The concentration of cations in the xylem sap influences the rate of xylem water flow in angiosperm plants. It has been speculated that this is due to the shrinking and swelling of pectins in the pit membranes. However, there is as yet minimal evidence for the presence of pectin in pit membranes of angiosperms. The little pectin that has been found at the pit membrane edges of some species might not be adequate to explain the swelling and shrinking phenomena. The presence of hemicelluloses is also not certain. Lignin, by contrast, seems to be sometimes present, apart from cellulose, which is the main component. An alternative hypothesis is formulated, which involves the shrinking of any polyelectrolyte polymers in the pit membrane and a change in volume of the mobile phase in the pit pores. These phenomena are the result of electrostatic events. Some pit membrane polymers are negatively charged because of proton dissociation from functional groups. This charge is compensated by cations in the aqueous phase, which form a diffuse double layer (DDL). Inside the pit pores, an increase of the electrolyte concentration in the xylem sap will reduce the extent of the DDL. This will result in an increase in water flow. Additional flow enhancement, upon increase of the cation concentration, can be due to shrinkage of all membrane polymers. This contraction will also lead to an increase of the pit pore diameter. These processes will only be partly counteracted by forces that decrease the diameter of the pit pore due to relaxation.
WATER TRANSPORT AND PIT MEMBRANES
Long-distance water transport in plants occurs in the nonliving xylem conduits (vessels and tracheids). Because most conduits are short in length (a few centimeters or less), they are interconnected to allow the upward flow of water. Very thin wall areas, called pit membranes, in the conduit walls allow the water to flow from conduit to conduit. The pit membranes have very small holes (typically 5–20 nm in diameter) that allow the passage of water. The pit membranes usually are the limiting factor for the rate of water flow in the xylem of angiosperm species because they account for approximately 50% to 90% of the hydraulic resistance, depending on the species (Wheeler et al., 2005; Choat et al., 2006; Hacke et al., 2006).
The hydraulic resistance in stems is often measured by cutting a segment and allowing water to flow through at a given pressure. However, when using distilled water, a steady decrease in the rate of water flow through the segment was observed. By contrast, a smaller decrease was found when using tap water. The effect of tap water was due to the presence of ions. It was suggested that “the phenomenon might be based upon swelling or shrinking of the vessel-to-vessel pit membranes” (Zimmermann, 1978).
The finding that ions in the water promote the flow rate in stem segments was corroborated by van Ieperen et al. (2000), Zwieniecki et al. (2001), and several others (for example, see López-Portillo et al., 2005; Domec et al., 2007; Gascó et al., 2007; Nardini et al., 2007b).
In intact plants, the positive effect of ions on the rate of xylem water flow increased exponentially with the number of air bubbles filling xylem conduits. The presence of air bubbles in plants has been reported to result in an increase in ion concentrations in the xylem sap. Therefore, an ion-mediated increase of hydraulic conductance might at least partially compensate for the loss of hydraulic conductance as a result of the formation of air bubbles (Gascó et al., 2006; Trifilò et al., 2008, 2011).
The data on the effect of ions on water flow rates in the xylem have been explained, hypothetically, by assuming that it is due to changes in the swelling of pectins in the pit membranes. This pectin-swelling hypothesis has not been challenged thus far. Here, some arguments will be raised against it, and an alternative mechanism will be suggested.
DATA ON STEM PERFUSION AND THE PIT MEMBRANE PECTIN-SWELLING HYPOTHESIS
Stem segments were cut from chrysanthemum (Chrysanthemum × morifolium) flowers. The inclusion of monovalent or divalent cations in the distilled water used to perfuse the segments immediately increased the rate of water flow, the anions being of no importance. Sugars were also without effect (van Ieperen et al., 2000; van Ieperen and van Gelder, 2006). Zwieniecki et al. (2001) found the same in stem segments of Laurus nobilis and Fraxinus americana. The effects were also found in dead branches and, thus, did not depend on physiological activity. In addition, evidence was given for the idea that the effect of ions on xylem flow in F. americana was mainly due to the presence of pit membranes. Data from Jansen et al. (2011) also suggested that the positive effect of cations on xylem water flow is in the pit membranes.
In an article in Science, the effects of cations on xylem water flow were explained by assuming a gel-like substance in the pit membranes (Zwieniecki et al., 2001). It was suggested that this gel limited the rate of water flow. The idea stemmed from the resemblance of the data on water flow rates in stem segments with those on swelling and shrinking of hydrogels (Zwieniecki et al., 2001). The hypothesis of gel swelling was tested using changes in pH, which would alter the effective ionization of the gel polymer network, and by using a change in solvent polarity, which would change polymer-polymer affinity. HCl given at a concentration that lowered the pH to 3.5 had a small positive effect on the rate of water flow, but HCl that lowered the pH to 2.5 had a large positive effect. The effect of solvent polarity was tested by including ethanol in the perfusion solution. Ethanol had no effect up to 50% (v/v) in water, but at 50% or more, it had a large flow-promoting effect, which was believed to be in agreement with the hydrogel-swelling hypothesis. The authors claimed that the pit membranes consist of cellulose, pectin, and hemicellulose, but unfortunately, the two references on which they based this claim do not support their contention. The authors further considered that pectins can form gels, and they hypothesized that the swelling/shrinking of pectins in the pit membranes were responsible for the cation effect (Zwieniecki et al., 2001). Several recent authors (such as van Ieperen, 2007; Espino and Schenk, 2010) have since explained the effect of cations on water flow rates in stem segments in terms of pit membrane pectin swelling.
