in maintaining ΨW, even though they partially wilted. After 84 h of prolonged drought, transgenic plants also suffered severe water stress. Soil water content greatly decreased during the early drought period, and the decline was similar between wild-type and transgenic plants (Fig. 2D). Although altered water status is a factor in a number of abiotic stresses, it is of most obvious importance in drought. The data clearly indicate that transgenic plants overexpressing PPO have a greater tolerance to drought-induced stress conditions compared with wild-type plants.
Figure 2.
Transgenic rice plants overexpressing M. xanthus PPO exhibited more tolerance to water deficit stress than wild-type (WT) rice plants. A, Phenotypes of wild-type and transgenic plants before and after water withholding for 48 to 84 h. B, Changes in ΨW. The results of shoot water parameters are given as a pooled mean of six separate pots with three replicates in each. C, RWC of leaves. D, Soil water content. Wild-type and transgenic rice plants expressing M. xanthus PPO were grown in the greenhouse under optimal conditions for 4 weeks. Well-watered plants were subjected to water stress by withholding irrigation. Cont, Nondesiccated controls; D-36, -48, -60, -72, and -84, water withholding for 36, 48, 60, 72, and 84 h, respectively; M4 and M7, homozygous lines of transgenic rice plants. Values are means ± se of six replicates from two independent experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Drought-Mediated Changes in Oxidative Stress, Redox State, and Transcript Levels of Some Stress-Related Genes
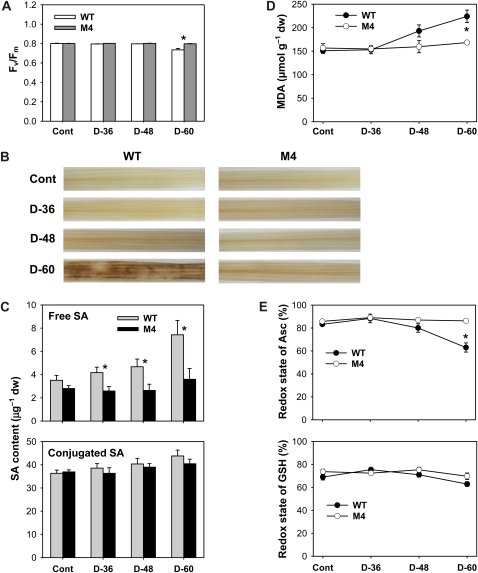
To gain an understanding of the physiological observations of the drought response summarized in Figure 2, a representative transgenic line (M4) was chosen for further in-depth analysis. The effect of drought on photosynthetic performance was verified by measuring changes in photochemical quantum efficiency (Fv/Fm), a trait positively correlated with the organization and vitality of PSII. Levels of Fv/Fm remained constant in both wild-type and transgenic plants until 48 h after drought and then decreased by 8% in wild-type plants 60 h after drought as compared with control plants (Fig. 3A). The accumulation of H2O2 and oxidatively damaged lipids is a marker of oxidative stress in plant tissues. To investigate whether the overexpression of M. xanthus PPO in rice reduces ROS generation in drought-treated tissues, control and drought-treated leaves were incubated with 3,3-diaminobenzidine (DAB) for the detection of H2O2 production. Whereas leaves from transgenic plants did not show any marked production of H2O2 before or during drought, leaves from wild-type plants exhibited dramatically increased H2O2 content 60 h after drought (Fig. 3B). Drought stress causes ROS accumulation that needs to be detoxified in order for plants to cope with photooxidative damage, and salicylic acid (SA) is known to induce ROS during the acquisition of freezing tolerance (Mora-Herrera et al., 2005). Levels of free SA accumulated with prolonged drought stress, with a greater increase in wild-type plants than in transgenic plants, although accumulations of conjugated SA were less than those of free SA in response to drought (Fig. 3C). We assayed rice plants for malondialdehyde (MDA) content, which is a measure of lipid peroxidation. MDA content in transgenic plants did not change during drought periods, whereas the MDA content increased up to 50% in wild-type plants after withholding water for 60 h (Fig. 3D). Membrane disruption by nonenzymatic lipid peroxidation destroys cellular compartments, causes loss of solutes and dehydration, and finally leads to cell death (Mock et al., 1999). To cope with increased levels of cellular oxidative stress, plants have evolved different antioxidant mechanisms.
Figure 3.
Oxidative metabolism and cellular redox state in leaves of wild-type (WT) and transgenic plants exposed to drought stress. A, Photosynthetic performance (Fv/Fm). B, H2O2-DAB staining in leaves. Brown spots represent H2O2 localization. C, Free and conjugated SA levels. D, MDA levels. E, Redox state of Asc and GSH, estimated as Asc × 100/Asct, where Asct = DHA + Asc, and GSH × 100/(oxidized GSH + GSH). The plants were subjected to the same treatments as in Figure 2, and treatment notations are the same as in Figure 2. Values are means ± se of six replicates from two independent experiments. * Significant differences between wild-type and transgenic samples as calculated by t test (P < 0.05). dw, Dry weight.
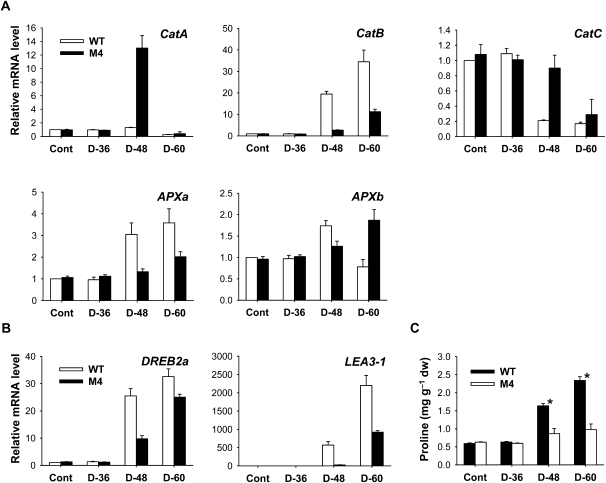
To test whether drought stress modifies cellular redox metabolism, Asc and GSH contents and antioxidant gene expression were used as markers. The redox state of Asc (estimated as Asc × 100/dehydroascorbate [DHA] + Asc) decreased significantly in wild-type plants after 60 h of drought, whereas transgenic plants maintained a constant redox state of Asc (Fig. 3E). Transgenic plants can rely on these antioxidative systems, which were less affected by the prolonged drought, to counteract the potentially harmful effects of drought. In contrast to Asc, the redox ratio of GSH was not greatly influenced by either moderate or severe drought stress in wild-type and transgenic plants. Transcript levels of genes encoding ROS-scavenging enzymes corresponded well with changes in redox state. In parallel with the decline in Asc redox state (Fig. 3E), both wild-type and transgenic plants responded to drought by up-regulating transcript levels of Catalase (Cat) B and Ascorbate Peroxidase (APX) a with a greater increase in the wild type (Fig. 4A). This can be seen as a strengthening in the capacity of the leaves to decompose H2O2. Transcript levels of CatA and CatC decreased in response to drought in wild-type and transgenic plants, with a greater decline in wild-type plants, except that CatA transcripts greatly increased in transgenic plants after 48 h of drought. Prolonged drought resulted in an increase in APXb transcript levels in transgenic plants, although the levels increased with mild water stress and then decreased with severe water stress in wild-type plants.
Figure 4.
Expression of stress-responsive genes and Pro induced by drought treatment in leaves of wild-type (WT) and transgenic rice plants. A, Expression analysis of ROS-scavenging genes by qRT-PCR. B, Expression analysis of drought-responsive genes by qRT-PCR. Total RNAs were purified from plants and reverse transcribed. The resultant cDNAs were used as templates for qRT-PCR using Actin as an internal control. The wild-type control was used for normalization, with the expression level of the sample set to 1. Error bars represent se, and representative data from three independent experiments are presented. C, Pro contents. Values are means ± se of six replicates from two independent experiments. * Significant differences between wild-type and transgenic samples as calculated by t test (P < 0.05). The plants were subjected to the same treatments as in Figure 2, and treatment notations are the same as in Figure 2. dw, Dry weight.
DREB2A and LEA3-1, which encode dehydration-responsive element-binding (DREB) and late embryogenesis abundant (LEA) proteins, respectively, were strongly induced in response to drought, with a greater increase in the wild type than in transgenic plants (Fig. 4B). DREB2s are transcription factors that specifically interact with DRE/CRT elements, which are involved in the expression of genes responsive to cold and drought stress in Arabidopsis (Arabidopsis thaliana; Stockinger et al., 1997; Qin et al., 2007). Plant adaptation to environmental stresses is often associated with mechanisms of dehydration avoidance through metabolic adjustment, such as the accumulation of Pro and soluble sugars (Ábrahám et al., 2003). To characterize the physiological basis for the improved stress tolerance of transgenic rice, we checked their Pro contents under normal growth and drought stress conditions. Under normal conditions, no difference in Pro content between the transgenic and wild-type plants was observed in the leaves (Fig. 4C). Under drought conditions, wild-type plants began to accumulate Pro after 48 h and further accumulated up to 4-fold higher Pro content after 60 h compared with levels prior to drought stress, whereas transgenic plants showed a 50% increase in Pro after 60 h. This result demonstrates that Pro accumulation does not correspond with the increased drought tolerance of transgenic plants.
