Abstract
Objective
There are numerous theories of panic disorder, each proposing a unique pathway of change leading to treatment success. However, little is known about whether improvements in proposed mediators are indeed associated with treatment outcomes and whether these mediators are specific to particular treatment modalities. Our purpose in this study was to analyze pathways of change in theoretically distinct interventions using longitudinal, moderated mediation analyses.
Method
Forty-one patients with panic disorder and agoraphobia were randomly assigned to receive 4 weeks of training aimed at altering either respiration (capnometry-assisted respiratory training) or panic-related cognitions (cognitive training). Changes in respiration (PCO2, respiration rate), symptom appraisal, and a modality-nonspecific mediator (perceived control) were considered as possible mediators.
Results
The reductions in panic symptom severity and panic-related cognitions and the improvements in perceived control were significant and comparable in both treatment groups. Capnometry-assisted respiratory training, but not cognitive training, led to corrections from initially hypocapnic to normocapnic levels. Moderated mediation and temporal analyses suggested that in capnometry-assisted respiratory training, PCO2 unidirectionally mediated and preceded changes in symptom appraisal and perceived control and was unidirectionally associated with changes in panic symptom severity. In cognitive training, reductions in symptom appraisal were bidirectionally associated with perceived control and panic symptom severity. In addition, perceived control was bidirectionally related to panic symptom severity in both treatment conditions.
Conclusion
The findings suggest that reductions in panic symptom severity can be achieved through different pathways, consistent with the underlying models.
Keywords: mediation, respiration, cognitions, panic, treatment
The efficacy of psychosocial treatment for panic disorder with or without agoraphobia (PD/A) has been well established (e.g., Barlow, Gorman, Shear, & Woods, 2000; Hofmann & Smits, 2008). However, the question of how and why these treatments work remains largely unanswered. The quest for extracting mediators that are central contributors to treatment success is vital for advancing intervention research. Investigating mediator candidates in PD/A is particularly intriguing given the wealth of interventions available, all with their own proposed mediators (Roth, Wilhelm, & Petitt, 2005). The mechanism of action for cognitive restructuring techniques (CT), for instance, is believed to be alterations in key cognitive processes (e.g., Clark et al., 1999; Gelder, Clark, & Salkovskis, 1993). The cognitive theory postulates that changes in catastrophic thinking are responsible for reductions in panic symptoms by attenuation of fearful interpretation of bodily symptoms (Clark, 1986). Thus, the alteration of symptom appraisal is presumed to be the primary mediator. A crucial first step in examining the validity of a proposed mediator requires the direct manipulation and repeated assessment of such mediators (Kazdin, 2007). Furthermore, to verify that change in symptom appraisal is indeed the proposed mediator, one must manipulate maladaptive cognitions in the absence of additional therapeutic interventions or techniques. However, CT is often combined with behavioral techniques (e.g., exposure), which complicates the direct testing of CT's specific pathways. Current evidence on the role of cognitive mediation on outcomes in PD is mostly limited to multicomponent interventions (e.g., Hoffart, Sexton, Hedley, & Martinsen, 2008; Hofmann et al., 2007; Teachman, Smith-Janik, & Marker, 2008). The one study that targeted changes in symptom appraisal in the absence of additional therapeutic techniques confirmed the validity of the proposed mediator (i.e., changes in daily ratings of beliefs about panic preceded changes in panic apprehension; Bouchard et al., 2007).
Another common intervention, based on an influential biological theory of panic, is breathing training. Breathing training is thought, contrary to CT, to derive its therapeutic effect through correction of a biological factor: a deranged respiratory control mechanism (Klein, 1993; Ley, 1985). These theories view hyperventilation or hypocapnia (lower than normal levels of carbon dioxide, or CO2) as the primary (Ley, 1985) or secondary (Klein, 1993) cause of panic symptoms. Hypocapnia can produce unpleasant bodily sensations, the fear of which may contribute to panic (Carr, Lehrer, & Hochron, 1992; Chambless, Caputo, Bright, & Gallagher, 1984), and has been linked to organ injury and a number of organic and mental illnesses (Laffey & Kavanagh, 2002; Meuret, in press). The correction of sustained hypocapnia by therapeutically increasing partial pressure of carbon dioxide (PCO2) should thereby lead to changes in panic symptoms. This theoretical framework was derived from cross-sectional research that identified hypocapnia as a critical biomarker in PD (e.g., Hegel & Ferguson, 1997; Papp, Klein, & Gorman, 1993). As noted earlier, a mandatory element of mechanism research is the successful manipulation of the proposed mediator. Surprisingly, the few studies examining the efficacy of traditional breathing training (Craske, Rowe, Lewin, & Noriega-Dimitri, 1997; Schmidt et al., 2000) have neither targeted nor assessed its proposed mechanism: the correction of hypocapnia. In contrast, the typical respiratory instructions (to breathe slowly and/or deeply; e.g., Craske et al., 1997; Schmidt et al., 2000) are likely to lead to compensatory deeper breathing, which in turn perpetuates hyperventilation and intensifies panic symptoms (Conrad et al., 2007; Meuret, Wilhelm, Ritz, & Roth, 2003).