It should be noted, nonetheless, that not all angiosperm species reacted the same to an increase in the cation concentration in the perfusion sap. In Salix alba, the hydraulic conductance was found to decrease by more than 30% when perfusing stem segments with 50 mm KCl, whereas in Prunus avium and Betula alba, this KCl concentration had no effect on water flow (Cochard et al., 2010). These data suggest a more complex interaction between ions in the xylem sap and hydraulic conductance than hitherto thought. The current theory does not predict a negative effect of cations on water flow.
The pit membrane in gymnosperms is different from that in angiosperms: It usually has a thick area in the middle (the torus) that is impermeable to water, and it has large holes in the periphery. Because of these rather large holes, the resistance to water flow in gymnosperm pit membranes is much lower (by a factor of more than 50) than that in angiosperms (Pittermann et al., 2005). The following discussion will therefore apply to angiosperms because the water flow through gymnosperm xylem seems less affected by the presence of pit membranes than the water flow through angiosperms.
IS THERE PECTIN IN THE PIT MEMBRANES?
A main problem with the pit membrane pectin-swelling hypothesis is that pectin may not be present in the pit membranes, at least in many species. Pectins are heterogeneous polysaccharides, forming a linear polymer. The backbone consists mainly of homogalacturonan and rhamnogalacturonans. The carboxyl groups of GalUA can be methylesterified. Acid pectins are often defined as having 0% to 50% methylesterification, whereas methylesterified pectins are defined as having more than 50% methylated carboxyl groups. Swelling and shrinking of pectins can only be due to the carboxyl groups that are not esterified because only these groups can be negatively charged.
Early research suggested that pit membranes in several species did not have pectins. O’Brien (1970) concluded that, at the time of the death of cells that produce the xylem conduits, any noncellulosic polysaccharides become hydrolytically removed from the pit membranes of willow (Salix babylonica) and lilac (Syringa vulgaris). The data of Butterfield and Meylan (1982), who studied Pseudowintera dandy, supported this idea. Other work, using staining techniques combined with electron microscopy, also suggested that all or almost all acidic pectins in the pit membranes of a number of angiosperm species were hydrolyzed by the time the xylem cell was dying, whereas highly methylated pectins remained (Czaninski, 1972, 1979; Catesson et al., 1979; Catesson, 1983).
More recent work has also suggested the absence of pectins. Sections from poplar (mainly Populus trichocarpa) wood were stained with Alcian blue, a dye with a high affinity for pectins. This dye is bound by electrostatic forces to the negative charge of pectin. Although the authors did not point this out, their data show no blue stain in the pit membranes between the xylem conduits (Arend et al., 2008). Staining with Alcian blue of pit membranes between xylem conduits was also absent in other species studied: Ampelocera dichotoma, Aphananthe aspera, Gironniera celtidifolia, Holoptelea integrifolia, Phyllostylon rhamnoides, Trema lamarckiana, Ulmus lanceifolia, and Ulmus mexicana (Jansen et al., 2004). These Alcian blue staining experiments suggest that there is little or no pectin in the pit membranes of the investigated species.
Absence of pectins in pit membranes is one possible explanation for the finding of Nardini et al. (2007a), who worked with transgenic tobacco (Nicotiana tabacum) lines with increased expression of endopolygalacturonase. These plants had significantly less acid pectin (less deesterified blocks of homogalacturonan) than the wild-type plants. No difference was found between the hydraulic conductance of the transgenic plants and the wild type when perfusing the stems with a solution in which the cation concentration had been increased.
In their literature review on pits in the xylem, Choat et al. (2008) concluded that there is no direct evidence that pectins are present in mature pit membranes of most angiosperm species. This conclusion still holds today. By using specific immunogold localization techniques, adequate proof can be obtained for the presence or absence of pectins. The monoclonal antibodies JIM5 and JIM7 detect unesterified (acidic) pectin and methylesterified pectin, respectively. Using JIM5 and JIM7, no pectins were found in the mature interconduit pit membranes of a hybrid poplar (Populus trichocarpa × deltoides; Plavcová et al., 2011). The interconduit pit membranes in Amelanchier alnifolia, Betula papyrifera, Populus balsamifera, and Prunus virginiana contained some acidic and some methylesterified pectin in a very small zone at the membrane edges (Plavcová and Hacke, 2011). It seems unlikely that this little acid pectin has an effect on swelling or shrinking of the pit membrane, but this hypothesis needs to be investigated in depth. Nardini et al. (2011) also briefly reviewed the evidence for the presence of pectin in mature pit membranes in angiosperms. They reiterated the idea that there is at present little evidence for its presence.
A recent publication, using dye techniques, suggested that there are acidic and methylesterified pectins in the pit membranes of some species of Lauraceae (L. nobilis, Lindera megaphylla, Litsea sericea, and Umbellularia californica; Gortan et al., 2011). The ionic effect on water flow in the stems was larger in species that apparently had more acidic pectins in their pit membranes. However, this finding needs further verification using immunolocalization techniques (S. Jansen, personal communication).
Using dye methods, Wisniewski and Davis (1995) found that pectin is absent from pit membranes in peach (Prunus persica) trees, although only if the individual trees had not been subjected to severe stress. When they had undergone such stress, pectins became secreted into the xylem by living xylem parenchyma cells. This type of secretion can apparently also occur after mechanical insult or after infection with microorganisms. The secreted material is a gel that is rich in pectin. Rioux et al. (1998) similarly observed that pectins can become secreted by xylem parenchyma cells in stressed plants of Hevea brasiliensis, Prunus pensylvanica, Sorbus americana, and Ulmus americana. The production of pectins was often so copious that it filled the whole lumen of the xylem conduit. Other examples are grapevine (Vitis vinifera; Sun et al., 2008) and the reed Phragmites australis (Soukup and Votrubová, 2005). These data indicate that pectins can be deposited on pit membranes between xylem conduits after serious physiological stress. It has been suggested that some pectins are present in pit membranes of unstressed grapevine (Pérez-Donoso et al., 2010; Sun et al., 2011), but we cannot be entirely sure that their presence was not due to stress.