Distribution of Tetrapyrrole Metabolites Is Greatly Altered during Drought
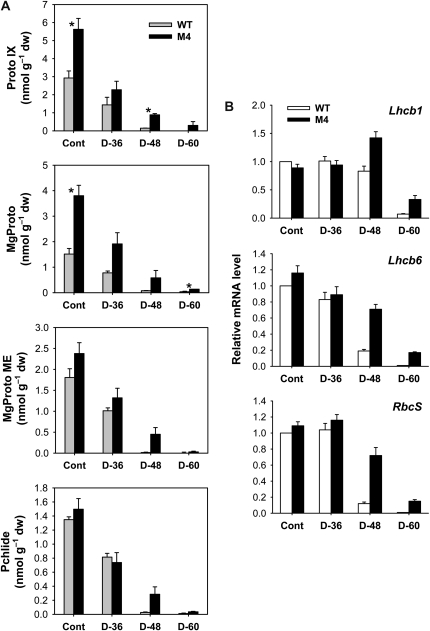
To assess the relationship between porphyrin biosynthesis and drought tolerance as well as the metabolic activities in the branched pathway of Mg- and Fe-porphyrin synthesis, we examined the effects of drought stress on the regulation of porphyrin biosynthetic intermediates in leaves. Strikingly, drought treatment in transgenic and wild-type plants caused a drastic and immediate decline in Proto IX, a common precursor for the chlorophyll and heme branches, 36 h after drought (Fig. 5A), although there was no indication of drought symptoms in the plants. After 60 h of dehydration, the potent photosensitizer Proto IX was not detected in wild-type plants, whereas transgenic plants retained Proto IX to some extent. Drought also decreased Mg-tetrapyrroles, including Mg-protoporphyrin IX (MgProto), MgProto methyl ester (MgProto ME), and Pchlide, with a greater decline in wild-type plants (Fig. 5A). After 84 h of drought, transgenic plants also showed drastic declines in these intermediates (Supplemental Fig. S1). Taken together, we conclude that Proto IX and Mg-porphyrin intermediate levels are all greatly reduced in plants following drought treatment. The degradation dynamics of these photosensitizing porphyrins may lead to reduced ROS production and an altered redox state of the plastid. Second, the sustained porphyrin level in the PPO-overexpressing transgenic plants coincides with their increased drought tolerance, although it remains necessary to explain how maintaining porphyrin levels leads to tolerance.
Figure 5.
Distribution of porphyrin metabolites and expression of nucleus-encoded photosynthetic genes in leaves of wild-type (WT) and transgenic plants. A, Proto IX and Mg-branch intermediates. Values are means ± se of six replicates from two independent experiments. *Significant differences between wild-type and transgenic samples as calculated by t test (P < 0.05). B, Expression analysis of nucleus-encoded photosynthetic genes by qRT-PCR. Total RNAs were purified from plants and reverse transcribed. The resultant cDNAs were used as templates for qRT-PCR using Actin as an internal control. The wild-type control was used for normalization, with the expression level of the sample set to 1. Error bars represent se, and representative data from three independent experiments are presented. The plants were subjected to the same treatments as in Figure 2, and treatment notations are the same as in Figure 2. dw, Dry weight.
The expression levels of nucleus-encoded photosynthetic genes were determined in order to evaluate their relationship with MgProto under drought stress. During drought, reduced accumulation of Mg-Proto corresponded with decreased expression of Lhcb1 and Lhcb6, the genes for Lhcb proteins of PSII, with a greater decline in wild-type leaves relative to transgenic leaves (Fig. 5B). However, Lhcb1 transcript increased in drought-stressed transgenic plants at 48 h. In response to drought, wild-type plants exhibited a greater decline in the RbcS gene encoding the small subunit of Rubisco compared with transgenic plants (Fig. 5B). These results provide an indication that nucleus-encoded photosynthetic genes can be down-regulated without an accumulation of MgProto under drought stress conditions. By contrast, Mg-porphyrin accumulation led to a down-regulation of Lhcb and other nuclear genes under oxidative stress conditions imposed by norflurazon (Strand et al., 2003).
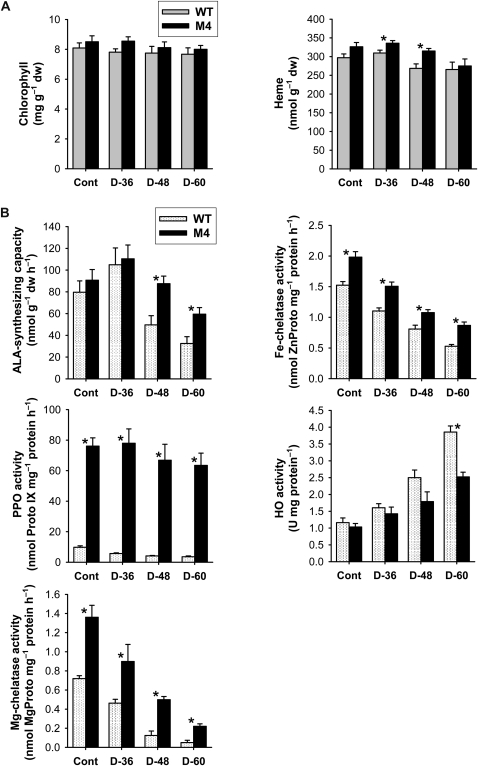
Despite the marked decline in the Proto IX and Mg-porphyrin intermediates, the end product of the Mg-porphyrin branch, chlorophyll, was not altered significantly in response to drought in leaves of wild-type and transgenic plants (Fig. 6A). Heme, the product of the Fe-porphyrin branch, was reduced merely 10% to 15% in transgenic and wild-type plants 60 h after drought (Fig. 6A), implying that plants retained the capacity to fulfill the demand for heme in drought-stressed plants. Noncovalently bound heme is the cofactor of hemoproteins, such as plastidic and mitochondrial cytochromes as well as peroxidases and catalases (Reinbothe and Reinbothe, 1996). Heme content in transgenic plants was 17% higher compared with that of wild-type plants after 48 h of drought. Under drought stress, transgenic plants thus retained a greater supply of porphyrin intermediates and processed them at a higher rate for heme metabolic flux than did wild-type plants.
Figure 6.
Effects of drought on metabolic activities of the porphyrin biosynthetic pathway in leaves of wild-type (WT) and transgenic plants. A, Chlorophyll and heme contents. B, Porphyrin-synthesizing enzyme activity. The plants were subjected to the same treatments as in Figure 2, and treatment notations are the same as in Figure 2. Values are means ± se of six replicates from two independent experiments. * Significant differences between wild-type and transgenic samples as calculated by t test (P < 0.05). dw, Dry weight.
Drought Treatment Has a Dramatic Effect on Gene Expression and Enzyme Activity in the Tetrapyrrole Metabolic Pathway
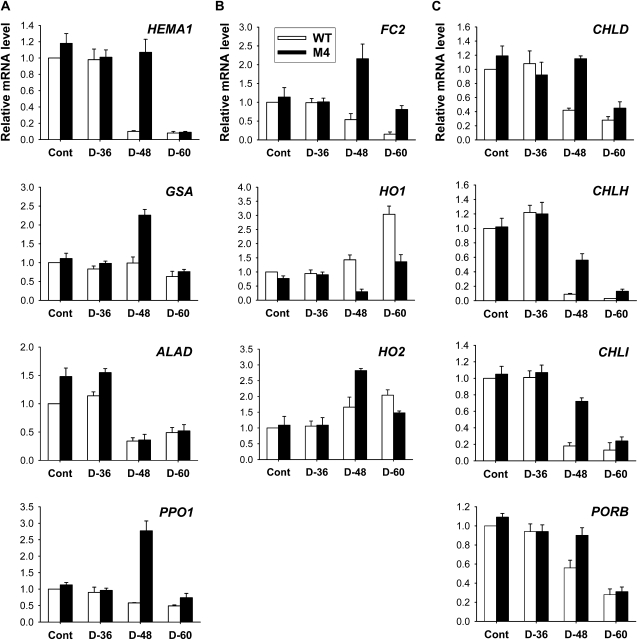
To gain insight into the molecular mechanisms underlying the PPO overexpression-induced changes in drought tolerance, we used enzyme assays and quantitative reverse transcription (qRT)-PCR to analyze the expression of the enzymes with known regulatory roles in tetrapyrrole biosynthesis. There was no marked difference in the expression of most porphyrin biosynthetic genes between wild-type and transgenic leaves before drought treatment (Fig. 7). To examine the drought-dependent regulation of the early biosynthetic steps before the branch point (Fig. 1), we assayed for the expression of genes involving ALA-synthesizing activity, HEMA and Glutamate 1-Semialdehyde Aminotransferase (GSA). Transcript levels of HEMA1 encoding glutamyl-tRNA reductase began to diminish greatly in wild-type and transgenic plants at 48 and 60 h after drought, respectively (Fig. 7). Although GSA transcript levels were slightly decreased in wild-type plants after 60 h of drought, they increased in transgenic plants after 48 h of drought but were not sustained at that level after 60 h of drought. ALAD, which encodes ALA dehydratase that converts ALA to porphobilinogen, was greatly suppressed with drought stress in wild-type and transgenic plants. The capacity of transgenic plants to synthesize ALA, a key precursor in the biosynthesis of porphyrins, was compared with that of wild-type plants under drought conditions. Unlike the transient ALA increase in the early stage of drought stress in wild-type and transgenic plants, the plants could not maintain the capacity to accumulate ALA with progressive dehydration (Fig. 6B), indicating that the drastic loss in Mg-tetrapyrrole-synthesizing activities during drought also causes an alteration in the control of ALA biosynthesis. The decline in ALA-synthesizing capacity was much greater in wild-type plants than in transgenic plants. Transcript levels of PPO1, encoding the enzyme for the last step before the branch point, decreased with the progression of dehydration in leaves of wild-type and transgenic plants, except that PPO1 dramatically increased in transgenic plants at 48 h of drought (Fig. 7). The ectopic expression of M. xanthus PPO was almost constant throughout drought periods in leaves of transgenic plants, with a slight decline after 48 h of drought (Supplemental Fig. S2). During drought stress, foliar levels of total PPO activity decreased greatly in wild-type plants but decreased slightly in transgenic plants. Overall, transgenic plants had greater levels of total PPO transcripts and about 18 times higher PPO activity compared with wild-type plants 60 h after drought.