Capnometry-assisted respiratory training, or CART, was developed to systematically change hypocapnia in patients with PD. CART is a brief, tightly controlled 4-week training that uses immediate feedback of end-tidal PCO2 to teach patients how to raise subnormal levels of PCO2, thereby gaining control over dysfunctional gas exchange and associated panic symptoms (e.g., shortness of breath, dizziness). Patients use a portable capnometer to continuously monitor the essential feature of hyperventilation, PCO2. Thus, CART differs substantially from traditional breathing retraining, as it assures the manipulation and assessment of respiratory physiology (Meuret et al., 2003). Due to the novelty of CART, empirical evidence is limited but promising. Preliminary results from a first randomized controlled trial study (CART vs. wait list; Meuret, Wilhelm, Ritz, & Roth, 2008) showed that clinically significant reductions in panic symptom severity were achieved by 68% of patients with PD/A at posttreatment (d = 2.21) and by 79% and 93% of such patients at 2-and 12-month follow-up; reductions were comparable to those achieved with cognitive behavior therapy or cognitive therapy (e.g., Barlow et al., 2000; Clark et al., 1999). These improvements were accompanied by sustained normalized levels of PCO2. Preliminary support for the proposed mediator, PCO2, was established with longitudinal mediation analysis that found PCO2 to mediate and drive changes in symptom appraisal (Meuret, Rosenfield, Suvak, Hofmann, & Roth, 2009).
Notwithstanding, secondary effects of PCO2 on symptom appraisal can be theorized, in that the attenuation of distressing respiratory symptoms may lessen threatening appraisals. Additionally, the ability to control respiratory activity may increase perceived control, an important modality-nonspecific mediator, by providing a strategy and a response plan (Craske & Hazlett-Stevens, 2002). It has been proposed that perceived control is an important determinant for the etiology and maintenance for all anxiety disorders (Barlow, 2002). Individuals with PD are believed to experience unexpected bursts of emotions (“true alarm” or “false alarms”). These emotional bursts (alarms) cause the vulnerable individuals to view their own emotions and bodily reactions as being out of their control. Individuals with PD are therefore believed to avoid agoraphobic situations or certain activities in part because they anticipate a lack of internal control over their emotional response on being exposed to perceived threat. These relations with other variables, and the effect of PCO2 on panic symptom severity over and above other potential mediators, remain untested. Likewise, secondary effects of symptom appraisal, the proposed mediator of CT, on a nonspecific mediator such as perceived control are plausible but have not been examined.
The aim in this study was to elucidate distinct and common mechanisms of change in two interventions for PD, CT and CART, and to determine their unique contribution to reductions in panic symptoms. Thus, the primary goal was to evaluate processes, rather than to compare outcomes, and to determine whether the coping skills taught in each treatment actually mediated the outcomes from each treatment. We took the following methodological steps to improve and extend prior research: (a) The interventions focused exclusively on the manipulation of the proposed mediator (symptom appraisal for CT and PCO2 for CART). By doing so, we avoided clouding the evaluation of the proposed mechanism of change with other manipulations. (b) We assessed intervention-specific mediators across multiple dimensions (both psychological and biological). If inconsistent results occurred, this would additionally inform us about method variance or the multifaceted nature of the constructs or dimensions. (c) We systematically examined a third, modality-nonspecific mediator, perceived control. As pointed out by Borden, Clum, and Salmon (1991), a general factor such as perceived control, common to many treatment modalities, “may be responsible for observed improvements in PD patients” (p. 259). Examining both modality-specific and modality-nonspecific mediators across different types of interventions offers insight as to whether these mediators emerge only when targeted directly by specific treatments or whether they also mediate relations in other treatment scenarios. The outlined methodological steps were based on the recommended guidelines for advanced meditational design (Kazdin, 2002).
Using longitudinal, moderated mediation analyses, we examined the following specific hypotheses:
Changes in respiratory regulation and symptom appraisal will be modality-specific mediators of outcome (panic symptom severity) in CT and CART, respectively.
Perceived control over emotional reactions and external threats will be a nonspecific mediator of outcomes across treatment modalities.
Among the mediators, PCO2 will partially mediate and precede changes in symptom appraisal and perceived control in CART, and symptom appraisal will partially mediate and precede changes in perceived control in CT.
Method
Participants
Forty-one patients with a principal diagnosis of panic disorder and agoraphobia (PDA), according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994), were enrolled at the Center for Anxiety and Related Disorders (CARD) in Boston, Massachusetts (N = 20), and the Anxiety, Stress, and Chronic Disease Research Program at Southern Methodist University (SMU) in Dallas, Texas (N = 21). The study was initiated at CARD (CT, N = 9; CART, N = 11) and completed at SMU (CT, N = 11; CART, N = 10) because the principal investigator (AEM) moved to Dallas. It was approved by the ethics committees of both universities, and informed consent was obtained from all patients. The approximate time frame of data collection was 1–1.5 years at each location.
Patients were recruited either from an outpatient clinic (CARD) or from the community via locally posted advertisements (SMU). At CARD, the principal diagnosis of PDA and other psychiatric diagnoses were determined with the Anxiety Disorders Interview Schedule for DSM–IV (ADIS–IV–L; Di Nardo, Brown, & Barlow, 1994). The diagnoses were established by consensus at a weekly staffing meeting. The diagnostic reliability of the ADIS–IV–L for principal DSM–IV anxiety and mood disorders is good to excellent in rater agreement (κ = .77 for PDA principal diagnosis; Brown, Di Nardo, Lehman, & Campbell, 2001).
At SMU, patients who appeared eligible on the basis of an initial telephone screen were invited for a diagnostic interview using the Structured Clinical Interview for DSM–IV, Patient Edition (SCID; First, Spitzer, Gibbon, & Williams, 1995). Diagnostic reliability was based on 20% of randomly selected interviews and was rated by a second independent assessor. Interrater reliability for the SCID was high (κ = .93 for principal DSM–IV anxiety and mood disorders). Both diagnostic interviews are well established and contain detailed diagnostic questions, based on the DSM–IV, about each anxiety disorder and other diagnostic categories that are important for differential diagnosis. The diagnostic interviews were followed by the clinician-administered Panic Disorder Severity Scale (PDSS; Shear et al., 1997), which was repeated at posttreatment. All interviews were conducted by clinicians trained and certified in the respective instruments.