Pectins were also apparently present in pit membranes of unstressed trees of the genera Ulmus and Zelkova because the pit membranes between xylem conduits in these species stained with Alcian blue. The pit membranes of these species had a thickening in the middle, called a torus, similar to the one often found in the pit membranes of gymnosperms (Jansen et al., 2004). Gymnosperm tori have been suggested to generally contain pectin (Bauch et al., 1968; 1972). True tori have been found in only a few species in five angiosperm families (Cannabaceae, Oleaceae, Rosaceae, Thymelaeaceae, and Ulmaceae) but are apparently absent from all other angiosperm families (Jansen et al., 2004, 2007; Rabaey et al., 2006; Dute et al., 2008b). It is currently unclear whether the pectin in angiosperm tori affects the rate of xylem water flow.
Taken together, the data show very little evidence for the presence of pectins in the pit membranes of angiosperm species. Exceptions are species with tori, which are rare, and some species, if they are subjected to major stress. Although the data suggest that pectins are virtually absent from the pit membranes of many angiosperm species, more detailed analysis (using monoclonal antibodies) will be necessary to confirm this.
AN ALTERNATIVE HYPOTHESIS
The next question is: If it turns out that that there are no pectins in pit membranes, can other polysaccharides, such as hemicellulose, lignin, or cellulose, take on their role? The first question to be answered is then: Have these compounds been shown in pit membranes?
Hemicelluloses apparently have not been reported in pit membranes of angiosperms, at least thus far. Although it was not the direct aim of the investigation, a recent publication that used antibodies for hemicellulose detection did not show any in the pit membranes between xylem conduits in Citrus sinensis (Alves et al., 2009). Older electron microscope work also suggested that no hemicelluloses were present in Fagus sylvatica pit membranes (Jayme and Azolla, 1965). If it is true that most noncellulosic polysaccharides in the pit membrane of angiosperms are hydrolyzed at the final phase of xylem element programmed cell death (O’Brien, 1970; Butterfield and Meylan, 1982), it would mean that hemicelluloses also disappear, along with pectins. For comparison, hemicelluloses reportedly were also removed from pit membranes of gymnosperms and Ginkgo biloba by the time of programmed cell death of the living tracheid cells (Imamura and Harada, 1973; Imamura et al., 1974; Dute, 1994; Dute et al., 2008a).
Although the data are still very scarce, they might suggest the absence of hemicelluloses from the pit membranes of angiosperms, but this obviously needs considerably more experimental work. If hemicelluloses are present, they can participate in the swelling and shrinking of pit membranes because of changes in cation concentration in the xylem sap (Li and Pan, 2010).
In contrast with hemicellulose, lignin was found in pit membranes of the angiosperm Drimys sp. (Boyce et al., 2004), Pseudowintera colorata (Meylan and Butterfield, 1982), Rhizophora mucronata (Schmitz et al., 2008), and F. sylvatica (Jayme and Azolla, 1965). Fromm et al. (2003) showed that the pit membranes in F. sylvatica latewood were heavily encrusted with lignin, but it is not clear whether the xylem conduits containing these pit membranes were still transporting water. Lignin has also been found in the pit membranes of several gymnosperm species (Jayme and Fengel, 1961; Sachs, 1963; Timell, 1973; Boyce et al., 2004) and in G. biloba (Eicke and Ehling, 1965). If lignin is present in pit membranes, it may well account for at least some of the swelling and shrinking phenomena (Li and Pan, 2010).
It is generally believed that the main component of pit membranes is cellulose. This follows from staining reactions (Jayme and Azolla, 1965; O’Brien, 1970; Catesson et al., 1979; Czaninski, 1979; Butterfield and Meylan, 1982; Catesson, 1983). It has been claimed that if a parenchyma cell wall contains both pectin and hemicellulose in addition to cellulose, the main swelling/shrinking effects are due to cellulose (Shomer et al., 1991). This claim seems unlikely and needs to be reconfirmed, in particular for pit membranes.
Ideal cellulose is a polymer with hydroxyl groups, whereby the OH content is approximately 18 mol/kg. Only a small fraction of these OH groups will dissociate at neutral pH, leading to charge development that may be in the order of 0.1 to 1 mol+/kg (mol+ is moles of elementary electric charges, even if negative). Full charging of the OH groups will only take place at high pH and at high salt concentrations (Sjöström, 1989). In addition, cellulose (at least that from fibers) contained carboxyl groups of having approximately 0.02 (Fält et al., 2003) or 0.05 mol+/kg (Ahola et al., 2008); it should be noted that the hemicellulose content of the celluloses used was about 6 and 14 weight %, respectively. This cellulose showed a little swelling and shrinking when treated with aqueous solutions of various pH or with such solutions containing various concentrations of cations. When the number of carboxyl groups in this cellulose was chemically increased (using carboxymethylation), it showed extensive swelling and shrinking after the cation or pH treatments (Grignon and Scallan, 1980; Fält et al., 2003; Ahola et al., 2008; El Seoud et al., 2008). To date, we do not yet know how many carboxyl groups are present in cellulose from interconduit pit membranes in angiosperms. Therefore, it remains unclear whether cellulose takes part in the swelling and shrinking phenomena related to changes in xylem sap cation composition.