Figure 7.
Drought-induced changes in the expression of genes encoding the porphyrin pathway enzymes in leaves of wild-type (WT) and transgenic plants. A, Common branch. B, Heme branch. C, Chlorophyll branch. The plants were subjected to the same treatments as in Figure 2, and treatment notations are the same as in Figure 2. Total RNAs were purified from plants and reverse transcribed. The resultant cDNAs were used as templates for qRT-PCR using Actin as an internal control. The wild-type control was used for normalization, with the expression level of the sample set to 1. Error bars represent se, and representative data from three independent experiments are presented.
As drought stress markedly affected the levels of Mg-tetrapyrrole intermediates, we considered whether the change in the chlorophyll-heme ratio could be explained by a regulatory switch in Proto IX distribution effected through regulating the genes encoding two enzymes at the branch point, Fe- and Mg-chelatase. During the progression of drought stress, down-regulation of Fe Chelatase2 (FC2), which encodes the plastidic isoform of Fe-chelatase, and its enzyme activity was noticeable in wild-type plants (Figs. 6B and 7), although there is the need to synthesize an elevated level of heme under stress conditions. Transgenic plants, however, revealed an increased level of FC2 transcript at 48 h of drought and a lesser decrease in Fe-chelatase activity than wild-type plants during drought stress. Transcript levels of HO1, encoding heme oxygenase (HO), which catalyzes the formation of biliverdin IXα from heme (Muramoto et al., 2002), greatly increased 60 h after drought stress in leaves of wild-type plants only, whereas HO2 transcript levels increased in both wild-type and transgenic plants. In contrast to the decreased activities of other porphyrin enzymes, HO activity continuously increased during drought periods, with a greater increase in wild-type plants than in transgenic plants (Fig. 6). In the chlorophyll branch, the genes encoding the three Mg-chelatase subunits CHLD, CHLH, and CHLI displayed similar expression profiles in response to drought. Parallel to the decrease in CHLD, transcript levels of CHLH and CHLI were reduced in the drought-stressed plants, while their levels in transgenic plants were higher in comparison with those of wild-type plants (Fig. 7). During drought, Mg-chelatase activity, which inserts Mg into Proto IX, exhibited a more drastic decline than did Fe-chelatase and PPO activity or ALA-synthesizing capacity (Fig. 6B). Wild-type plants decreased the transcript level of Protochlorophyllide Oxidoreductase B (PORB), encoding the enzyme that generates chlorophyllide from Pchlide, faster than did transgenic plants in response to drought stress (Fig. 7). These data are consistent with the observation that drought-treated plants have a markedly reduced capacity to synthesize porphyrin intermediates. In addition to their higher PPO activity, transgenic plants also maintained higher activities of Mg-chelatase and Fe-chelatase than wild-type plants under both optimal and drought conditions (Fig. 6B). It is possible that the strong expression of PPO1 and FC2 in transgenic plants ensured the cofactor supply for hemoproteins that may be required in response to severe oxidative stress imposed by drought.
Root Response to Drought and Its Relation to Heme Metabolism
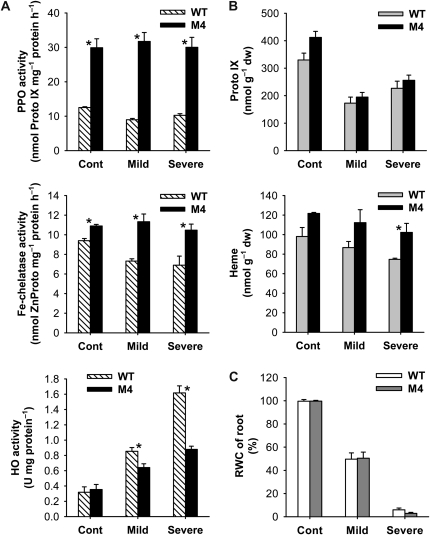
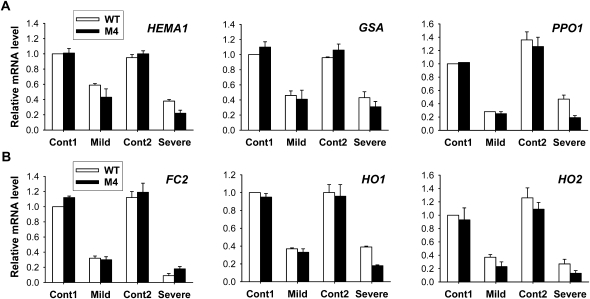
Roots are the very place where plants first encounter water stress and are able to sense and respond to the stress condition. Because of the distinct drought responses between the wild-type and transgenic plants, we tested whether PPO overexpression affected the morphological characteristics of the roots by measuring root weight, number, and length in plants grown under optimal conditions, due to the difficulty in observing alterations of root growth in soil during drought periods. Roots of the transgenic plants contained 5% less dry matter and nearly 10% increased numbers of roots compared with those of the wild type (Supplemental Fig. S3). Under drought stress imposed by air-drying treatment, there was no significant difference in the RWC of roots between wild-type and transgenic plants (Fig. 8). Since the plant heme oxygenases are involved in root development (Cao et al., 2007), we investigated the regulatory role played by HO and/or heme in root morphology as well as the drought-dependent regulation of tetrapyrrole metabolism in the root system. Transcript levels of all tetrapyrrole synthesis genes examined significantly decreased in roots subjected to drought stress by air drying (Fig. 9). After exposure to severe drought, transgenic roots exhibited greater declines in transcript levels of most genes, except FC2, compared with wild-type roots (Fig. 9). Transcript levels of M. xanthus PPO in transgenic roots increased with mild drought but decreased with severe drought (Supplemental Fig. S2), whereas transcript levels of OsPPO greatly decreased during drought. Although total PPO activity in roots of both wild-type and transgenic plants remained almost constant throughout the drought periods, transgenic roots had a 3-fold higher PPO activity than wild-type roots (Fig. 8). Unlike in leaves, levels of Proto IX in roots were much lower and not drastically scavenged during drought stress in wild-type and transgenic plants. In drought-stressed roots, HO enzyme activity increased continuously with prolonged drought stress, with a greater increase in wild-type plants than in transgenic plants, whereas transcript levels of HO1 and HO2 were greatly decreased compared with those of controls (Figs. 8 and 9). In response to drought, wild-type roots had slightly decreased heme contents as a result of a slight decrease in Fe-chelatase activity. Transgenic roots maintained a constant Fe-chelatase activity level and a slightly decreased heme content during drought, but their levels were greater than those of wild-type plants (Fig. 8). This work contributes to our understanding of regulatory functions of HO for root development as well as root responses to drought. The above results indicate that growth characteristics of the PPO-overexpressing plants (i.e. the development of more roots) are not likely to be the physiological basis for the enhanced performance of plants exposed to water stress conditions.
Figure 8.
Drought responses of the root system in the porphyrin biosynthetic pathway. A, Metabolic enzyme activities. B, Metabolic intermediates. C, RWC of roots. Three-week-old plants were transferred from soil to half-strength Hoagland solution, hydroponically grown for 1 week, and then subjected to drought stress by air-drying treatment. Cont, Nondesiccated controls; Mild, mild drought stress under air drying for 4 h; Severe, severe drought stress under air drying for 8 h. Values are means ± se of six replicates from two independent experiments. * Significant differences between wild-type (WT) and transgenic samples as calculated by t test (P < 0.05). dw, Dry weight.
Figure 9.
