The primary purpose of the eyelids is to ensure corneal integrity. An important component of this protective function is maintaining the corneal tear film. Blink characteristics can determine tear film stability. Increasing blink amplitude thickens the lipid layer that overlies the aqueous layer of the tear film. This thickening reduces evaporation of the aqueous layer. Because each blink reforms the tear film, increasing blink frequency reduces tear film break-up.1–4 Both innocuous stimuli such as air across the eyelashes, and noxious stimuli such as touching the cornea, elicit trigeminal reflex blinks. If abnormal corneal afferent activity caused by corneal irritation acts as an “error signal” that adjusts the amplitude and frequency of reflex blinks evoked by both innocuous and corneal stimuli, then corneal irritation creates an adaptive blink response regardless of whether a corneal or an innocuous trigeminal stimulus elicits the blink.
An investigation of the effects of aging on trigeminal blinks5 supports the hypothesis that corneal irritation modifies trigeminal reflex blinks evoked by innocuous stimuli. In people over age 40 years, a single innocuous, supraorbital (SO) nerve stimulus frequently evokes a reflex blink and additional blinks that occur at a nearly constant interval relative to the onset of the preceding blink, blink oscillations. Because blink oscillations are more frequent and larger than reflex blinks, this modification increases tear film stability. Peshori and colleagues5 propose that the development of consistent blink oscillations in many individuals over age 40 years is a blink adaptation that compensates for the subclinical reduction in corneal wetting that accompanies aging.6 This hypothesis predicts that innocuous SO stimuli should produce more blink oscillations in people with dry eye than in age-matched control subjects. We test this prediction by comparing the SO-evoked blinks of individuals with dry eye with those of age-matched controls. Given the frequent occurrence of dry eye at the onset of benign essential blepharospasm (BEB),7 blink modifications associated with dry eye may play a role in the origin of BEB. We present a hypothesis that links the adaptive processes initiated by dry eye with the origin of BEB.
METHODS
Measurements of eyelid movement and orbicularis oculi electromyographic (OOemg) activity were made on seven subjects, ranging in age from 44 to 72 years of age. Five female subjects had been diagnosed with dry eye at the SUNY Stony Brook Ophthalmology Clinic. The data from these subjects were compared with those from age-matched control subjects in another study.5 Two normal subjects, a man and a woman, participated in an experimental paradigm described below. Apart from dry eye, no subject had any ocular disorders other than the need for corrective lenses. A previous study8 detailed measurement of lid movements and OOemg used in the current study. Upper eyelid position was measured with the magnetic search coil technique. The OOemg was recorded with a pair of miniature silver plates attached to the medial and lateral aspects of the upper eyelid, and was amplified and filtered (0.3–2 kHz). To evoke reflex blinks, an electrical stimulus was presented through a pair of electrodes placed over the SO nerve. Threshold was defined as the smallest intensity stimulus of 170-µsec duration that reliably elicited a blink. For data collection, SO stimuli were always twice threshold intensity (2T). No subject reported these stimuli to be uncomfortable. Subjects received 28 trials containing pairs of 2T SO stimuli with interstimulus intervals of 250, 500, 750, or 1000 msec and 10 single, 2T SO stimulus trials. Trials occurred every 25 ± 5 sec.
To test whether Aδ- and C-fiber activation such as occurs with corneal stimulation modifies the blinks evoked by subsequent innocuous stimuli, we presented high-intensity SO stimuli to two normal subjects. These subjects received 28 trials containing pairs of 2T SO stimuli with interstimulus intervals of 250, 500, 750, or 1,000 msec before and immediately following five, high-intensity (10T) SO stimuli at 4 Hz. The same procedure was repeated 24 hours later for each subject.
For each trial, a period of 1.4 seconds of upper eyelid position and OOemg activity were digitized at 2 kHz per channel and stored for later off-line analysis. By using laboratory-created software, we analyzed blink magnitude, duration, and latencies and calculated the magnitude of EMG activity by integrating the rectified OOemg activity.