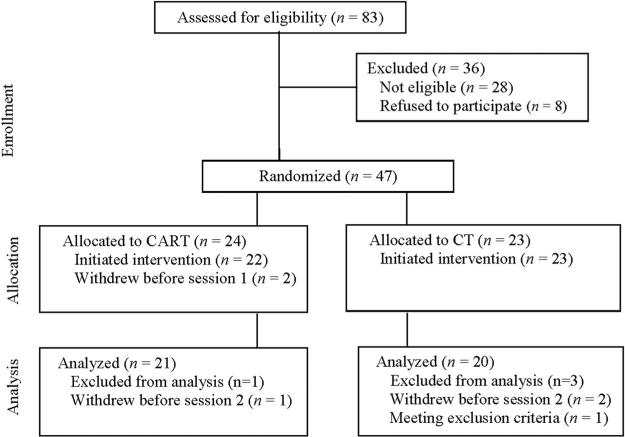
Forty-seven patients (CART = 24; CT = 23) met inclusion criteria and were randomized to treatment. Two patients withdrew before initiating treatment (CART = 2), and 45 initiated treatment. An additional three patients dropped out between Sessions 1 and 2 due to scheduling conflicts (CART = 1; CT = 2), and one patient (CT = 1) was diagnosed with asthma during treatment, a condition meeting exclusion criteria. The remaining 41 patients, who completed two or more treatment sessions (CART = 21; CT = 20), were included in the analyses, which allowed inclusion of subjects with incomplete data.1 Five more patients withdrew after Session 2 (CART = 3; CT = 2), bringing the overall attrition rate (for treatment initiators) to 18.2% for CART (4 of 22) and 17.4% for CT (4 of 23; see Figure 1 for details). The sample was predominantly female (n = 34), Caucasian (n = 36), and well educated (M = 15.8 years of education, SD = 2.4). The mean age of those sampled was 33.2 years (SD = 9.9, range = 20 –57), and 43.9% were married. Other ethnic origins included Hispanic (n = 2), African American (n = 2), and other (n = 1). Agoraphobic avoidance was reported by all patients (2.4% mild, 24.4% moderate, 58.5% severe, and 14.6% extreme, as measured by Item 4 of the PDSS). Diagnostic interviews revealed that 43.9% of the sample had at least one additional current DSM–IV Axis I diagnosis. Of these, 66.7% had an additional anxiety disorder, 11.1% had an additional mood disorder, and 22.2% had both an additional anxiety and a mood disorder. More than half of the participants (n = 28) were on a stable dose of psychotropic medication (antidepressants = 15, benzodiazepines = 19, beta-blockers = 2; see Table 1). Inclusion criteria were as follows: (a) 18 years or older; (b) current DSM–IV diagnosis of PDA that is designated by the patient as the most important source of current distress or interference; (c) agreement not to seek further psychological treatment until after the 2-month follow-up; (d) if on psychotropic medication, to have been on a stable dose for at least 3 months before study began and to maintain this dose until the 2-month follow-up. Exclusion criteria included a history of bipolar disorder, psychosis or delusional disorders, substance abuse or dependence; suicidality; presence of an organic mental disorder, severe unstable medical illness, chronic respiratory disease, or seizures.
Figure 1.
Participant flow diagram. CART = capnometry-assisted respiratory training; CT = cognitive skill training.
Table 1.
Pretreatment Levels of Demographic and Clinical Characteristics
| CART (n = 21) |
CT (n = 20) |
|||||
|---|---|---|---|---|---|---|
| Variable | N (%) | M (SD) | N (%) | M (SD) | χ2(df) | t(df) |
| Participant characteristics | ||||||
| Male | 4 (19.1) | 3 (15.0) | 0.12 (1) | |||
| Age (years) | 31.42 (8.93) | 35.00 (10.64) | 1.13 (39) | |||
| White | 18 (85.7) | 18 (90.0) | 0.98 (3) | |||
| Family and social background | ||||||
| Education (years) | 16.05 (2.40) | 15.55 (2.44) | 0.66 (39) | |||
| Married | 10 (47.6) | 8 (40.0) | 4.89 (4) | |||
| Additional Axis I diagnosis | 8 (40.0) | 10 (58.8) | 1.30 (1) | |||
| Use of psychotropic medication | 13 (61.9) | 15 (75.0) | 0.81 (1) | |||
| Clinical characteristics | ||||||
| PCO2 | 34.34 (3.82) | 33.49 (4.75) | 0.63 (39) | |||
| RR | 15.57 (3.08) | 13.41 (3.74) | 1.98 (37) | |||
| ASI | 35.34 (9.39) | 35.75 (12.44) | 0.45 (37) | |||
| BSQ | 2.17 (0.64) | 2.25 (0.86) | –0.33 (37) | |||
| ASI/BSQ | 0.02 (1.57) | –0.02 (2.05) | 0.07 (37) | |||
| ACQ | 69.74 (15.34) | 74.42 (14.49) | –0.98 (37) | |||
| PDSS | 17.72 (3.10) | 16.95 (5.36) | 0.57 (39) | |||
Note. None of the differences between treatment groups were significant. CART = capnometry-assisted respiratory training; CT = cognitive skill training; SD = standard deviation; PCO2 = partial pressure of carbon dioxide; RR = respiration rate; ASI = Anxiety Sensitivity Index; BSQ = Body Sensations Questionnaire; ASI/BSQ = composite of z-scored Anxiety Sensitivity Index and Body Sensations Questionnaire; ACQ = Anxiety Control Questionnaire; PDSS = Panic Disorder Severity Scale.