Drought responses of the root system in the expression of genes encoding the porphyrin enzymes related to heme metabolism. A, Common branch. B, Heme branch. The plants were subjected to the same treatments as in Figure 8. Cont1 and Cont2, Nondesiccated controls for mild and severe drought stress, respectively; Mild, mild drought stress under air drying for 4 h; Severe, severe drought stress under air drying for 8 h. Total RNAs were purified from plants and reverse transcribed. The resultant cDNAs were used as templates for qRT-PCR using Actin as an internal control. The wild-type (WT) control was used for normalization, with the expression level of the sample set to 1. Error bars represent se, and representative data from three independent experiments are presented.
DISCUSSION
Considering its importance as the most crucial component in energy transfer, catalysis, and signal transduction, porphyrin metabolism that links with other cellular processes needs to be better characterized. Both transgenic and wild-type plants responded to the onset of drought stress by drastically scavenging porphyrin intermediates. All living organisms must have evolved strategies to quench the potentially harmful porphyrin compounds or their derived ROS. In most cases, photodestruction is prevented by a tight control of porphyrin metabolism (Reinbothe and Reinbothe, 1996) as well as by the induction of several protective antioxidants (Asada, 1999; Niyogi, 1999; Holt et al., 2005). During drought periods, the M. xanthus PPO-expressing transgenic rice plants were more resistant to drought and maintained a higher level of porphyrin intermediates than wild-type plants, demonstrating that porphyrin status is tied to drought tolerance.
Overexpression of PPO Confers Drought Tolerance and Altered Redox Status in Transgenic Rice
Under drought stress, which is another source of ROS production, a decrease in ΨW makes it more difficult for plants to take up water, and this elicits responses that allow the plant to avoid water loss, continue water uptake at a reduced ΨW, or tolerate reduced tissue water content (Verslues et al., 2006). Prolonged drought stress treatments showed that transgenic rice plants were better able to maintain shoot ΨW during drought than wild-type rice plants, even though they partially wilted after 60 h of drought and wilted to a high extent after 84 h (Fig. 2). The RWC of wild-type plants also decreased more quickly than that of transgenic plants, resulting in severe wilting during drought. As a consequence of PPO overexpression, transgenic plants suffered lower levels of free radical reactions in tissues, as indicated by smaller increases in H2O2 and MDA, as well as a lesser decline in photosynthetic performance, Fv/Fm, compared with wild-type plants (Fig. 3). Accumulating light-absorbing porphyrins evoke the generation of ROS, which can add directly to the double bonds of polysaturated fatty acids to form lipid peroxides (Hess, 2000; Girotti and Kriska, 2004) and possibly signaling cascades that stimulate gene activation (op den Camp et al., 2003). Overall, our results support that overexpression of M. xanthus PPO confers drought tolerance in transgenic rice.
In response to abiotic stresses, plants have evolved an array of protective mechanisms (e.g. accumulation of compatible osmolytes) to adjust the intracellular osmotic potential and act as scavengers of ROS (Smirnoff and Cumbes, 1989; Hare et al., 1998). Under drought, both transgenic and wild-type plants accumulated Pro to avoid cellular damage by stress avoidance, although wild-type plants accumulated a higher level of Pro than did transgenic plants (Fig. 4C). The accumulation of Pro thus does not explain the enhanced drought tolerance of PPO-overexpressing plants. Another aspect of dehydration tolerance is the control of the ROS level or the limitation of the damage caused by ROS. The steady-state levels of ROS depend on the balance between generation and removal, which is facilitated by the ROS-scavenging system of the cell (Bartoli et al., 2004; Gechev et al., 2006). In parallel with the induction of H2O2, free SA greatly accumulated only in the wild type (Fig. 3), implying that wild-type plants were experiencing severe drought stress. The up-regulation of DREB2A and LEA3-1, known to accumulate in response to decreases in tissue water content (Close, 1997), was coincident with SA accumulation in drought-stressed rice plants, with a stronger induction in the wild type (Figs. 3C and 4B). The application of SA to Arabidopsis also triggered a significant accumulation of DREB2A, which might result from SA-amplified ROS synthesis (Chini et al., 2004). These results demonstrate that SA-dependent ROS signaling is critical for plant responses to dehydration. The Asc redox ratio, indicative of the cellular redox balance, substantially decreased in wild-type plants at 60 h of drought (Fig. 3E), accounting for ROS perception, especially in the control of H2O2 levels. The severe drought symptoms in wild-type plants can be interpreted as a deficiency in meeting the increased requirement for redox equivalents during drought. However, the PPO-overexpressing transgenic plants maintained their Asc redox ratio throughout drought periods, and this efficient scavenging of ROS is likely a contributing factor to plant survival during drought. Cells express a set of H2O2-decomposing enzymes, namely catalase and ascorbate peroxidase, which are heme enzymes with Proto IX moieties (Feierabend, 2005; Mittler and Poulos, 2005). Alleviation of drought symptoms in transgenic plants was accompanied by greater transcript levels of stress-responsive transcripts such as CatA, CatC, and APXb compared with those of wild-type plants (Fig. 4A), possibly contributing to the suppressed ROS levels and enhanced tolerance in transgenic plants exposed to drought. During drought stress, unbound heme must be incorporated into a multitude of ROS scavengers distributed in different compartments of plant cells.
Why Does Drought Cause a Marked Scavenging of Tetrapyrrole Photosensitizers?
A controlled flow of metabolites in the tetrapyrrole biosynthetic pathway is essential for the fitness of photosynthetic organisms (Lermontova and Grimm, 2006), and plants suffer severe photodynamic damage if these control mechanisms are circumvented, for example, in plants treated with peroxidizing herbicides (Ichinose et al., 1995; Ha et al., 2004; Jung et al., 2004) or plants with deregulation of porphyrin biosynthetic genes (Lermontova and Grimm, 2006; Jung et al., 2008; Peter et al., 2010). Our data provide evidence that drought treatment has striking effects on the regulatory mechanism of tetrapyrrole biosynthesis in plants. Proto IX, MgProto, MgProto ME, and Pchlide in leaves of wild-type and transgenic plants decreased markedly following the onset of drought stress compared with the nonstressed plants (Fig. 5A), showing that those plants cannot maintain flux through the tetrapyrrole pathway. The rapid disappearance of Proto IX and Mg-tetrapyrrole intermediates may result from tetrapyrrole-degrading processes, which allow the maintenance of cellular integrity and resistance against phototoxic intermediates, as well as increased conversion of the intermediates into chlorophyll and/or heme. Increased demand for ROS-scavenging enzymes may contribute to porphyrin scavenging related to heme supply during drought. Plants can also use strategies to avoid their formation by down-regulating porphyrin production. The decline of photosensitizing, harmful porphyrins is obviously important to avoid porphyrin-mediated photodynamic damage under excess ROS levels imposed by drought. By contrast, roots did not show a drastic decline in Proto IX throughout drought periods (Fig. 8B), suggesting that the scavenging of porphyrin intermediates under drought is light dependent. Free porphyrins are extremely harmful compounds, as they can become cytotoxic by producing powerful radicals in the presence of light (Wagner et al., 2004; Mochizuki et al., 2010). Since tetrapyrrole intermediates were efficiently removed during drought stress, the sensing and signaling mechanism that detects changes in plant water status might also act as a porphyrin scavenger. Considering the absence or very low levels of porphyrin intermediates in wild-type plants, transgenic plants were markedly less affected by the same drought treatment, suggesting that overexpression of M. xanthus PPO might protect the plants from porphyrin-induced cytotoxicity. Our results also establish that the tetrapyrrole biosynthetic pathway is regulated by environmental factors that cause an imbalance in substrate flow through the pathway. This is corroborated by other studies that found Mg-Proto accumulation and Pchlide decline in chilling-stressed Chlorella vulgaris cells (Wilson et al., 2003) and in chill- and heat-stressed cucumber (Cucumis sativus) seedlings (Tewari and Tripathy, 1998), respectively. Inconsistent with the effects of drought stress, the production of ROS in response to the exposure of plants to intense light causes Proto IX to accumulate (Aarti et al., 2006).
Tetrapyrrole intermediates and end products not only possess regulatory functions within their pathway but also play roles in interorganellar signaling (Poyton and McEwen, 1996; Strand et al., 2003; Nott et al., 2006; Ankele et al., 2007). MgProto accumulates under norflurazon-induced oxidative stress conditions, is exported from the chloroplast, and transmits the plastid signal to the cytosol, negatively regulating nuclear photosynthetic gene expression. By contrast, we found that MgProto failed to accumulate under drought stress conditions and was at levels well below that of the control in wild-type and transgenic plants (Fig. 5). Sensing of the reduced levels of tetrapyrrole intermediates may be partially responsible for the down-regulation of the nuclear photosynthetic genes Lhcb and RbcS in drought-stressed plants. Since the expression of the chlorophyll-binding proteins was inhibited, thus channeling the available Proto IX toward the heme branch, the flux of Proto IX toward the chlorophyll branch was substantially reduced during drought stress. Our results are in accordance with the finding that the norflurazon-induced down-regulation of nuclear gene expression can occur without any accumulation of MgProto (Moulin et al., 2008). MgProto cannot itself be acting as a signaling intermediate, and possible candidates for the signal might be a degradation derivative or side products (e.g. ROS) of MgProto (Mochizuki et al., 2008). Apart from these functions, tetrapyrrole intermediates may also regulate a variety of cellular processes, such as the drought responses in our study.