RESULTS
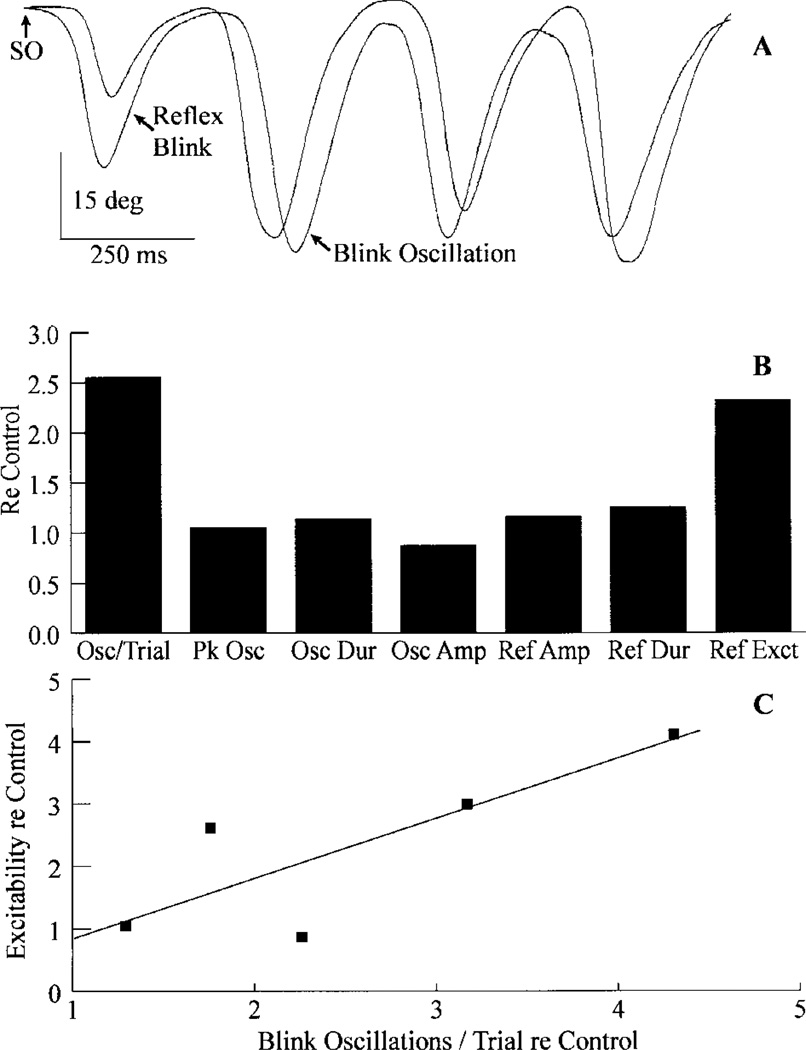
A dry eye condition modified trigeminal blinks in two ways. First, people with dry eye exhibited frequent blink oscillations. For example, a single 2T SO stimulus evoked three or more blink oscillations with a nearly constant interblink interval (Fig. 1A). To estimate the frequency of blink oscillations, we divided the total number of blink oscillations by the number of trials. Overall, dry eye subjects generated 2.55 ± 0.6 times more blink oscillations/trial than age-matched controls (Fig. 1B, Osc/Trial). Thus, dry eye converted the reaction of the reflex blink circuit to an innocuous stimulus from a single response to an oscillatory pattern. Second, dry eye patients often exhibited increased trigeminal reflex blink excitability as measured with the paired stimulus paradigm (Fig. 1B, Ref Exct). Averaged over all four interstimulus intervals, the five subjects were 2.33 ± 0.79 times more excitable than age-matched controls. Other than increased excitability and frequency of blink oscillations, however, trigeminal blink characteristics were the same in patients with dry eye and age-matched controls (Fig. 1B).
FIG. 1.