Interventions
This intervention was a two-phase study in which patients were randomized (within each site) to receive five individual, weekly, 1-hr sessions of respiratory skill training (CART) or cognitive skill training (CT) (Phase I, Skill Acquisition Training), followed by three weekly sessions of in vivo exposure (Phase II, Skill Application Training). The present article exclusively reports the data collected prior to exposure training. The results of Phase II will be the subject of a separate report (procedure overview can be found in the online supplemental materials). All patients were informed that Phase I was skill acquisition training and that Phase II was skill application training. Randomization software was used to assign patients to condition. The interventions were conducted by four trained therapists (one doctoral-level and three master's-level clinicians) who followed structured manuals.
CART
CART is based on the theory that sustained levels of hypocapnia contribute to symptom development and maintenance of PD (Meuret et al., 2008). The CART intervention employed in the present study is the same as the one used in a previous investigation conducted on a different sample (Meuret et al., 2008, 2009). The training included four components: (a) educating patients about the exacerbation of panic symptoms through hypocapnia; (b) directing patients’ attention to potentially detrimental respiratory patterns; (c) teaching patients techniques to control their respiration, in particular end-tidal PCO2; and (d) instructing patients in between-session exercises. Between-session exercises using a portable capnometer were to be performed twice a day for 17 min at home or elsewhere. The exercises consisted of three parts: (a) a 2-min physiological baseline recording, during which the patients sat quietly with their eyes closed; (b) a 10-min period during which the patients breathed in synchrony with recorded tones (weekly target: 13, 11, 9, 6 breath/min) while monitoring their PCO2 levels and respiratory rate (RR) and (c) a 5-min transfer period with visual feedback only. The patient's goal was to breathe shallowly and regularly with the tones while increasing or maintaining a PCO2 of 40 +/–3 mm Hg. At each weekly session, therapists downloaded and printed out the physiological data from the exercises during the previous week and presented the data in graphs to the patients (see Meuret, Wilhelm, & Roth, 2004, for a detailed description of the CART protocol).
CT
CT is based on the theory that maladaptive thoughts contribute to symptom development and maintenance of panic (Clark, 1986). The training was based on an adapted version of Craske, Barlow, and Meadows’ (2000) cognitive therapy module (Chapters 7–8) and included four components: (a) educating patients about exacerbating panic symptoms through catastrophic thoughts (vicious cycle), (b) identifying negative cognitions associated with physical sensation triggers of recent panic attacks, (c) practicing replacement of maladaptive cognitions with non-catastrophic explanations, and (d) instructing patients in between-session exercises. The treatment rationale emphasized catastrophic cognitions and the benefits of controlling thinking. Patients were asked to describe examples of recent panic attacks and to identify maladaptive thoughts and consequences associated with them. Patients were instructed in methods of examining realistic odds and generating alternative, noncatastrophic explanations for the sensations that they feared. As in CART, the homework included twice-daily 17-min practice sessions. They consisted of two parts: (a) a 2-min baseline physiological recording and (b) 15 minutes during which patients thought about or imagined recent or upcoming panic-related events, identified panic-related catastrophic thoughts and cognitive errors associated with their thoughts, and generated noncatastrophic and more realistic expectations and appraisals. These were recorded in writing (homework logs). At each weekly session, therapists reviewed the written materials from each between-session exercise to assist patients in identifying examples of successful cognitive restructuring and to further improve skills.2 Patients were instructed to run the capnometry device throughout homework exercises to obtain respiratory data. The digital display was set to mask numeric feedback, and the recorded values were neither discussed nor disclosed.
Treatment Integrity
All sessions were audio- or video-taped and discussed in the weekly supervision meetings by expert clinicians to ensure that therapists adhered to the treatment protocol. A random sample of 10% of all recorded treatment sessions (10 CART and 10 CT sessions) was evaluated blindly for protocol adherence by two independent, experienced master's-level clinicians. In addition, 50% of the rated sessions (5 CART and 5 CT) were randomly selected and rated by another master's-level and one doctoral-level clinician to assess interrater reliability. All raters had extensive training in the administration of CT and/or CART. Therapist adherence for CT was rated against an adherence checklist that was based on the Mastery of Your Anxiety and Panic (MAP-3) treatment protocol (Craske et al., 2000) and was created to assess adherence in the study by Barlow et al. (2000). It contains items pertaining to how well the therapist covered elements central to cognitive therapy of panic (e.g., concept of probability overestimation as a cognitive error, countering strategy of evaluating evidence). Items were rated on a 7-point Likert scale (1 = not covered to 7 = thoroughly covered). The overall adherence rating reflected the coder's impression of the degree to which the therapist adhered to the given treatment protocol/therapeutic model (7-point Likert scale; 0 = poor adherence/substantial deviation to 7 = excellent adherence/no deviations). Deviations were defined as any systematic reference to the rationale/skills of any other therapeutic treatments (e.g., exposure instructions, relaxation). An adapted version was used for the adherence ratings for CART. Adherence to the given model/protocol was rated high for both conditions (CART: M = 6.25, SD = 0.92; CT: M = 5.50, SD = 1.20), and ratings were not significantly different between conditions. Interrater agreement was calculated with intraclass correlation coefficients. The results suggest that coders showed high agreement (intraclass correlation [2, 1] = 0.85; Shrout & Fleiss, 1979).
Self-Report Instruments
All measures, except the PDSS, were assessed at pretreatment and at Sessions 2, 3, 4, and 5 (end of Phase I). PD severity was measured at pretreatment and Session 5 (4 weeks apart).