Metabolic Control of the Porphyrin Biosynthetic Pathway at the Branch Point
Expression levels of most genes in the porphyrin biosynthesis pathway were strongly down-regulated by drought stress in both leaves and roots, which is consistent with the need to eliminate the synthesis of potentially dangerous tetrapyrroles. The scavenging of Mg-porphyrin intermediates in drought-stressed leaves may result from the suppressed expression of CHLD, CHLH, CHLI, and PORB as well as the marked decrease in Mg-chelatase activity (Figs. 5–7). Notably, most genes in the chlorophyll branch maintained higher expression levels in transgenic plants relative to those of wild-type plants during drought, sustaining a higher Mg-chelatase activity. In addition to its enzymatic functions as a subunit of Mg-chelatase in producing the photosynthetic apparatus, CHLH has a key function in mediating plastid-to-nucleus retrograde signaling by controlling the metabolism of the tetrapyrrole signal MgProto or by sensing the signal (Mochizuki et al., 2001; Strand et al., 2003; Nott et al., 2006). In order to cope with the decline of tetrapyrrole intermediates, early drought stress transiently stimulated ALA-synthesizing capacity in leaves of wild-type and transgenic plants. During prolonged drought, HEMA1 and GSA transcript levels were down-regulated, and consequently, reduced levels of ALA and other porphyrin metabolites were found in wild-type and transgenic plants. Down-regulation of the porphyrin biosynthesis genes, including GSA, HEMA, ALAD, CHLI, and FC, has also been shown in chill-stressed seedlings (Mohanty et al., 2006). Despite the disappearance of tetrapyrrole intermediates, the levels of the end product of the pathway, chlorophyll, were maintained during drought in transgenic and wild-type plants. It may be that chlorophyll catabolism was not much affected by the degradation activity of tetrapyrrole intermediates during early drought. Remarkably, overexpression of M. xanthus PPO led to a transient increase of OsPPO and M. xanthus PPO transcripts in transgenic leaves and roots, respectively, during drought, sustaining much higher PPO activity compared with those of wild-type plants (Figs. 6–8; Supplemental Fig. S2). This alleviated the reduction of tetrapyrrole intermediates in transgenic plants, suggesting that PPO might play an important role as a key regulatory enzyme when the tetrapyrrole biosynthetic pathway is disrupted under drought stress.
Unlike the large reduction in the Mg-porphyrin intermediates during drought, leaves of wild-type and transgenic plants showed a transient increase in heme content (Figs. 5 and 6), which may fulfill physiological purposes related to the inevitable oxidative stress accompanying water stress. Heme is the cofactor of many enzymes participating in the cellular respiration and detoxification of ROS and lipophilic xenobiotics (Beale and Weinstein, 1990; Papenbrock and Grimm, 2001). During prolonged drought stress, a slight decrease in heme was observed with a lesser decrease in Fe-chelatase activity in wild-type and transgenic leaves, which was distinguished from the drastic loss of Mg-chelatase activity. In drought-stressed transgenic leaves, the higher level of heme coincided with the lesser decline in FC2 and HEMA1 transcripts and Fe-chelatase activity as compared with wild-type leaves; this is likely needed for enhanced drought tolerance. Also, roots of transgenic plants maintained higher levels of heme and Fe-chelatase activity than those of wild-type plants during drought (Fig. 8). As a result, transgenic plants appeared to keep porphyrin intermediates at certain levels, redirecting retained intermediates for heme synthesis to respond to stress conditions. During stress, the sustained heme level in the plastid is required to protect cellular structures from ROS damage. Heme may leave the plastid and be allocated to heme-binding proteins, including ROS-scavenging enzymes, in various compartments of the cell (Vanhee et al., 2011). ROS was found to be involved in the induction of AtHEMA2 and AtFC1 in photosynthetic tissues under ozone-induced oxidative stresses (Nagai et al., 2007), and SA treatment was also able to replace the stress-induced AtFC1 expression (Singh et al., 2002). These results suggest that the requirement for heme synthesis is part of the defense response, probably for defensive hemoproteins. In our study, however, increased levels of SA and H2O2 were determined independently of the induction of HEMA1 and FC2 in drought-stressed plants, and levels of free SA were inversely related to levels of tetrapyrrole intermediates.
Additionally, heme is suggested to be degraded in the plastid by HO to generate biliverdin IXα, a known antioxidant molecule (Otterbein et al., 2003; Balestrasse et al., 2005). The amount of tomato (Solanum lycopersicum) HO1 protein and transcript increases in parallel with lateral root development (Guo et al., 2008; Yang et al., 2008), and heme-related proteins, including RLF, which has a cytochrome b5-like heme/steroid-binding domain (Ikeyama et al., 2010), and GLB1 (a class 1 hemoglobin protein; Hunt et al., 2002), are also proposed to be involved in lateral root development. We characterized whether the overexpression of PPO causes alterations in root growth and morphology by influencing the balance between leaves and roots in terms of heme metabolism. Compared with wild-type plants, slightly higher levels of heme in leaves and roots, rather than phenotypes of less dry matter and a large number of roots under nonstress conditions, may confer some degree of tolerance in transgenic plants when exposed to stress conditions (Fig. 8; Supplemental Fig. S3). In both roots and leaves, HO enzyme activities continuously increased during the progression of drought stress, with a greater increase in the wild type. Although transcript levels of HO1 and HO2 decreased in drought-stressed roots, their transcript levels greatly increased in drought-stressed leaves (Figs. 6–9). Based on these data, we suggest that HO is involved in drought stress responses through modulating redox signaling by interaction with heme molecules and in possible relation to root development. The highly expressed HO1 isoform in plants is up-regulated by various abiotic stresses and abscisic acid (Shekhawat and Verma, 2010). On the other hand, HO2 is suggested to function as a Proto IX storage protein or a carrier protein for Mg- and/or Fe-chelatase through HO2 binding to Proto IX, not to heme (Gisk et al., 2010). Taken together, redistribution of heme through leaf-to-root communication may take part in the transcriptional regulation of tetrapyrrole biosynthesis genes for tolerating stress conditions. The signal from sensing altered tetrapyrrole status could trigger a regulator that either ties in gene expression with the metabolic pathway of tetrapyrroles or is part of an intracellular regulatory network of drought stress responses. Although an overlapping regulatory role of heme and MgProto in transcription within the nucleus has been suggested by von Gromoff et al. (2008), the nature of the signal that communicates between the chelating enzyme activities at the branch point is still in question.
Do Sustained Porphyrin Levels in Transgenic Plants Have a Role in Tolerance to Drought Stress?
Drought stress itself leads to a substantial accumulation of ROS that needs to be detoxified (Chaves and Oliveira, 2004; Wang et al., 2005); therefore, even the normal level of photosensitizing tetrapyrrole intermediates can be excessive under drought conditions. Our study demonstrates that rice plants responded to drought stress with a fast decline in tetrapyrrole intermediates, which occurred even in the absence of a detectable change in ΨW and oxidative metabolism. A minor change in the status of water or ROS within plant cells can help trigger distinct alterations in tetrapyrrole metabolism. Due to the photosensitizing property of the intermediates, their decline may have an immediate role in mitigating further damage caused by drought. In addition to a marked decline in Mg-chelatase activity, the lowering of MgProto/MgProto ME/Pchlide pool levels in drought-stressed plants can be expected to correlate with the reduced expression of genes in the chlorophyll branch, allowing a sustained flux through the heme biosynthetic pathway. Scavenging of porphyrins might be required immediately after exposure to drought; however, in the long term, these activities are likely to be detrimental to plants by affecting photosynthesis, which is a physiological process closely related to this pathway, and by competing for heme allocation to ROS scavengers. During early drought, inactivation of photochemical efficiency, as indicated by a decline in Fv/Fm, was substantially lower, considering the great reduction in porphyrin intermediates. Therefore, we conclude that the sustained levels of chlorophyll and heme during drought are prerequisites for plants to maintain photosynthesis and defense responses to some extent. Based on our findings that transgenic rice plants expressing M. xanthus PPO are more tolerant to drought than wild-type plants, we suppose that the regulatory mechanism for porphyrin metabolism is part of the complex protective system against water stress. It is possible that PPO overexpression as well as higher transcript levels of HEMA1, GSA, and FC2 make the transgenic plants direct higher pools of ALA and Proto IX to the heme branch, leading to an increased production of ROS scavengers in drought-treated leaves. Most importantly, however, their porphyrin intermediates should be at levels below the threshold that can cause porphyrin-mediated photosensitization. Sustained high levels of PPO and Fe-chelatase activities as well as heme in drought-treated leaves and roots of transgenic plants further substantiate this proposition.