Thus, we suggest here that the swelling/shrinking phenomena that have been solely ascribed by Zwieniecki et al. (2001) to pectin apply just as much to any other polyelectrolyte in the pit membrane. It is concluded that, apart from pectin, there can be other polyelectrolytes, such as lignins and hemicelluloses. There might even be a remote possibility that cellulose is involved.
CATIONS AND WATER FLOW: ELECTROSTATIC EVENTS
For a description of the effect of cations on water flow through pores in pit membranes, we will distinguish three situations. First, we will consider the pores at a constant diameter. Second, we will reflect on possible changes in the diameter solely due to processes that occur within the pore channel. Third, the effects of processes within the pit membrane matrix on pore size will be taken into account. The present model is conceptually relatively simple; it is meant as a first approximation only.
Constant Pore Size in the Pit Membrane
The interactions between dissolved cations and negatively charged polyelectrolyte pore wall surfaces can be described using the DDL theory (Bolt, 1982). The DDL has also been called (diffuse) electrical double layer. The electrostatic field of the pore wall is neutralized by counter ions (e.g. Ca2+, K+) and coions (e.g. NO3−HCO3−) in solution, leading to a diffuse distribution of ions in the interface. For example, the negative charges at the surface of the pit pore walls can be counteracted by K+ ions (the counter ions), each with their shell of water molecules. Because of the shells of water molecules, the DDL mainly consists of water.
The counter ion concentration decreases with the distance from the surface, ultimately reaching the same concentration as in the free solution. The extent of the DDL (away from the charged surface) depends on the ion concentration in the free solution. For a linear field, the extent of the DDL is inversely proportional with the root of the ionic strength I. According to Bolt (1982), the extent of the DDL can be defined as:
where β is a constant (=1019 m/kmol), and I ≡ 1/2Σzi2Ci, with zi as the valence, and Ci as the molar concentration of an ion i. For example, for I = 0.001 mol/L, the corresponding κ−1 = 10 nm, and for I = 0.01 mol/L, κ−1 = 3 nm. Thus, the extent of the DDL diminishes at higher ionic strength. This is due to more efficient screening of the electrostatic field at the surface by the available counter ions.
In a charged interface, water molecules are electrically neutral entities. This might suggest that there is little or no impediment for the flow of water molecules in the DDL. However, in the DDL, the viscosity of the solution is strongly enhanced and fluidity reduced (Goli et al., 2010) because the local ion concentration of the DDL can be very high, in particular at the high electrical charge in narrow pores. The increase of viscosity and the corresponding reduction of the mobility of water also depends on the type of counter ion. The experimental order of increase of viscosity, at negatively charged synthetic membranes, was Ca2+ > Na+ > K+ (Goli et al., 2010). In addition, water becomes increasingly structured near a charged surface, but the thickness of the layer of water structuring is only less than 0.5 to 1.0 nm, whereas the DDL might extend to as much as 10 nm (Hiemstra and Van Riemsdijk, 2006a). Therefore, the increase in viscosity is the main impediment to water flow in the DDL.
The above-calculated values of the extent of the DDL (κ−1) can be compared with the pore size of pit membranes in plant stems. The pore size can be assessed using perfusion with spherical molecules of various diameters. This yielded an average pore diameter of 5 to 20 nm, depending on the species (Choat et al., 2003; Pérez-Donoso et al., 2010). It should be remembered that these data on pore size can be influenced by DDL processes that affect the path of water flow in the pores. Nonetheless, if this order of magnitude is correct, the DDL of the pit membrane pores, which might stretch about 3 to 10 nm into the solution from all sides of the walls, can be an obstacle for water flow if an electrolyte solution has to pass.
The Hagen-Poiseuille equation assumes laminar flux in an ideal cylinder. Such cylinders are usually not found in nature, but we can use the equation conceptually. If applied to a single cylindrical pore with a radius r, the expression for the discharge Q (m3 s−1) at a laminar flow can be given as Q = −1/(8η)πr4ΔP, in which ΔP (Newtons [N]m−2 m) is the pressure difference per unit pore length and η (Nm−2 s) is the viscosity of the mobile phase (Koorevaar et al., 1983). For a tissue containing n such pores per unit surface area (m−2) and having volumetric water content θ (= nπr2), the flux density (q ≡ nQ) is:
Equation 2 shows that at a given water content, the water flux is proportional to the square of the pore radius. Thus, a 10-fold smaller radius leads to a 100-fold smaller longitudinal water flux. It illustrates the well-known fact that the largest pores will dominate in water transport.
In case part of the intrinsic pore space with radius ro becomes immobile over distance κ−1, the effective radius decreases, leading at the same overall water content θ to:
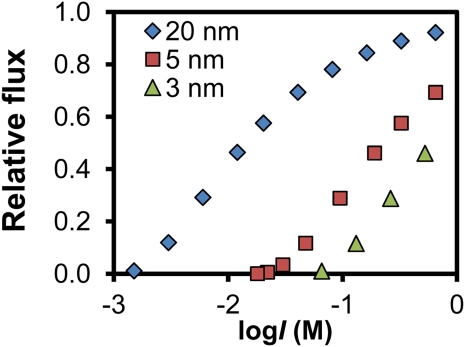
Figure 1 shows the model fluxes q relative to the initial flux without DDL (qo) for various pore sizes as a function of the ionic strength. The decrease is due to the formation of a stagnant phase. The calculations show a 50% reduction of the relative flux (q/qo) for pores of 20 and 5 nm at I ≈ 0.01 m and I ≈ 0.2 m, respectively. The 20-nm pores will exhibit a clear variation of the relative water flux due to swelling and shrinking as a result of a change of ionic strength (Fig. 1). Water in small pores undergoes a large friction due to the wall (Poiseuille’s law). In addition, small pores have a significant double layer overlap. Both will strongly reduce longitudinal water flow (Fig. 1).