Body Sensations Questionnaire (BSQ)
The BSQ (Chambless et al., 1984) assesses the degree to which sensations associated with autonomic arousal (e.g., “feeling short of breath,” “heart palpitations”) are experienced as frightening. The items are rated on a 5-point Likert-type scale (1 = not frightened or worried by this sensation to 5 = extremely frightened by this sensation). The questionnaire has shown good internal consistency (α = .87), moderate test–retest reliability (rtt = .67), and good discriminative validity for individuals with agoraphobia in comparison to healthy control subjects (Chambless et al., 1984). The reliability for the present sample was α = .90.
Anxiety Sensitivity Index (ASI)
The 16-item ASI (Reiss, Peterson, Gursky, & McNally, 1986) is designed to assess the beliefs that unexplained somatic sensations are dangerous and may cause deleterious physical, psychological, or social consequences beyond any immediate physical discomfort. The index is based on a 5-point Likert-type scale (range 0 = very little to 4 = very much). Examples include “It scares me when I become short of breath” or “Other people notice when I feel shaky.” The index has shown good internal consistency (α = .82; Telch, Shermis, & Lucas, 1989), test–retest reliability (rtt = .71–.75; Reiss et al., 1986), and criterion validity by significantly discriminating between patients with anxiety disorders and college students, as well as patients with agoraphobia and patients with other anxiety disorders (Reiss et al., 1986). The reliability for the present sample was α = .87.
Combined measure of symptom appraisal (ASI/BSQ)
Although some researchers have suggested that the ASI and BSQ reflect differing aspects of the “fear of anxiety” construct (e.g., Reiss et al., 1986), others (e.g., McNally & Lorenz, 1987) have argued that the ASI and BSQ measure the same construct. This conceptual similarity, coupled with the high correlation of these measures in this and previous studies (r = .63), led us to combine the ASI and BSQ into a single composite. Scores on these two measures were first standardized and then added together to form our composite measure of symptom appraisal. Henceforward, ASI/BSQ will be referred to as symptom appraisal.
Anxiety Control Questionnaire (ACQ)
The 30-item ACQ (Rapee, Craske, Brown, & Barlow, 1996) measures the degree to which individuals perceive that they have control, or lack control, over aversive events. These events can be external threats (e.g., “The extent to which a difficult situation resolves itself has nothing to do with my actions”) or internally generated sensations (“My emotions seem to have a life on their own”). On a 6-point Likert-type scale ranging from 0 (strongly disagree) to 5 (strongly agree), patients were asked to indicate how much they thought each statement/event was typical for them. The questionnaire has shown good internal consistency (α = .87–.89), test–retest reliability (rtt = .82–.88; Rapee et al., 1996), convergent validity (r = .46–.77), and discriminative validity (Rapee et al., 1996). For the present sample, the Cronbach's alpha was .72.
PDSS
The PDSS (Shear et al., 1997) is a semistructured, seven-item clinician-administered interview scale that assesses overall panic disorder severity. It includes ratings, on a 0 to 4 scale (none, mild, moderate, severe, extreme), for panic frequency and severity, anticipatory anxiety, avoidance of sensations and situations, and panic-related impairment in work and social situations. The ratings on the seven scales are summed to form a total severity score. The scale has moderate internal consistency (α = .65), acceptable convergent validity (r = .55; Shear et al., 1997), and good discriminative validity for patients with PD/A in comparison to individuals without PD/A (Shear et al., 2001). As originally recommended, the PDSS was administered in a time-sensitive interval (i.e., 4 weeks apart) to allow for a stable estimation of panic frequency and severity. Two raters independently conducted the interviews, with the second rater being uninformed as to the patient's group assignment. Interrater reliability of the pre- and posttreatment assessments was excellent (intraclass correlations [2, 1] = 0.97 and 0.96, respectively; Shrout & Fleiss, 1979). Hence-forward, the PDSS is referred to as panic symptom severity.
Credibility/Expectancy Questionnaire (CEQ)
The CEQ (Devilly & Borkovec, 2000) contains a total of six items; four measure expectancy, and two measure credibility of treatment. Patients are asked to rate items on a scale of 1 to 9 with anchors provided for 1 (not at all logical), 5 (somewhat logical), and 9 (very logical). The scale has shown good internal consistency (expectancy, α = .79–.90; credibility, α = .81–.86) and test–retest reliability (expectancy, rtt = .82; credibility, rtt = .75; Devilly & Borkovec, 2000). The Cronbach's alpha was .83 for expectancy and .76 for credibility in this sample.
Measures of Respiratory Mediators
End-tidal PCO2(PCO2)
PCO2 was assessed with a portable, battery-operated capnometry device (Tidal Wave Sp, Respironics) that samples exhaled gas through a nasal cannula. This device provides digital displays of PCO2 and respiration rate, which are stored along with the time, date, and duration of the entire exercise. Data from the individual exercises can be downloaded and analyzed. Levels of basal pretreatment PCO2 were in a hypocapnic range (below 36 mm Hg) in both conditions (mean CART = 34.3 mm Hg, SD = 3.82; mean CT = 33.5 mm Hg, SD = 4.75).
Respiration rate (RR)
The same device was used to measure RR. The goal with RR was to gradually reduce the rate to lower levels. The pacing tones were set to correspond to an RR of 13, 11, 9, and 6 breaths per minute in successive weeks.
The present data reflect baseline recordings for PCO2 and RR of the last between-session exercise of each week. Patients received in-depth training on how to operate the device. The Tidal Wave Sp meets international accuracy standards (Biedler et al., 2003). Although PCO2 is the hypothesized mediator of CART, RR was also tested as a mediator because other breathing treatments have emphasized the importance of RR and not PCO2.