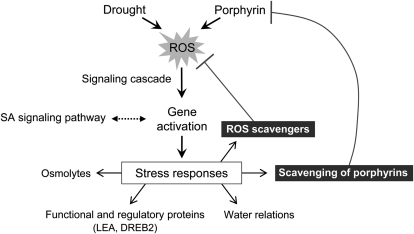
In conclusion, we have presented strong evidence that water stress controls metabolites of the porphyrin biosynthetic pathway through their scavenging to cope with excited-state dynamics of tetrapyrroles in the cell, consequently attenuating the photodynamic stress imposed by drought. Porphyrin intermediates may act as a possible switch in ROS and/or dehydration-mediated stress responses and may be involved in the complex network controlling stress-responsive genes, which may also be mediated by SA (Fig. 10). If drought and porphyrin simultaneously trigger the production of ROS to a certain level, the signal could mediate the signaling cascade for plants to achieve efficient down-regulation of porphyrin biosynthetic genes. This work also provides new approaches for engineering the porphyrin biosynthesis of crop plants with improved drought tolerance. Although the mechanism underlying how surplus PPO expression mediates drought tolerance is still not clear, overexpression of PPO alleviates drought-induced stress in transgenic rice, and their porphyrin status is partly implicated in drought tolerance. This work proposes a potential role for tetrapyrrole intermediates in signaling the metabolic state of porphyrin biosynthesis under drought stress conditions.
Figure 10.
A model explaining the role of porphyrins in plant responses to drought. Both drought and porphyrins trigger the accumulation of ROS, which activates plant responses to drought stress, while suppressing porphyrin levels. ROS initiate signaling cascades that stimulate gene activation. The possible association of drought responses with the SA signaling pathway is shown with dotted arrows, and the negative influence on porphyrin and ROS accumulation is shown with T-bars. The drought-mediated suppression of porphyrin biosynthetic pathway transcripts and intermediates is proposed as a mechanism leading to reduced damage during dehydration. Unbound hemes are used to supplement the level of ROS scavengers inside and outside of the plastids.
MATERIALS AND METHODS
Plant Growth Conditions and Drought Treatment
Transgenic rice (Oryza sativa ‘Dongjin’) plants expressing Myxococcus xanthus PPO were generated using Agrobacterium tumefaciens-mediated transformation (Jung et al., 2004). Rice seedlings of homozygous transgenic lines (T7–T9 generations) were grown for 4 weeks in a greenhouse at 28°C to 30°C. Four-week-old plants were transferred to a growth chamber maintained at day/night temperatures of 28°C/25°C under a 14-h-light/10-h-dark cycle (7:00 am–9:00 pm) with a 200 μmol m–2 s–1 photosynthetic photon flux density. After 3 d of acclimation in the growth chamber, plants were exposed to drought by withholding water for 84 h, and the youngest, fully expanded leaf tissues were sampled at 36 h (9:00 am), 48 h (9:00 pm), 60 h (9:00 am), 72 h (9:00 pm), and 84 h (9:00 am) after drought treatment. Control plants with sufficient water supply were harvested at the same time as the drought-treated plants for 36 h. To exclude the influence of diurnal regulation on the expression of genes tested in this study, transcript levels by RT-PCR were analyzed in wild-type rice plants grown under a 14-h-light/10-h-dark cycle (Supplemental Fig. S4). For experiments on the root system, 3-week-old plants were transferred from soil to half-strength Hoagland solution, hydroponically grown for 1 week, and then subjected to drought stress by air-drying treatment, due to difficulty in observing alterations of root growth in soil during drought periods.
Measurements of Shoot Water Parameters
ΨW was evaluated immediately as the plant stem xylem-pressure potential using a pressure chamber (PMS Instrument Co.). For RWC measurement, shoots were excised, and their fresh weight was scored immediately. After floating them in deionized water at 4°C overnight, their rehydrated weight was determined. Finally, they were dried at 80°C for 48 h and weighed. The RWC was calculated as follows: RWC (%) = (fresh weight – dry weight)/(rehydrated weight – dry weight) × 100. To monitor soil water content in the pots, the soil samples obtained were dried at 80°C, and the water content was calculated from the difference between the initial and dried soil weights.
Determination of Pro
Pro was extracted and quantified by the method of Bates et al. (1973). Leaf segments were homogenized with 3% sulfosalicylic acid, and the homogenate was centrifuged at 3,000g for 20 min. The supernatant was treated with acetic acid and acid ninhydrin and boiled for 1 h, and then the A520 was determined. Pro concentration was calculated using l-Pro as a standard.
Measurement of Photosynthetic Activity
Chlorophyll a fluorescence was measured in vivo using a pulse amplitude modulation fluorometer (Handy PEA; Hansatech Instruments) after dark adaptation for 20 min. The minimal fluorescence yield (Fo) was obtained upon excitation with a weak measuring beam from a pulse light-emitting diode. The maximal fluorescence yield (Fm) was determined after exposure to a saturating pulse of white light to close all reaction centers. The ratio of Fv to Fm, representing the activity of PSII, was used to assess the functional damage to the plants.
Measurement of SA
Leaf tissue samples were sequentially extracted with 90% and 100% methanol (Raskin et al., 1989). After centrifugation, supernatants were dried under N2. The residue was resuspended either in 5% TCA for free SA or in hot water at 80°C for total SA (free SA plus glucosyl SA). Enzymatic hydrolysis was performed at 37°C in 0.1 m sodium acetate buffer (pH 5) containing β-glucosidase (22 units mL−1). The reaction was stopped with the addition of 10% TCA and centrifuged. The supernatant was partitioned with 2 volumes of a 1:1 (v/v) mixture of ethyl acetate and cyclopentane containing 1% (v/v) isopropanol. The resulting top organic phase was dried under N2 and resuspended by HPLC in the mobile phase of 40 mm sodium acetate (pH 3.5):methanol (75:25, v/v). SA was quantified as described previously by Koch et al. (2000).
In Vivo Detection of H2O2
H2O2 was visually detected in the leaves using DAB (Thordal-Christensen et al., 1997). The leaves were cut with a razor blade and incubated in a 1 mg mL–1 solution of DAB (pH 3.8) for 4 h in light at 25°C. The experiment was terminated by boiling the leaves in ethanol for 10 min. This treatment decolorized the leaves, with the exception of the deep-brown polymerization product produced by the reaction of DAB with H2O2. After cooling, the leaves were washed at room temperature with fresh ethanol for 4 h and photographed.
Lipid Peroxidation
Lipid peroxidation was estimated by the level of MDA production using a slight modification of the thiobarbituric acid method, as described previously (Buege and Aust, 1978). Leaf tissues were homogenized in a solution of 0.5% thiobarbituric acid in 20% TCA. After centrifugation, the supernatants were heated in a boiling-water bath for 25 min. Following centrifugation, the resulting supernatants were used for spectrophotometric determination of MDA content at 532 nm.
Determination of Asc and GSH
For the Asc assay, tissues were homogenized in 5% metaphosphoric acid and centrifuged at 13,000g for 15 min (Law et al., 1983). To chromatically measure total Asc (reduced Asc plus oxidized DHA) and the amount of reduced Asc alone, we mixed 100 μL of the supernatant with 250 μL of 0.15 m K2HPO4 buffer (containing 5 mm EDTA, pH 7.4) in the presence of either 50 μL of 10 mm dithiothreitol (DTT; for total Asc) or the same volume of water (for the reduced Asc). Samples were incubated for at least 10 min at room temperature before 50 μL of 0.5% N-ethylmaleimide was added. A color-developing solution containing 200 μL of 10% TCA, 200 μL of 44% O-phosphoric acid, 200 μL of 4% α,α′-dipyridyl in 70% ethanol, and 11 μL of 30% FeCl3 was added to each of the above mixtures. These were then vigorously mixed and incubated at 37°C for 60 min before A525 was measured.
GSH and sulfur compounds were analyzed by a modified method of Koprivova et al. (2002). Tissues were homogenized in 0.1 n HCl and then centrifuged at 16,000g for 10 min. Aliquots of 180 μL of the supernatant were mixed with 180 μL of 0.2 m CHES [2-(cyclohexylamino)-ethansulfonic acid], pH 9.3. Reduction of thiols was initiated by the addition of 30 μL of 5 mm DTT and terminated after 30 min by the addition of 20 μL of 30 mm monobromobimane for derivatization. After 15 min, derivatization was stopped by acidification with 240 μL of 10% (v/v) acetic acid that also stabilizes the monobromobimane thiol derivatives. Thiols were separated and quantified by HPLC using fluorescence detection.
RNA Extraction and qRT-PCR
Total RNA was prepared from leaf tissues using TRIZOL Reagent (Invitrogen), and 5 μg of RNA from each sample was used for the RT reaction (SuperScript III First-Strand Synthesis System; Invitrogen). Subsequently, 50 ng of cDNA was used for qRT-PCR analysis. The qRT-PCR analysis was carried out with the 7300 Real-Time PCR System (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems) and specific primers for genes (Supplemental Table S1). The qRT-PCR program consisted of 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 1 min at 60°C. A melting curve analysis was performed after every PCR to confirm the accuracy of each amplified product. All reactions were set up in triplicate. The wild-type control sample was used as the calibrator, with the expression level of the sample set to 1. Actin was used as the internal control.
