Svensson and colleagues advocated for an extended accreditation cycle for well-established pharmacy programs.1 They argued that the extensive time and resources necessary during the 12 to 18 months prior to reaccreditation as part of the self-study process may reduce or delay other important initiatives, including academic initiatives. Therefore, Svensson and colleagues suggested that the Accreditation Council for Pharmacy Education (ACPE) consider lengthening their customary 6-year accreditation cycle, especially for programs that have been successful through 3 or more continuous full-accreditation cycles.1,2 Subsequently, the ACPE Board of Directors approved an 8-year accreditation cycle for fully accredited programs seeking continuing accreditation at or after the January 2012 Board meeting.3
The ACPE will now reaffirm full accreditation status for a period of 8 years; however, it reserves the right to allow for shorter intervals.2 Between onsite evaluations, institutions must provide annual reviews, interim reports, reports of any substantive changes, and a full self-study immediately prior to an evaluation visit. The self-study is the most time-intensive part of the process and it is recommended that the college or school start it 18 to 24 months prior to the accreditation visit. The self-study is composed of a summary evaluation of each of the accreditation standards and guidelines and any progress and changes that have occurred since the last accreditation visit, with documentation, data, and descriptive text provided to support each.4 Based on findings from the self-study, the college or school notes whether its program is compliant, compliant with monitoring, partially compliant, or noncompliant with each accreditation standard. Ideally, the purpose of this process is to promote self-evaluation and continuous quality improvement in programs. However, it may be conducive for some programs to only think about quality improvement and self-evaluation during the time of the self-study.
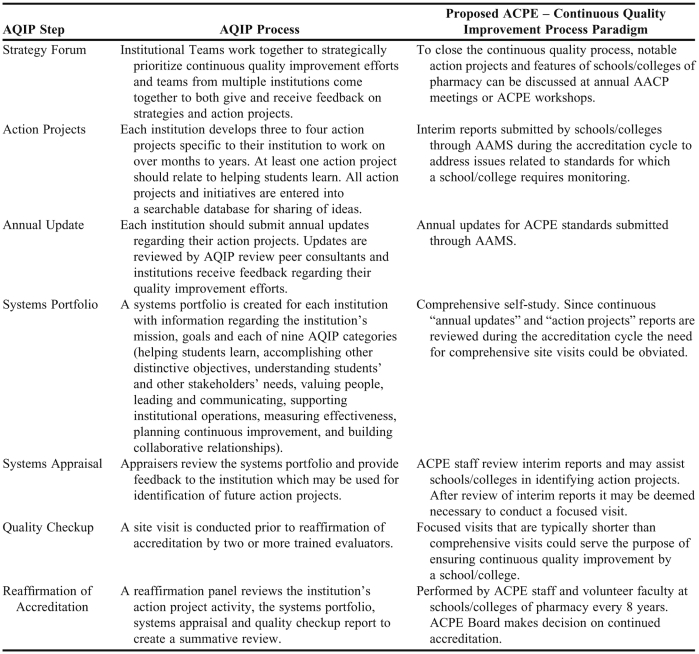
We would like to propose an alternative accreditation process that incorporates an alternative continuous quality improvement model. Our institution, Southern Illinois University Edwardsville, maintains accreditation through the Academic Quality Improvement Program (AQIP) of The Higher Learning Commission. This process requires that the institution provide evidence of continuous improvement through 7 core processes: strategy forum, action projects, annual update, systems portfolio, systems appraisal, quality checkup, and reaffirmation of accreditation.5 These processes are further described in Table 1. Each institution continually maintains 3 to 4 action projects related to continuous improvement efforts that should be completed within months to years depending on the scope of the project. Progress on the action projects is updated in the systems’ portfolio and feedback is given to the organization through the portfolio and strategy forum through a peer review process.
Table 1.
AQIP Core Processes5 and the Proposed ACPE-Continuous Quality Improvement Process Paradigm
The AQIP process charges programs to continually assess and improve based on outcomes and requires constant resources to maintain the portfolio and work on action projects.5 While this process still includes a reaccreditation or reaffirmation process every 7 years, the process is much less time intensive during the time immediately prior to reaccreditation because of the continuous efforts throughout the accreditation cycle. Such an evidence-based approach requires significant dedication and infrastructure to support these institutional initiatives. Moreover, it encourages sustained attention and action from administrators, faculty members, and staff members, and facilitates the university's achievement of its long-term goals. Arguably, this process helps foster a culture focused on continuous improvement, rather than focusing stakeholders’ attention on accountability in the short-term.