Blink modifications associated with dry eye. A: A single, twice threshold intensity stimulus to the supraorbital branch of the trigeminal nerve (SO↑) evokes a reflex blink and additional blinks, blink oscillations, which occur with a nearly constant interblink interval. Each trace is a single trial showing upper eyelid position. B: Graph showing the average number of blink oscillations per trial (Osc/Trial), interblink oscillation interval (Pk Osc), blink oscillation duration (Osc Dur), amplitude of blink oscillations (Osc Amp), amplitude of reflex blinks (Ref Amp), reflex blink duration (Ref Dur), and reflex blink excitability (Ref Exct) for five dry eye subjects relative to age-matched controls.5 C: Average reflex blink excitability for all four interstimulus intervals relative to age-matched control subjects as a function of blink oscillations per trial relative to age-matched control subjects5 for the five dry eye subjects. The line is the best-fit linear regression (y = 0.933, x = 0.056, r2 = 0.67).
The frequency of blink oscillations/trial varied among the subjects with dry eye. The subject illustrated in Figure 1A averaged 3.2 blink oscillations each trial. The subject with the fewest blink oscillations showed an average of only 0.96 blink oscillations each trial. Overall, the five subjects produced an average of 1.9 ± 0.45 blink oscillations each trial. For these five dry eye subjects, reflex blink excitability and blink oscillations per trial relative to age-matched control subjects increased together (Fig. 1C). These data suggested that dry eye links reflex blink excitability and the frequency of blink oscillations. Thus, the activation of corneal Aδ- and C-afferents associated with the irritation of dry eye modified the excitability of trigeminal blink circuits to innocuous SO stimuli.
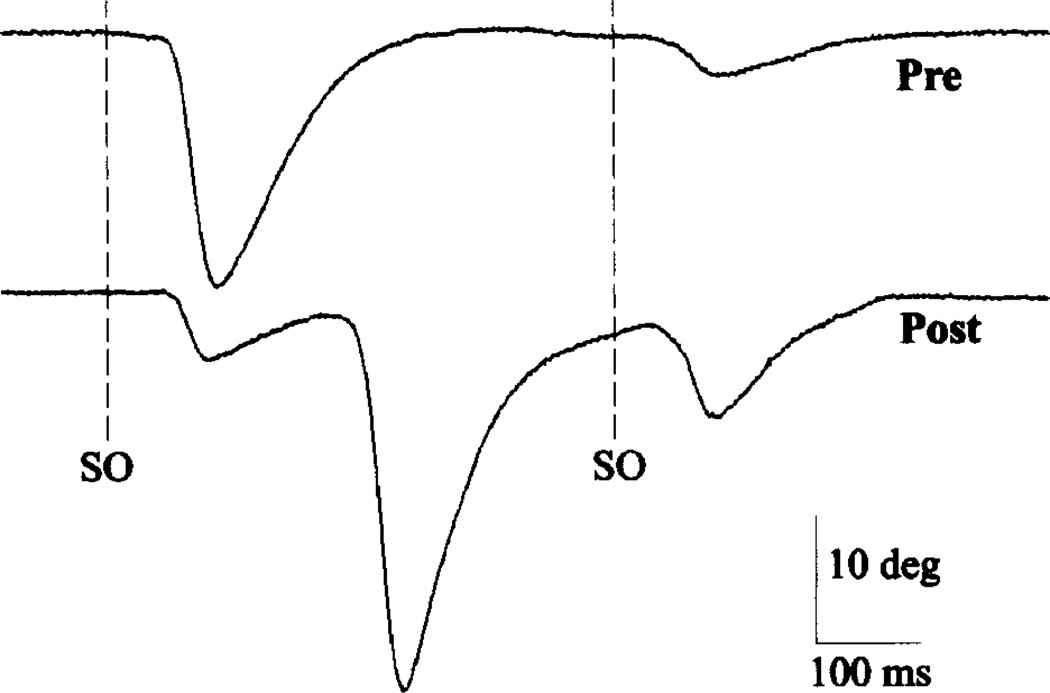
To investigate whether transient activation of Aδ- and C-fibers was sufficient to modify subsequent innocuous SO-evoked blinks in normal subjects, we presented five high-intensity (10T) SO stimuli and examined subsequent blinks evoked by 2T SO stimuli. Consistent with high-intensity stimuli activating small diameter SO fibers, subjects reported that these stimuli were painful. Immediately after high-intensity stimulation, an innocuous stimulus evoked 1.13 to 8.4 times more blink oscillations per trial than before the high-intensity treatment, with a mean increase of 3.38 times more oscillations per trial (n = 4 experiments; Fig. 2). In one subject, high-intensity treatment also increased reflex blink excitability. For the four interstimulus intervals, the average excitability increased by factors of 1.35 and 3.29 immediately after high intensity stimuli in the 2 days of testing. High-intensity treatment also decreased the amplitude of blinks evoked by 2T SO stimuli relative to blink amplitude before treatment (Fig. 2). One subject exhibited a 25% and 27% decrease in blink amplitude in the two experiments. The subject showing increased excitability exhibited a 53% and 40% decrease.