Determination of Porphyrins
For the measurement of porphyrin content, plant tissue was ground in methanol:acetone:0.1 n NaOH (9:10:1, v/v) and the homogenate was centrifuged at 10,000g for 10 min (Lermontova and Grimm, 2006). Porphyrin was separated by HPLC using a Novapak C18 column (4-μm particle size, 4.6 × 250 mm; Waters) at a flow rate of 1 mL min–1. Porphyrins were eluted with a solvent system of 0.1 m ammonium phosphate (pH 5.8) and methanol. The column eluate was monitored using a fluorescence detector (2474; Waters) at excitation and emission wavelengths of 400 and 630 nm for Proto IX, 440 and 630 nm for Pchlide, and 415 and 595 nm for MgProto and MgProto ME, respectively. The chlorophyll content was spectrophotometrically determined according to the method of Lichtenthaler (1987).
Determination of Heme
Heme was extracted as described previously (Schneegurt and Beale, 1986). Acid-acetone extracts were extracted again with diethyl ether and put on a QMA SepPak column (Waters). Protoheme was further purified and separated by HPLC on a Novapak C18 column (Waters) with a solvent system of ethanol:acetic acid:water (66.5:17:16.5, v/v) using detection at 402 nm.
ALA-Synthesizing Capacity
ALA-synthesizing capacity was measured as described by Papenbrock et al. (1999). Leaf discs were incubated in 20 mm phosphate buffer containing 40 mm levulinic acid in the light for 6 h. Samples were homogenized, resuspended in 1 mL of 20 mm K2HPO4/KH2PO4 (pH 6.9), and centrifuged at 10,000g. The 500-μL supernatant was mixed with 100 μL of ethylacetoacetate, boiled for 10 min, and cooled for 5 min. An equal volume of modified Ehrlichs reagent was added, and the absorption of the chromophore was determined at 553 nm with the spectrophotometer.
Assays for Enzyme Activities of Tetrapyrrole Biosynthesis
For assaying PPO, leaves or roots were homogenized in homogenization buffer (0.5 m sorbitol, 0.1 m Tris-HCl, pH 7.5, 1 mm DTT, and 0.1% bovine serum albumin [BSA]). The mixture was centrifuged at 5,000g for 10 min. The crude chloroplast pellet was resuspended in assay buffer (0.1 m Tris-HCl, pH 7.5, 5 mm DTT, 1 mm EDTA, and 0.03% Tween 80). The substrate Protogen IX was prepared by chemical reduction of Proto IX with sodium mercury amalgam (Sigma-Aldrich). The PPO activity was determined using the method of Lermontova and Grimm (2000). The enzyme reaction was set up, incubated at 30°C for 5 min, and stopped by adding ice-cold methanol:dimethyl sulfoxide (DMSO; 8:2, v/v). Proto IX was separated by HPLC as described above.
Mg-chelatase was assayed as described by Lee et al. (1992), with some modifications. Leaf tissue was homogenized in the homogenization buffer consisting of 0.5 m sorbitol, 50 mm Tricine, pH 7.8, 0.1% BSA, 1 mm MgCl2, and 1 mm DTT and centrifuged at 5,000g for 10 min. Crude plastids were incubated in a homogenization buffer (without BSA) containing 4 mm MgATP in a regenerating system (60 mm phosphocreatine/creatine phosphokinase; 10 units mL−1) and 10 mm MgCl2. Reactions were started by adding DMSO-solved Proto IX to a final concentration of 100 μm and stopped after 60 min at 30°C. The MgProto in hexane-washed water-acetone extracts was determined by fluorescence detection at excitation and emission wavelengths of 415 and 595 nm.
Fe-chelatase activity was measured using the protocol from Papenbrock et al. (1999). Crude plastids from leaves or roots were lysed in 0.1 m Tris-HCl buffer (pH 7.3), 0.5% Triton X-100, and 1 mm DTT, and membranes were spun down, resuspended in the same buffer, and recentrifuged. Two-hundred-microliter aliquots of the supernatant were mixed with 4 μL of 6 mm DMSO-solved Proto IX, 2 μL of 0.5 m ZnSO4, and 2 μL of 100 mm palmitic acid in the dark at 30°C for 45 min. Assay samples were extracted twice with acetone:0.1 n NH4OH (9:1, v/v). The ZnProto in hexane-washed water-acetone extracts was determined by fluorescence detection at excitation and emission wavelengths of 416 and 589 nm.
For assaying heme oxygenase, leaves or roots were homogenized in 0.25 m Suc solution containing 1 mm phenylmethylsulfonyl fluoride, 0.2 mm EDTA, and 50 mm potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 15,000g for 30 min, and the supernatant was used for activity determination. HO activity was assayed as described previously with minor modifications (Muramoto et al., 2002). The assays (1 mL final volume) contained 250 μL of extract (0.5 mg of protein), 10 μm hemin, 0.15 mg mL−1 BSA, 50 μg mL−1 (4.2 μm) spinach (Spinacia oleracea) ferredoxin, and 0.025 units mL−1 spinach ferredoxin-NADP+ reductase. The reaction was started by adding NADPH to a final concentration of 100 μm, samples were incubated at 37°C for 1 h, and biliverdin IXα formation was calculated using the absorbance change at 650 nm.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Distribution of tetrapyrrole metabolites in leaves of wild-type and transgenic rice plants during prolonged drought periods.
Supplemental Figure S2. Effect of drought treatment on the expression of M. xanthus PPO and diurnal regulation of M. xanthus PPO and OsPPO in transgenic plants.
Supplemental Figure S3. Effect of PPO overexpression on the morphological characterization and physiology of root growth in wild-type and transgenic plants.
Supplemental Figure S4. Diurnal regulation of porphyrin biosynthetic genes, ROS-scavenging genes, and nucleus-encoded photosynthetic genes in wild-type rice plants grown under a 14-h-light/10-h-dark cycle.
Supplemental Table S1. Primers used for qRT-PCR and RT-PCR assays.
References
- Aarti DP, Tanaka R, Tanaka A. (2006) Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol Plant 128: 186–197 [Google Scholar]
- Ábrahám E, Rigó G, Székely G, Nagy R, Koncz C, Szabados L. (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51: 363–372 [DOI] [PubMed] [Google Scholar]
- Ankele E, Kindgren P, Pesquet E, Strand A. (2007) In vivo visualization of Mg-protoporphyrin IX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell 19: 1964–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Balestrasse KB, Noriega GO, Batlle A, Tomaro ML. (2005) Involvement of heme oxygenase as antioxidant defense in soybean nodules. Free Radic Res 39: 145–151 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Gómez F, Martínez DE, Guiamet JJ. (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55: 1663–1669 [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207 [Google Scholar]
- Beale SI, Weinstein JD. (1990) Tetrapyrrole metabolism in photosynthetic organisms. In HA Daily, ed, Biosynthesis of Heme and Chlorophyll. McGraw-Hill, New York, pp 287–391 [Google Scholar]
- Bray EA. (1997) Plant responses to water deficit. Trends Plant Sci 2: 48–54 [Google Scholar]
- Buege JA, Aust SD. (1978) Microsomal lipid peroxidation. Methods Enzymol 52: 302–310 [DOI] [PubMed] [Google Scholar]
- Cao ZY, Xuan W, Liu ZY, Li XN, Zhao N, Xu P, Guan RZ, Shen WB. (2007) Carbon monoxide promotes lateral root formation in rapeseed. J Integr Plant Biol 49: 1070–1079 [Google Scholar]
- Capell T, Bassie L, Christou P. (2004) Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc Natl Acad Sci USA 101: 9909–9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM. (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55: 2365–2384 [DOI] [PubMed] [Google Scholar]
- Chini A, Grant JJ, Seki M, Shinozaki K, Loake GJ. (2004) Drought tolerance established by enhanced expression of the CC-NBS-LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J 38: 810–822 [DOI] [PubMed] [Google Scholar]
- Close TJ. (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100: 291–296 [Google Scholar]
- Dolphin D. (1994) Photomedicine and photodynamic therapy. Can J Chem 72: 1005–1013 [Google Scholar]
- Feierabend J. (2005) Catalases in plants: molecular and functional properties and role in stress defence. Smirnoff N, , Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing, Oxford, pp 101–140 [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28: 1091–1101 [DOI] [PubMed] [Google Scholar]
- Girotti AW, Kriska T. (2004) Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid Redox Signal 6: 301–310 [DOI] [PubMed] [Google Scholar]
- Gisk B, Yasui Y, Kohchi T, Frankenberg-Dinkel N. (2010) Characterization of the haem oxygenase protein family in Arabidopsis thaliana reveals a diversity of functions. Biochem J 425: 425–434 [DOI] [PubMed] [Google Scholar]
- Guo K, Xia K, Yang Z-M. (2008) Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. J Exp Bot 59: 3443–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N, Zhang JZ. (2004) Genomics applications to biotech traits: a revolution in progress? Curr Opin Plant Biol 7: 226–230 [DOI] [PubMed] [Google Scholar]
- Ha SB, Lee SB, Lee Y, Yang K, Lee N, Jang SM, Chung JS, Jung S, Kim YS, Wi SG, et al. (2004) The plastidic Arabidopsis protoporphyrinogen IX oxidase gene, with or without the transit sequence, confers resistance to the diphenyl ether herbicide in rice. Plant Cell Environ 27: 79–88 [Google Scholar]
- Hare PD, Cress WA, Van Staden J. (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21: 535–553 [Google Scholar]
- Hendry GA, Finch-Savage WE, Thorpe C, Atherton NM, Buckland S, Nilsson KA, Seel WE. (1992) Free radical processes and loss of seed viability during desiccation in the recalcitrant species Quercus robur L. New Phytol 122: 273–279 [DOI] [PubMed] [Google Scholar]
- Hess FD. (2000) Light-dependent herbicides: an overview. Weed Sci 48: 160–170 [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR. (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436 [DOI] [PubMed] [Google Scholar]