Figure 1.
Relative flux (q/qo) at a given water content in membranes with various fixed pore diameters (3, 5, or 20 nm), as a function of ionic strength I. It is assumed that a stagnant phase exists over a distance κ−1 (Eq. 3) because of formation of the DDL inside the pores. q/qo is flux q relative to the initial flux without DDL (qo). [See online article for color version of this figure.]
Changes of Pore Size by DDL Overlap in the Pore Space
Another mechanism could be at work inside the pore that may change the pore diameter. At relatively low ionic strength, a charged flat surface will radiate an electrostatic field. This field can be considered to be a collection of field lines. If the surface is curved to a cylinder, as in the case of the pit pore, the field lines are concentrated toward the center, creating a double layer overlap that increases the free energy of the interface. For this reason, there will be a spontaneous tendency to decrease field curvature, i.e. to increase the pore diameter. The force that tends to increase the pore diameter will be more strongly counteracted if the rigidity of the polymer network in the wall is higher.
When the ionic strength in the xylem sap is increased, an opposing set of forces will occur. The force field is annihilated by the increase in the concentration of neutralizing counter ions (as discussed in the previous section). Therefore, the increase of the ionic strength will tend to contract the pore because of relaxation of the polymer network that forms the pore wall. The degree of pore contraction will depend on the degree the pore had widened at low ionic strength; thus, it depends on the rigidity of the polymers in the pit membrane. This pore contraction due to an increase of the ionic strength can counteract the widening of the effective size of the pore due to an increase in ionic strength as described above. It is not clear at this point how large this countereffect is because we do not know the rigidity of the pit membrane polymers. It is possible that this negative term accounts for the negative effect of cations on water flow, as found in some tree species (Cochard et al., 2010).
Changes of Pore Size Due to Processes Occurring in the Surrounding Pit Membrane
It is well known that a mixture of pectin polymers exhibit swelling and shrinking that depend on the salt concentration of the aqueous solution that flows through. The same is true for any other polyelectrolyte. The polymer network of these compounds is charged because of deprotonation of functional groups. As mentioned, the pore diameter in pit membranes is estimated to be 5 to 20 nm, which is only slightly larger than the estimated pore diameter in cellulose in cell walls (3–5 nm; Shomer et al., 1991). Therefore, at ionic strength I = 0.001 to 0.01 m, complete or significant overlap of the DDL may be assumed. In that case, the double layer properties can be described with the Donnan model (Grignon and Scallan, 1980; Hiemstra and van Riemsdijk, 2006b). A mathematical description of this model is given below.
The Donnan model predicts that an increase of the ionic charge leads to shrinking of the polyelectrolyte. When the polyelectrolyte in the pit membrane shrinks, a force will act on the pore walls to pull it back. This will tend to enlarge the pore diameter. This force will counteract the previously discussed force within the pore. Because double layer overlap within the polymer network of the membrane is much stronger (due to smaller pore size) than in the cylindrical pores of the pit, it may be expected that the polymer matrix in the pit membrane will shrink considerably when the ionic strength is increased. Thus, this will lead to an increase in pore diameter. The magnitude of this change will again depend on the rigidity of the polymer network in the pit membrane. In combination with the increase of the free pore space due to a decrease of the DDL, the increase of the ionic strength will allow a larger water flux through the pit membrane pores.
It should be noted that our alternative hypothesis is not fundamentally different from one proposed by Zwieniecki et al. (2001). Still, the alternative hypothesis pinpoints other causes and is more complex. The two modifications on the Zwieniecki et al. (2001) hypothesis are: 1) that swelling and shrinking are extended to all polyelectrolytes in the pit membrane (at least hemicellulose and lignin) and thus that swelling and shrinking do not depend on pectin, and 2) that the DDL processes within the pit pore channels can also inhibit or promote local water flow. The latter effect consists of two opposing forces. For example, increasing the ion concentration in the xylem sap will lead to an increase of the effective space in the pore that can be used for rapid water transport. This effect is opposed by the relaxation of the pore walls that had been pushed back by the large DDL in the case of low ionic strength. This relaxation will result in a smaller pore diameter.
Mathematical Description of the Donnan Model Applied to Polyelectrolyte Swelling
The negative charge (σ) of the (polyelectrolyte) polymers in the pit membrane is compensated by accumulated cations in an aqueous Donnan phase with concentration (CD) and volume (VD). The Donnan concentration (CD) differs from the concentration of the free solution (Co), giving rise to an electrostatic potential (ψ; in V). This leads to (Hiemstra and van Riemsdijk, 2006b):
where z is the valence of the ion (mol+/mol), F is the Faraday constant (96,485 C/mol+), R is the gas constant (8.31 J/mol/K), and T is the absolute temperature. The total charge of the cations in the Donnan phase equals the polymer charge. Simplifying to the presence of a single type of cation, one may write σ = −zVDCD (Hiemstra and van Riemsdijk, 2006b). Introduction into Equation 4 leads to:
where B is called the Boltzmann factor. At a given charge (σ) and Donnan volume (VD), a decrease of the ion concentration in the free solution (Co) will increase the Boltzmann factor (B) and the corresponding electrostatic potential ψ. In other words, the electrostatic effects will increase due to more overlap of the DDL. This overlap of the double layer can be counteracted by an increase of the Donnan volume (VD). This increase in Donnan volume will result in swelling of the polymer matrix, which occurs as far as allowed by the bonds of the polymer network. By contrast, an increase of the ionic charge leads to shrinking of the polymer matrix.