Analytic Procedure
We used multilevel modeling (MLM), employing the program HLM 6.06 (Raudenbush, Bryk, Cheong, & Congdon, 2004), to analyze the data, as this method allows all participants to be included in the analyses, regardless of missing data/assessments. It is considered the preferred method for analyzing longitudinal psychiatric data (Gibbons et al., 1993). Longitudinal MLM analyses also permit us to examine relations (e.g., mediation and cross lag relations) within individuals over time, instead of across individuals. In longitudinal MLM analyses, relationships between variables (e.g., a mediator and outcome) are calculated within individuals over time, and then these relations are aggregated over all individuals, weighted by number of assessments, to form an overall relation between the variables.
The first analyses compared the slope of change in the measures over time across the two treatment groups to verify treatment efficacy and to investigate treatment differences. Treatment condition and pretreatment level of the outcome were included as Level 2 predictors of the slope of change. A significant treatment effect would indicate different rates of improvement between the two treatment conditions. We also report the standardized effect size for the within-subject slope of change over time in each condition. This effect size (ES) was derived by Raudenbush and Liu (2001) and is equivalent to a Cohen's d extended to correlated (longitudinal) data.
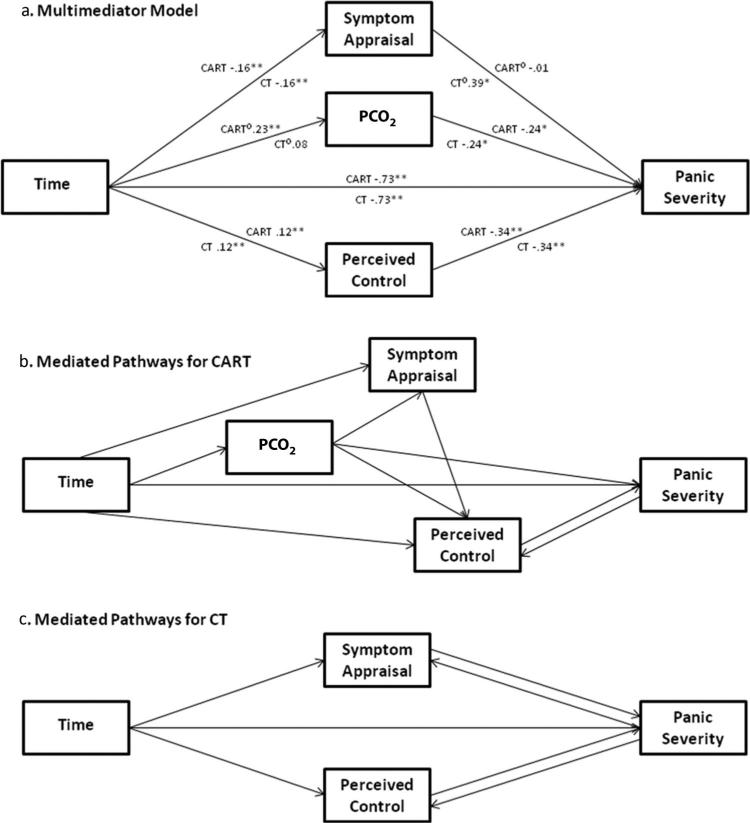
The second set of analyses examined the mediators of the changes in panic symptom severity over time. To assess mediation, we employed the MLM longitudinal mediation model used in other recent treatment outcome studies (e.g., Meuret et al., 2009; Smits, Rosenfield, McDonald, & Telch, 2006). All variables were z-scored for all mediation analyses to facilitate interpretation of the path coefficients. The path diagram illustrating our multimediator model is displayed in Figure 2a. This mediation model tests all three proposed mediators (PCO2, symptom appraisal, and perceived control) simultaneously. As with standard multiple regression analysis, including the three predictors (mediators) of PDSS simultaneously allows one to assess the effect of each predictor (mediator) while controlling for the other mediators. If testing the mediators separately (one at a time), one might find a relation between a mediator (e.g., perceived control) and PDSS simply because that mediator was related to PCO2 which in turn was related to PDSS. We used the asymmetric distribution of products test for mediation (MacKinnon, 2008), in which the product of the two segments of the mediated pathway is calculated, and around which a 95% confidence interval is computed (with the program PRODCLIN; MacKinnon, Fritz, Williams, & Lockwood, 2007). If the confidence interval (CI) does not include 0, the mediated pathway is significant. This test has greater power and more appropriate Type 1 error rates than the Baron and Kenny (1986) approach (MacKinnon, 2008). For each mediated pathway, we calculated the proportion of the effect of the treatment that was accounted for by the mediator (PM = a*b/c; MacKinnon, 2008), which is a commonly used measure of effect size for mediation.
Figure 2.
The multimediator model (a) and models summarizing the significant mediated pathways for CART and CT (b and c). Each measure was standardized for this analysis. The path coefficients shown are the regression coefficients from the MLM analyses represented in the path diagram. These coefficients are equivalent to standardized regression coefficients, as the variables were standardized. If a path is moderated by treatment condition, both CT and CART are marked with a degree sign. PCO2 = partial pressure of carbon dioxide; CART = capnometry-assisted respiratory training; CT = cognitive skill training; MLM = multilevel modeling. * p < .05. ** p < .01.