- Hsiao TC. (1973) Plant responses to water stress. Annu Rev Plant Physiol 24: 519–570 [Google Scholar]
- Hunt PW, Klok EJ, Trevaskis B, Watts RA, Ellis MH, Peacock WJ, Dennis ES. (2002) Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 17197–17202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose K, Che F-S, Kimura Y, Matsunobu A, Sato F, Yoshida S. (1995) Selection and characterization of protoporphyrinogen oxidase inhibiting herbicide (S23142) resistant photomixotrophic cultured cells of Nicotiana tabacum. J Plant Physiol 146: 693–698 [Google Scholar]
- Ikeyama Y, Tasaka M, Fukaki H. (2010) RLF, a cytochrome b(5)-like heme/steroid binding domain protein, controls lateral root formation independently of ARF7/19-mediated auxin signaling in Arabidopsis thaliana. Plant J 62: 865–875 [DOI] [PubMed] [Google Scholar]
- Jung S, Lee H-J, Lee Y, Kang K, Kim YS, Grimm B, Back K. (2008) Toxic tetrapyrrole accumulation in protoporphyrinogen IX oxidase-overexpressing transgenic rice plants. Plant Mol Biol 67: 535–546 [DOI] [PubMed] [Google Scholar]
- Jung S, Lee Y, Yang K, Lee SB, Jang SM, Ha SB, Back K. (2004) Dual targeting of Myxococcus xanthus protoporphyrinogen oxidase into chloroplasts and mitochondria and high-level oxyfluorfen resistance. Plant Cell Environ 27: 1436–1446 [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET. (2005) Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keetman U, Mock H-P, Grimm B. (2002) Kinetics of antioxidative defense responses to photosensitisation in porphyrin-accumulating tobacco plants. Plant Physiol Biochem 40: 697–707 [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR. (2000) Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid: the role of programmed cell death in lesion formation. Plant Physiol 123: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivova A, Meyer AJ, Schween G, Herschbach C, Reski R, Kopriva S. (2002) Functional knockout of the adenosine 5′-phosphosulfate reductase gene in Physcomitrella patens revives an old route of sulfate assimilation. J Biol Chem 277: 32195–32201 [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. (1983) Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J 210: 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ball MD, Parham R, Rebeiz CA. (1992) Chloroplast biogenesis 65: enzymic conversion of protoporphyrin IX to Mg-protoporphyrin IX in a subplastidic membrane fraction of cucumber etiochloroplasts. Plant Physiol 99: 1134–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lermontova I, Grimm B. (2000) Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol 122: 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lermontova I, Grimm B. (2006) Reduced activity of plastid protoporphyrinogen oxidase causes attenuated photodynamic damage during high-light compared to low-light exposure. Plant J 48: 499–510 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148: 350–382 [Google Scholar]
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R, Poulos TL. (2005) Ascorbate peroxidase. Smirnoff N, , Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing, Oxford, pp 87–100 [Google Scholar]
- Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. (2001) Arabidopsis genomes uncoupled 5 (gun5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98: 2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ. (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15: 488–498 [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A. (2008) The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc Natl Acad Sci USA 105: 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock H-P, Heller W, Molina A, Neubohn B, Sandermann H, Jr, Grimm B. (1999) Expression of uroporphyrinogen decarboxylase or coproporphyrinogen oxidase antisense RNA in tobacco induces pathogen defense responses conferring increased resistance to tobacco mosaic virus. J Biol Chem 274: 4231–4238 [DOI] [PubMed] [Google Scholar]
- Mohanty S, Grimm B, Tripathy BC. (2006) Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 224: 692–699 [DOI] [PubMed] [Google Scholar]
- Mora-Herrera ME, Lopez-Delgado H, Castillo-Morales A, Foyer CH. (2005) Salicylic acid and H2O2 function by independent pathways in the induction of freezing tolerance in potato. Physiol Plant 125: 430–440 [Google Scholar]
- Moulin M, McCormac AC, Terry MJ, Smith AG. (2008) Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc Natl Acad Sci USA 105: 15178–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T. (2002) Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 130: 1958–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S, Koide M, Takahashi S, Kikuta A, Aono M, Sasaki-Sekimoto Y, Ohta H, Takamiya K-I, Masuda T. (2007) Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol 144: 1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, et al. (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 104: 16450–16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Nott A, Jung H-S, Koussevitzky S, Chory J. (2006) Plastid-to-nucleus retrograde signaling. Annu Rev Plant Biol 57: 739–759 [DOI] [PubMed] [Google Scholar]
- op den Camp RG, Ochsenbein C, Przybyla D. (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455 [DOI] [PubMed] [Google Scholar]
- Papenbrock J, Grimm B. (2001) Regulatory network of tetrapyrrole biosynthesis: studies of intracellular signalling involved in metabolic and developmental control of plastids. Planta 213: 667–681 [DOI] [PubMed] [Google Scholar]
- Papenbrock J, Mock H-P, Kruse E, Grimm B. (1999) Expression studies in tetrapyrrole biosynthesis: inverse maxima of magnesium chelatase and ferrochelatase activity during cyclic photoperiods. Planta 208: 264–273 [Google Scholar]
- Peter E, Rothbart M, Oelze M-L, Shalygo N, Dietz K-J, Grimm B. (2010) Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol 51: 1229–1241 [DOI] [PubMed] [Google Scholar]
- Poyton RO, McEwen JE. (1996) Crosstalk between nuclear and mitochondrial genomes. Annu Rev Biochem 65: 563–607 [DOI] [PubMed] [Google Scholar]
- Qin F, Kakimoto M, Sakuma Y, Maruyama K, Osakabe Y, Tran L-SP, Shinozaki K, Yamaguchi-Shinozaki K. (2007) Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J 50: 54–69 [DOI] [PubMed] [Google Scholar]
- Raskin I, Turner IM, Melander WR. (1989) Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. Proc Natl Acad Sci USA 86: 2214–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C. (1996) The regulation of enzymes involved in chlorophyll biosynthesis. Eur J Biochem 237: 323–343 [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Apel K, Lebedev N. (1996) Evolution of chlorophyll biosynthesis: the challenge to survive photooxidation. Cell 86: 703–705 [DOI] [PubMed] [Google Scholar]
- Schneegurt MA, Beale SI. (1986) Biosynthesis of protoheme and heme a from glutamate in maize. Plant Physiol 81: 965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat GS, Verma K. (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 61: 2255–2270 [DOI] [PubMed] [Google Scholar]
- Singh DP, Cornah JE, Hadingham S, Smith AG. (2002) Expression analysis of the two ferrochelatase genes in Arabidopsis in different tissues and under stress conditions reveals their different roles in haem biosynthesis. Plant Mol Biol 50: 773–788 [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes OJ. (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060 [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand A, Asami T, Alonso J, Ecker JR, Chory J. (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421: 79–83 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Tanaka A. (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58: 321–346 [DOI] [PubMed] [Google Scholar]
- Tewari AK, Tripathy BC. (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117: 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tommasi F, Paciolla C, Arrigoni O. (1999) The ascorbate system in recalcitrant and orthodox seeds. Physiol Plant 105: 193–198 [Google Scholar]
- Valenzeno DP. (1987) Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol 46: 147–160 [DOI] [PubMed] [Google Scholar]
- Vanhee C, Zapotoczny G, Masquelier D, Ghislain M, Batoko H. (2011) The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 23: 785–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lis R, Atteia A, Nogaj LA, Beale SI. (2005) Subcellular localization and light-regulated expression of protoporphyrinogen IX oxidase and ferrochelatase in Chlamydomonas reinhardtii. Plant Physiol 139: 1946–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu J-K. (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539 [DOI] [PubMed] [Google Scholar]
- von Gromoff ED, Alawady A, Meinecke L, Grimm B, Beck CF. (2008) Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell 20: 552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, et al. (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162: 465–472 [DOI] [PubMed] [Google Scholar]
- Wilson KE, Sieger SM, Huner NPA. (2003) The temperature-dependent accumulation of Mg-protoporphyrin IX and reactive oxygen species in Chlorella vulgaris. Physiol Plant 119: 126–136 [Google Scholar]
- Yang JH, Kim KD, Lucas A, Drahos KE, Santos CS, Mury SP, Capelluto DGS, Finkielstein CV. (2008) A novel heme-regulatory motif mediates heme-dependent degradation of the circadian factor period 2. Mol Cell Biol 28: 4697–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Greenberg JT. (2006) Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 18: 397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]