The American Association of Colleges of Pharmacy (AACP) developed the Assessment and Accreditation Management System (AAMS) which is required for submission of accreditation documents to ACPE.6 The advantage of the AAMS is that data can be continuously compiled and updated. Interim reports that are submitted by schools during the accreditation cycle to address issues related to standards for which a college or school requires monitoring could serve as “action projects” in the continuous quality improvement process paradigm. “Annual updates” in this paradigm could be created from the continuously updated information in the AAMS. The regular updating of information for action projects and annual updates should greatly reduce the time and resource commitment before the comprehensive review.
The systems appraisal process is already conducted by the ACPE staff when they review interim reports and determine if a focused visit is necessary. This would constitute a quality checkup in the continuous quality improvement process paradigm. Such focused visits, which are typically shorter than comprehensive visits, could serve the purpose of ensuring continuous quality improvement by a college or school. These focused visits would include faculty members at other colleges and schools of pharmacy in addition to ACPE staff members. The systems portfolio in the AQIP paradigm would constitute the comprehensive self-study. Because continuous annual updates and action project reports are reviewed during the accreditation cycle, the need for comprehensive site visits could be obviated. The “reaffirmation of accreditation” in the continuous quality improvement process paradigm could therefore be performed by ACPE staff members and volunteer faculty members at colleges and schools of pharmacy. The ACPE Board would make a decision about continued accreditation of a college or school at this point. To close the loop of the continuous quality improvement process, notable action projects and features of colleges and schools of pharmacy could be discussed at annual AACP meetings or ACPE workshops. This would be similar to the strategy forum in the continuous quality improvement process. The strategy forum would serve as a strategic planning meeting, allowing colleges and schools of pharmacy to review unit data and strategically prioritize continuous quality efforts for the upcoming accreditation cycle.
This process would likely not be appropriate for every pharmacy program, but may be a realistic/viable alternative for established programs with sufficient resources and administrative support. This process could be used as a parallel method to the existing process as a means to enhance continuous quality improvement in colleges and schools of pharmacy. It would be fair for ACPE to consider the financial implications of the system proposed here. Appropriate adjustments in ACPE annual fees, focused visit fees, and reaffirmation of accreditation fees would be needed.
A proposed ACPE process similar to the AQIP core process is presented in Table 1.5 We believe that this process would reduce the amount of time and resources that colleges and schools of pharmacy spend on compiling a comprehensive self-study document every 8 years and may assist schools in cultivating a continuous quality improvement focus.
REFERENCES
- 1.Svensson CK, Speedie MK, Roberts JC, Letendre DE, Brueggemeier RW, Bauman JL, Ascione FJ. Reconsidering the length of program accreditation. Am J Pharm Educ. 2011;75(1):Article 6. doi: 10.5688/ajpe7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Policies and Procedures for ACPE Accreditation of Professional Degree Programs. Accreditation Council for Pharmacy Education. http://www.acpe-accredit.org/pdf/CS_PoliciesandProcedures.pdf. Accessed February 15, 2012.
- 3. Accreditation Council for Pharmacy Education Report of Proceedings – June 22-26, 2011 Meeting. http://www.acpe-accredit.org/pdf/ReportofProceedingsJune2011.pdf. Accessed February 15, 2012.
- 4. Self-Assessment Instrument for the Professional Degree Program of Colleges and Schools of Pharmacy. Version 4.0 Standards 2007/Guidelines 2.0. Accreditation Council for Pharmacy Education. http://www.acpe-accredit.org/pdf/rubric2007guidelines20version40schoolversionforrelease.pdf. Accessed February 15, 2012.
- 5. Introduction to AQIP. Academic Quality Improvement Program, The Higher Learning Commission. http://www.asis.org/IPA/Introduction_to_AQIP.pdf. Accessed February 15, 2012.
- 6. Assessment & Accreditation Management System (AAMS). American Association of Colleges of Pharmacy (AACP). http://www.aacp.org/resources/education/peas/Pages/AAMS.aspx. Accessed February 15, 2012.