We also examined the possibility that treatment condition moderated each mediated pathway, indicating specificity of the mediation to a particular condition. If moderated mediation was present, we calculated the mediated effect separately for each treatment condition (MacKinnon, 2008). However, methodologists have pointed out that a significant mediated pathway does not necessarily mean that the proposed mediation model is correct (e.g., Kazdin, 2007). For example, finding that PCO2 mediates changes in panic symptom severity could actually result from the latter causing changes in PCO2 (“reverse mediation”). To evaluate this possibility, we also tested for reverse mediation to determine whether a mediated effect is unidirectional (no reverse mediation) or bidirectional (reverse mediation also occurs). Post hoc power analyses using the multilevel power analysis program PinT (Snijders & Bosker, 1993) confirmed that we had sufficient power (>.80) to detect a mediated pathway comprising paths that were of medium effect sizes and to detect a medium effect size for treatment condition as a moderator of the mediated pathways.
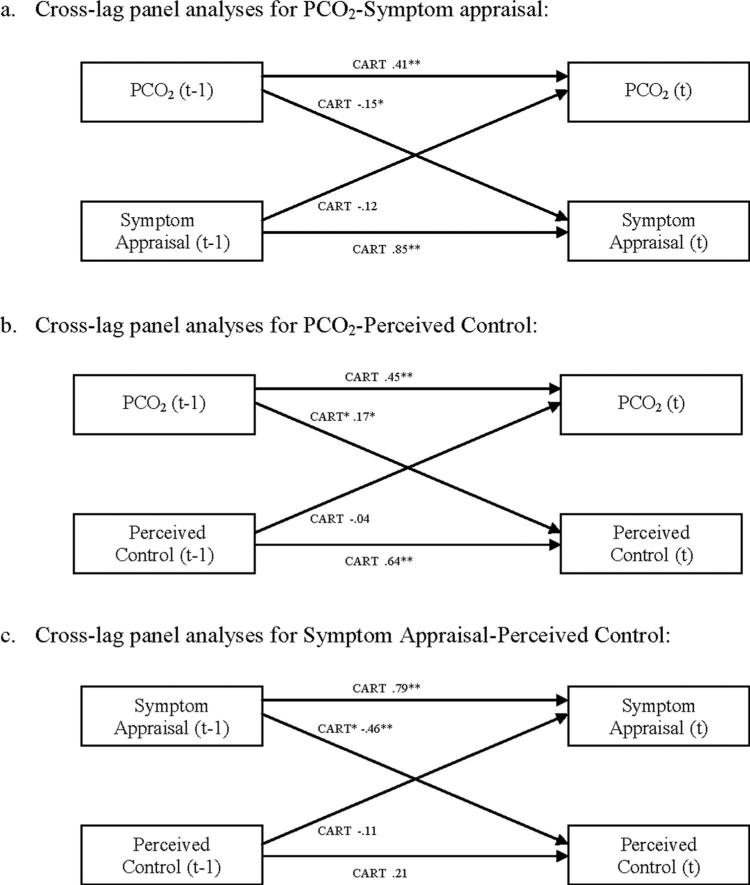
We conducted within-subject, cross-lag panel analyses using MLM to test for temporal precedence (see Figure 3). Accordingly, separate analyses were conducted for each of the two dependent variables in the each of the models. For example, in Figure 3c, perceived control (ACQ) at time t was predicted by ACQ and ASI/BSQ at time t-1, and ASI/BSQt was predicted by ACQt-1 and ASI/BSQt-1. To compute the cross-lag analysis for ACQ, the variables of interest were nested within individuals as follows (one data “line” for each of the 4 time points, t = 2, 5): ACQ2, ACQ1, and ASI/BSQ1; ACQ3, ACQ2, and ASI/BSQ2; ACQ4, ACQ3, and ASI/BSQ3; and ACQ5, ACQ4, and ASI/BSQ4. The analysis computes the regression of ACQt on ACQt-1 and ASI/BSQt-1, across the four time points (within each subject), and then “averages” that relation over the sample. This approach allows us to evaluate the extent to which mediators at earlier points predict outcomes at later points, or vice versa, capturing the relations longitudinally (as opposed to examining the relations among the mediators and outcomes cross-sectionally at each time point). Examples of this application can be found in the studies by Meuret et al. (2009) and Smits et al. (2006). Post hoc power analyses (using PinT) indicated that power was >.90 to detect a medium effect for the cross-lag paths and >.80 to detect a moderator of the cross-lag paths.
Figure 3.
Models representing cross-lag panel analyses for CART. Each measure was standardized for these analyses. The path coefficients shown are the regression coefficients from the MLM analyses represented in the path diagram. These coefficients are equivalent to standardized regression coefficients, as the variables are z-scored. All coefficients are for the CART condition. None of the cross-lag coefficients were significant for CT. PCO2 = partial pressure of carbon dioxide; CART = capnometry-assisted respiratory training; CT = cognitive skill training; MLM = multilevel modeling. * p < .05. ** p < .01.
Results
Treatment Credibility and Compliance
Treatment credibility ratings were completed after the educational session (Session 1). Patients’ ratings for both treatment expectancy and credibility were high and were not significantly different between the two groups (p = .45 for treatment expectancy; p = .14 for treatment credibility). Mean (SD) ratings for the CART condition were 7.54 (0.98) and 6.83 (1.44) for expectancy and credibility; ratings in the CT condition were 7.75 (0.83) for expectancy and 7.43 (0.99) for credibility. Treatment compliance was assessed by matching the digital capnometry records with the homework log entries. Patients in both conditions completed about 64% of the 52 assigned homework exercises (CART = 33.8, SD = 13.3; CT = 32.8, SD = 16.7). Condition, site, or comorbid diagnosis did not result in differences in homework compliance, exclusion, attrition rate, or outcome (PDSS; p > .05). Multivariate analyses of variance, logistic regressions, t tests, and chi-squares confirmed that there were no treatment condition or site differences for the demographic or clinical variables (see Table 1).3
Overall Treatment Effects
Respiratory measures
PCO2 increased and RR decreased over time in CART (ps < .01) but not in CT (p > .21), with significant slope differences between treatment conditions on both measures (ps < .05; see Table 2). Effect sizes for the slope of improvement for both PCO2 and RR were large (ES = .97 and .80) for CART and small (ES = .33 and .42) for CT.