FIG. 2.
Effect of high-intensity stimulation (10× threshold) to the supraorbital branch of the trigeminal nerve on subsequent reflex blinks. Before high-intensity treatment (Pre), pairs of twice threshold intensity stimuli to the supraorbital branch of the trigeminal nerve (SO dashed lines) with a 500-msec interstimulus interval elicited unequal amplitude reflex blinks. After high-intensity treatment (Post), the same stimuli elicited nearly equal amplitude reflex blinks and a blink oscillation. Each trace is a single record of upper eyelid position.
DISCUSSION
Blink oscillations compensate for dry eye. Because an innocuous stimulus evokes multiple blinks instead of a single reflex blink, this adaption improves tear distribution across the cornea and reduces tear film break-up.2 Moreover, because blink oscillations are larger than reflex blinks (Figs. 1A, 2), the increased amplitude of blinks evoked by an innocuous stimulus thickens the lipid layer, thereby reducing evaporation of the tear film’s aqueous layer.1,3 These observations and the increased frequency of blink oscillation with dry eye relative to age-matched control subjects support the hypothesis that the activation of corneal Aδ- and C-afferents by dry eye modifies the reflex blink circuit’s response to innocuous stimuli.
Wide dynamic range (WDR) neurons present a means through which corneal afferents can modify the response of reflex-blink circuits to innocuous, Aβ blink-evoking stimuli. WDR neurons are a component of the SO-evoked trigeminal reflex blink circuit, and these neurons respond to both small- and large-diameter afferents.9,10 In dry eye syndromes, innocuous blink-evoking stimuli arrive at WDR neurons while these neurons receive a continuous barrage of excessive Aδ- and C-fiber inputs produced by the corneal irritation. This summation of Aβ- with Aδ- and C-fiber inputs may modify the discharge of WDR neurons to an innocuous SO stimulus so that the reflex-blink circuit generates blink oscillations and exhibits increased blink excitability. It is also possible that large fiber inputs to WDR neurons can produce blink oscillations without summing with a continual barrage of small fiber inputs. If WDR neurons undergo long-term potentiation (LTP)- or long-term depression (LTD)-like modifications of the synaptic strength of their Aβ input caused by pairing with the Aδ- and C-fiber inputs, then a WDR neuron should respond to an innocuous stimulus alone in the same way as it did in response to the Aβ-fiber input combined with the Aδ- and C-fiber inputs. If blink oscillations result from LTP- or LTD-like modifications of WDR neurons’ response to Aβ inputs, then the blink modifications associated with dry eye are an example of motor learning.