CONCLUSION
Several arguments can be raised against the hypothesis that the shrinking of pectin in the pit membranes is responsible for the increase in the rate of water flow through the xylem that is induced by cations or protons. One argument is that there often seems to be no pectin. An alternative hypothesis was formulated based on the presence of charge in any polyelectrolyte in the pit membranes. In the channel of a charged pit pore, a DDL will develop, creating a layer of water that is less mobile and more viscous. This can physically reduce or inhibit the flow of water in the very narrow pores of the angiosperm pit membrane. Upon an increase in salt concentration, this inhibitory effect becomes less, thus allowing a higher rate of water flow. Furthermore, increasing the salt concentration induces shrinking of the pit membrane polymers. This results in a larger diameter of the pores and in higher water flux. Both effects are only partly counteracted by relaxation of the polymers in the wall of the pit pore, decreasing the pore diameter. Together, these effects likely largely explain the effects of cations on the water flow in the xylem.
Acknowledgments
We thank Steven Jansen (Ulm, Germany) for very useful comments during the preparation of the manuscript and for sending a then-unpublished paper.
References
- Ahola S, Salmi J, Johansson LS, Laine J, Österberg M. (2008) Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions. Biomacromolecules 9: 1273–1282 [DOI] [PubMed] [Google Scholar]
- Alves E, Leite B, Pascholati SF, Ishida ML, Andersen PC. (2009) Citrus sinensis leaf petiole and blade colonization by Xylella fastidiosa: details of xylem vessel occlusion. Sci Agric (Braz) 66: 218–224 [Google Scholar]
- Arend M, Muninger M, Fromm J. (2008) Unique occurrence of pectin-like fibrillar cell wall deposits in xylem fibres of poplar. Plant Biol (Stuttg) 10: 763–770 [DOI] [PubMed] [Google Scholar]
- Bauch J, Liese W, Scholz F. (1968) Ueber die Entwicklung und stoffliche Zusammensetzung der Hoftüpfelmembranen von Längstracheiden in Coniferen. Holzforschung 22: 145–153 [Google Scholar]
- Bauch J, Liese W, Schultze R. (1972) The morphological variability of the bordered pit membranes in gymnosperms. Wood Sci Technol 6: 165–184 [Google Scholar]
- Bolt GH. (1982) The ionic distribution in the diffuse double layer. Bolt GH, , Soil Chemistry, Ed 2 Elsevier, Amsterdam, pp 1–26 [Google Scholar]
- Boyce CK, Zwieniecki MA, Cody GD, Jacobsen C, Wirick S, Knoll AH, Holbrook NM. (2004) Evolution of xylem lignification and hydrogel transport regulation. Proc Natl Acad Sci USA 101: 17555–17558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield BG, Meylan BA. (1982) Cell wall hydrolysis in the tracheary elements of the secondary xylem. Baas P, , New Perspectives in Plant Anatomy. Martinus Nijhoff/Dr. W. Junk Publishers, The Hague, The Netherlands, pp 71–84 [Google Scholar]
- Catesson AM. (1983) A cytochemical investigation of the lateral walls of Dianthus vessels. Differentiation and pit-membrane formation. IAWA Bull 4: 89–101 [Google Scholar]
- Catesson AM, Czaninski Y, Moreau M, Peresse M. (1979) Conséquences d’une infection vasculaire sur la maturation des vaisseaux. Rev Mycol 43: 239–243 [Google Scholar]
- Choat B, Ball M, Luly J, Holtum J. (2003) Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiol 131: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Brodie TW, Cobb AR, Zwieniecki MA, Holbrook NM. (2006) Direct measurements of intervessel pit membrane hydraulic resistance in two angiosperm tree species. Am J Bot 93: 993–1000 [DOI] [PubMed] [Google Scholar]
- Choat B, Cobb AR, Jansen S. (2008) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177: 608–625 [DOI] [PubMed] [Google Scholar]
- Cochard H, Herbette S, Hernández E, Hölttä T, Mencuccini M. (2010) The effects of sap ionic composition on xylem vulnerability to cavitation. J Exp Bot 61: 275–285 [DOI] [PubMed] [Google Scholar]
- Czaninski Y. (1972) Observations ultrastructurales sur l’hydrolyse des parois primaries des vaisseaux chez le Robinia pseudo-acacia L. et l’Acer pseudoplatanus L. C R Acad Sci (Paris) 275: 361–363 [Google Scholar]
- Czaninski Y. (1979) Cytochimie ultrastructurel des parois du xylème secondaire. Biol Cell 35: 97–102 [Google Scholar]
- Domec JC, Meinzer FC, Lachenbruch B, Housset J. (2007) Dynamic variation in sapwood specific conductivity in six woody species. Tree Physiol 27: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Dute RR. (1994) Pit membrane structure and development in Ginkgo biloba. IAWA J 15: 75–90 [Google Scholar]
- Dute RR, Hagler L, Black A. (2008a) Comparative development of intertracheary pit membranes in Abies firma and Metasequoia glyptostroboides. IAWA J 29: 277–289 [Google Scholar]
- Dute RR, Jansen S, Holloway C, Paris K. (2008b) Torus-bearing pit membranes in selected species of the Oleaceae. J Ala Acad Sci 79: 12–21 [Google Scholar]
- Eicke R, Ehling E. (1965) Die Ausbildung der jungen Tracheiden von Ginkgo biloba. Ber Deutsch Botan Gesellsch 78: 326–333 [Google Scholar]
- El Seoud OA, Fidale LC, Ruiz N, D’Almeida MLO, Frollini E. (2008) Cellulose swelling by protic solvents: which properties of the biopolymer and the solvent matter? Cellulose 15: 371–392 [Google Scholar]
- Espino S, Schenk HJ. (2011) Mind the bubbles: achieving stable measurements of maximum hydraulic conductivity through woody plant samples. J Exp Bot 62: 1119–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fält S, Wågberg L, Vesterlind EL. (2003) Swelling of model films of cellulose having different charge densities and comparison to the swelling behavior of corresponding fibers. Langmuir 19: 7895–7903 [Google Scholar]
- Fengel D. (1966) Entwickling und Ultrastruktur de Pinaceen-Hoftüpfel. Svensk Papperstidning 69: 232–241 [Google Scholar]
- Fromm J, Rockel B, Lautner S, Windeisen E, Wanner G. (2003) Lignin distribution in wood cell walls determined by TEM and backscattered SEM techniques. J Struct Biol 143: 77–84 [DOI] [PubMed] [Google Scholar]
- Gascó A, Nardini A, Gortan E, Salleo S. (2006) Ion-mediated increase in the hydraulic conductivity of Laurel stems: role of pits and consequences for the impact of cavitation on water transport. Plant Cell Environ 29: 1946–1955 [DOI] [PubMed] [Google Scholar]
- Gascó A, Salleo S, Gortan E, Nardini A. (2007) Seasonal changes in the ion-mediated increase of xylem hydraulic conductivity in stems of three evergreens: any functional role? Physiol Plant 129: 597–606 [Google Scholar]
- Goli E, Hiemstra T, Van Riemsdijk WH, Rahnemaie R, Malakouti MJ. (2010) Diffusion of neutral and ionic species in charged membranes: boric acid, arsenite, and water. Anal Chem 82: 8438–8445 [DOI] [PubMed] [Google Scholar]
- Gortan E, Nardini A, Salleo S, Jansen S. (2011) Pit membrane chemistry influences the magnitude of ion-mediated enhancement of xylem hydraulic conductance in four Lauraceae species. Tree Physiol 31: 48–58 [DOI] [PubMed] [Google Scholar]
- Grignon J, Scallan AM. (1980) Effect of pH and neutral salts upon the swelling of cellulose gels. J Appl Polym Sci 25: 2829–2843 [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. (2004) Analysis of circular bordered pit function II. Gymnosperm tracheids with torus-margo pit membranes. Am J Bot 91: 386–400 [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26: 689–701 [DOI] [PubMed] [Google Scholar]
- Hiemstra T, Van Riemsdijk WH. (2006a) On the relationship between charge distribution, surface hydration, and the structure of the interface of metal hydroxides. J Colloid Interface Sci 301: 1–18 [DOI] [PubMed] [Google Scholar]
- Hiemstra T, van Riemsdijk WH. (2006b) Biogeochemical speciation of Fe in ocean water. Mar Chem 102: 181–197 [Google Scholar]
- Imamura Y, Harada H. (1973) Electron microscopic study on the development of the bordered pit in coniferous tracheids. Wood Sci Technol 7: 189–205 [Google Scholar]
- Imamura Y, Harada H, Saiki H. (1974) Embedding substances of pit membranes in softwood tracheids and their degradation by enzymes. Wood Sci Technol 8: 243–254 [Google Scholar]
- Jansen S, Choat B, Vinckier S, Lens F, Schols P, Smets E. (2004) Intervascular pit membranes with a torus in the wood of Ulmus (Ulmaceae) and related genera. New Phytol 163: 51–59 [DOI] [PubMed] [Google Scholar]
- Jansen S, Gortan E, Lens F, Lo Gullo MA, Salleo S, Scholz A, Stein A, Trifilò P, Nardini A. (2011) Do quantitative vessel and pit characters account for ion-mediated changes in the hydraulic conductance of angiosperm xylem? New Phytol 189: 218–228 [DOI] [PubMed] [Google Scholar]
- Jansen S, Sano Y, Choat B, Rabaey D, Lens F, Dute RR. (2007) Pit membranes in tracheary elements of Rosaceae and related families: new records of tori and pseudotori. Am J Bot 94: 503–514 [DOI] [PubMed] [Google Scholar]
- Jayme G, Azolla FK. (1965) Textur und Topochemie der Tüpfel und Tüpfelschliesshäute von Buchenholzzellen (Fagus sylvatica). Holz als Roh und Werkstoff 23: 41–49 [Google Scholar]
- Jayme G, Fengel D. (1961) Beitrag zur Kenntniss der Fichtenholztracheiden. II. Beobachtungen an Ultradünnschnitten von delignifiziertem Holz und Ligningerüsten. Holzforschung 16: 98–102 [Google Scholar]
- Koorevaar P, Menelik G, Dirksen C. (1983) Elements of Soil Physics. Elsevier, Amsterdam [Google Scholar]
- Li X, Pan X. (2010) Hydrogels based on hemicellulose and lignin from lignocellulose biorefinery: a mini-review. J Biobased Mat Bioenergy 4: 289–297 [Google Scholar]
- López-Portillo J, Ewers FW, Angeles G. (2005) Sap salinity effects on xylem conductivity in two mangrove species. Plant Cell Environ 28: 1285–1292 [Google Scholar]
- Meylan BA, Butterfield BG. (1982) Pit membrane structure in the vessel-less wood of Pseudowintera dandy (Winteraceae). IAWA Bull New Ser 3: 167–175 [Google Scholar]
- Milne CJ, Kinniburgh DG, Tipping E. (2001) Generic NICA-Donnan model parameters for proton binding by humic substances. Environ Sci Technol 35: 2049–2059 [DOI] [PubMed] [Google Scholar]