Two lines of evidence suggest that the blink modifications associated with dry eye are not simply a response to continuous corneal stimulation but are an example of motor learning initiated by the error signal created by corneal irritation. First, the increase in blink oscillations occurring after the cessation of high intensity SO stimuli demonstrates that blink oscillations outlast the transient activation of Aδ- and C-fibers generated by the high-intensity stimuli (Fig. 2). Thus, blink oscillations remain elevated in the absence of constant Aδ- and C-fiber activation. Second, creating mild corneal irritation with unilateral lid restraint in normal subjects increases blink oscillations and reflex excitability. These modifications remain for 15 to 20 minutes after the eyelid returns to normal motility.11 Thus, the pairing of Aβ- with Aδ- and C-fiber inputs that occurs with dry eye can cause modifications that outlast the dry eye.
Interpreting some BEB characteristics12 as exaggerations of the blink adaptations initiated by dry eye points to a possible mechanism for the development of BEB. Reducing the time between blink oscillations with dry eye (Fig. 1A) will create the spasms of lid closure of BEB that result from repetitive bursts of orbicularis oculi activity. The elevated trigeminal reflex blink excitability of BEB can be an exaggeration of the increased blink excitability associated with dry eye (Fig. 1C). Consistent with this interpretation, the frequency of blink oscillations and excitability increase together. The frequent occurrence of dry eye before or coincident with the onset of BEB,7 supports the hypothesis that BEB stems from the nervous system’s attempt to compensate for dry eye. In individuals predisposed to BEB, the nervous system may lose its ability to regulate adjustment of trigeminal circuits, so that the blink modifications initiated by dry eye undergo unchecked increases, creating the involuntary spasms of lid closure and hyperexcitability that characterize BEB.
Acknowledgments
C.E. was supported by a grant from the National Eye Institute (EY07391).
REFERENCES
- 1.Korb DR, Baron DF, Herman JP, Finnemore VM, Exford JM, Hermosa JL, Leahy CD, Glonek T, Greiner JV. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359. doi: 10.1097/00003226-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Tseng SCG, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Opthalmol. 1997;124:825–835. doi: 10.1016/s0002-9394(14)71700-3. [DOI] [PubMed] [Google Scholar]
- 3.Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function and control. Adv Exp Med Biol. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- 4.Tutt R, Bradley A, Begley C, Thibos LN. Optical and visual impact of tear break-up in human eyes. Invest Ophthalmol Vis Sci. 2000;41:4117–4123. [PubMed] [Google Scholar]
- 5.Peshori KR, Schicatano EJ, Gopalaswamy R, Sahay E, Evinger C. Aging of the trigeminal blink system. Exp Brain Res. 2001;136:351–363. doi: 10.1007/s002210000585. [DOI] [PubMed] [Google Scholar]
- 6.van Haeringen NJ. Aging and the lacrimal system. Br J Ophthalmol. 1997;81:824–826. doi: 10.1136/bjo.81.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elston JS, Marsden CD, Granadas F, Quinn NP. The significance of ophthalmological symptoms in idiopathic blepharospasm. Eye. 1988;2:435–439. doi: 10.1038/eye.1988.79. [DOI] [PubMed] [Google Scholar]
- 8.Evinger C, Manning KA, Sibony PA. Eyelid movements: mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387–400. [PubMed] [Google Scholar]
- 9.Pantaleo T, Duranti R, Bellini F. Effects of heterotopic ischemic pain on muscular pain threshold and blink reflex in humans. Neurosci Lett. 1988;85:56–60. doi: 10.1016/0304-3940(88)90428-4. [DOI] [PubMed] [Google Scholar]
- 10.Ellrich J, Treede R-D. Characterization of blink reflex interneurons by activation of diffuse noxious inhibitory controls in man. Brain Res. 1998;803:161–168. doi: 10.1016/s0006-8993(98)00646-5. [DOI] [PubMed] [Google Scholar]
- 11.Schicatano EJ, Peshori KR, Henriquez VM, Evinger C. Lid restraint in normal humans mimics the effects of facial nerve palsy. Soc Neurosci Abstr. 1996;22:2035. [Google Scholar]
- 12.Berardelli A, Rothwell JC, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108:593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]