- Nardini A, Gascó A, Cervone F, Salleo S. (2007a) Reduced content of homogalacturonan does not alter the ion-mediated increase in xylem hydraulic conductivity in tobacco. Plant Physiol 143: 1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Gascò A, Trifilò P, Lo Gullo MA, Salleo S. (2007b) Ion-mediated enhancement of xylem hydraulic conductivity is not always suppressed by the presence of Ca2+ in the sap. J Exp Bot 58: 2609–2615 [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Jansen S. (2011) More than just a vulnerable pipeline: xylem physiology in the light of ion-mediated regulation of plant water transport. J Exp Bot 62: 4701–4718 10.1093/jxb/err208 [DOI] [PubMed] [Google Scholar]
- O’Brien TP. (1970) Further observations on hydrolysis of the cell wall in the xylem. Protoplasma 69: 1–14 [Google Scholar]
- Pérez-Donoso AG, Sun Q, Roper MC, Greve LC, Kirkpatrick B, Labavitch JM. (2010) Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected grapevines. Plant Physiol 152: 1748–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J, Sperry JS, Hacke UG, Wheeler JK, Sikkema EH. (2005) Torus-margo pits help conifers compete with angiosperms. Science 310: 1924. [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hacke UG. (July 29, 2011) Heterogeneous distribution of pectin epitopes and calcium in different pit types of four angiosperm species. New Phytol http://dx.doi.org/10.1111/j.1469-8137.2011.03842.x [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hacke UG, Sperry JS. (2011) Linking irradiance-induced changes in pit membrane ultrastructure with xylem vulnerability to cavitation. Plant Cell Environ 34: 501–513 [DOI] [PubMed] [Google Scholar]
- Rabaey D, Lens F, Smets E, Jansen S. (2006) The micromorphology of pit membranes in tracheary elements of Ericales: new records of tori or pseudotori? Ann Bot 98: 943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux D, Nicole M, Simard M, Ouellette GB. (1998) Immunocytochemical evidence that secretion of pectin occurs during gel (gum) and tylosis formation in trees. Phytopathology 88: 494–505 [DOI] [PubMed] [Google Scholar]
- Sachs IB. (1963) The torus of the bordered pit membrane in conifers. Nature 198: 906–907 [Google Scholar]
- Schmitz N, Koch G, Schmitt U, Beeckman H, Koedam N. (2008) Intervessel pit structure and histochemistry of two mangrove species as revealed by cellular UV microspectrophotometry and electron microscopy: intraspecific variation and functional significance. Microsc Microanal 14: 387–397 [DOI] [PubMed] [Google Scholar]
- Shomer I, Frenkel H, Polinger C. (1991) The existence of a diffuse electric double layer at cellulose fibril surfaces and its role in the swelling mechanism of parenchyma plant cell walls. Carbohydr Polym 16: 199–210 [Google Scholar]
- Sjöström E. (1989) The origin of charge on cellulosic fibers. Nordic Pulp Paper Res J 4: 90–93 [Google Scholar]
- Soukup A, Votrubová O. (2005) Wound-induced vascular occlusions in tissues of the reed Phragmites australis: their development and chemical nature. New Phytol 167: 415–424 [DOI] [PubMed] [Google Scholar]
- Sun Q, Greve LC, Labavitch JM. (2011) Polysaccharide compositions of intervessel pit membranes contribute to Pierce's disease resistance of grapevines. Plant Physiol 155: 1976–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA. (2008) Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): tyloses in summer and gels in winter. Am J Bot 95: 1498–1505 [DOI] [PubMed] [Google Scholar]
- Timell TE. (1973) Studies on opposite word in conifers: III. Distribution of lignin. Wood Sci Technol 7: 163–172 [Google Scholar]
- Trifilò P, Lo Gullo MA, Salleo S, Callea K, Nardini A. (2008) Xylem embolism alleviated by ion-mediated increase in hydraulic conductivity of functional xylem: insights from field measurements. Tree Physiol 28: 1505–1512 [DOI] [PubMed] [Google Scholar]
- Trifilò P, Nardini A, Raimundo F, Lo Gullo MA, Salleo S. (2011) Ion-mediated compensation for drought-induced loss of xylem hydraulic conductivity in field-growing plants of Laurus nobilis. Funct Plant Biol 38: 606–613 [DOI] [PubMed] [Google Scholar]
- van Ieperen W. (2007) Ion-mediated changes of xylem hydraulic resistance in planta: fact or fiction? Trends Plant Sci 12: 137–142 [DOI] [PubMed] [Google Scholar]
- van Ieperen W, van Gelder A. (2006) Ion-mediated flow changes suppressed by minimal calcium presence in xylem sap in Chrysanthemum and Prunus laurocerasus. J Exp Bot 57: 2743–2750 [DOI] [PubMed] [Google Scholar]
- van Ieperen W, van Meeteren U, van Gelder H. (2000) Fluid ionic composition influences hydraulic conductance of xylem conduits. J Exp Bot 51: 769–776 [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N. (2005) Intervessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ 28: 800–812 [Google Scholar]
- Wisniewski M, Davis G. (1995) Immunogold localization of pectins and glycoproteins in tissues of peach with reference to deep supercooling. Trees Struct Func 9: 253–260 [Google Scholar]
- Zimmermann MH. (1978) Hydraulic architecture of some diffuse-porous trees. Can J Bot 56: 2286–2295 [Google Scholar]
- Zwieniecki MA, Melcher PJ, Michele Holbrook NM. (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291: 1059–1062 [DOI] [PubMed] [Google Scholar]