Table 2.
Slope of Change Over Time
| Slope estimates |
ESa |
||||
|---|---|---|---|---|---|
| Variable | CART (n = 21) | CT (n = 20) | Slope differences | CART (n = 21) | CT (n = 20) |
| PCO2 | 1.02** | 0.35 | –0.66* | 0.97 | 0.34 |
| RR | –0.89** | 0.47 | 1.36** | 0.80 | 0.42 |
| ASI/BSQ | –0.30** | –0.30** | –0.00 | 0.74 | 0.75 |
| ACQ | 2.17* | 2.02* | –0.15 | 0.66 | 0.61 |
| PDSS | –0.72** | –0.95** | –0.24 | 2.68 | 2.25 |
Note. Raw scores were used for these analyses (except for the ASI/BSQ). Slope estimates represent the change per week within a group. Slope differences represent differences in changes over time between groups. CART = capnometry-assisted respiratory training; CT = cognitive skills training; PCO2 = partial pressure of carbon dioxide; RR = respiration rate; ASI/BSQ = composite of z-scored Anxiety Sensitivity Index and Body Sensations Questionnaire; ACQ = Anxiety Control Questionnaire; PDSS = Panic Disorder Severity Scale.
We calculated the standardized effect size for the linear change over time within each treatment group. This effect size is Cohen's standardized effect size (d) extended to clustered data (Raudenbush & Liu, 2001).
p ≤ .05.
p < .01.
Psychological measures
Symptom appraisal, perceived control, and panic symptom severity improved significantly in both treatments (see Table 2). No slope differences between treatment conditions were observed (ps > .87). Within-group effect sizes were medium to large (ES = .61–.75) for symptom appraisal and perceived control, and very large (ES = 2.25–2.68) for PDSS.
Mediation of Changes in Panic Symptom Severity over Time
Our multimediator model is displayed in Figure 2a. This model examines the three potential mediators of changes in PDSS simultaneously, thus calculating the effects of each while controlling for the effects of the other mediators. Below, we present the results for each mediator in turn, discussing its significance as a mediator and its specificity to treatment condition.
Examining the results for each mediator, we found that PCO2 was a moderated mediator of PDSS. The a path (from Time to PCO2) was moderated by treatment condition, bdiff = –.16, t(36) = –2.15, p < .05. The a path was significant for those in CART, b = .23, t(36) = 6.47, p < .01, but not for those in CT, b = .08, t(36) = 1.25, p = .21. The b path (from PCO2 to PDSS) was not moderated by treatment condition (p = .57) and was significant, b = –.24, t(57) = –2.39, p < .05. Following Tein, Sandler, MacKinnon, and Wolchik (2004), we calculated the magnitude and significance of the mediated pathway from time to PDSS through PCO2 separately for each treatment condition. As expected, PCO2 was a significant mediator of changes in PDSS over time for patients in the CART condition, a*b = –.070, 95% CI [–.139, –.009], PM = .27, but not for those in the CT condition, a*b = –.016, 95% CI [–.054, .008], PM = .06. Reverse mediation analysis (employing PDSS as the mediator and PCO2 as the outcome) was not significant, suggesting that the mediation was unidirectional.
Symptom appraisal was also a moderated mediator of PDSS, because the path from ASI/BSQ to PDSS was moderated by treatment condition, bdiff = .40, t(57) = 2.55, p = .01. This path was significant for those in CT, b = .39, t(57) = 3.10, p < .005, but not for those in CART, b = –.01, t(57) = –.08, p = .93. The path from Time to ASI/BSQ was not moderated by treatment (p = 1.00) and was significant, b = –.16, t(38) = –4.19, p < .01. Calculating the significance of the mediated pathway (from Time to PDSS through ASI/BSQ) for each treatment condition, we found it to be significant for patients in the CT condition, a*b = –.059, 95% CI [–.118, –.014], PM = .23, but not for those in the CART condition, a*b = .002, 95% CI [–.037, .041], PM = –.01. Reverse mediation analysis indicated the PDSS was a moderated mediator of symptom appraisal, such that PDSS mediated changes in symptom appraisal in CT (p < .05) but not in CART (p = .65). Thus, the effect of symptom appraisal on PDSS appears to be specific to CT and bidirectional.
Neither segment of the mediated pathway involving perceived control (ACQ) was moderated by treatment (p = .92 for path a; p = .77 for path b). Therefore ACQ was not a moderated mediator of PDSS. However, both segments of the mediated pathway linking Time and PDSS through ACQ were significant: path a, b = .12, t(38) = 3.07, p < .01; path b, b = –.34, t(57) = –4.17, p < .001. The mediated pathway was significant across treatment conditions, a*b = –.039, 95% CI [–.077, –.011], PM = .15 for CART; a*b = –.045, 95% CI [–.093, –.010], PM = .17 for CT. Reverse mediation analyses indicated that PDSS also mediated changes in ACQ (regardless of condition, ps < .05), indicating that the relation between ACQ and PDSS was bidirectional.
Because a previous study indicated that RR was not related to symptom appraisal in CART (Meuret et al., 2009), we sought to replicate these findings by adding RR to our mediation model for PDSS. Results confirmed that RR did not mediate changes in PDSS over time, a*b = –.006, 95% CI [–.049, .034], PM = .02 for CART; a*b = .003, 95% CI [–.018, .027], PM = –.01 for CT.
Finally, individual mediator analyses were conducted to examine whether each of these mediators by themselves, without controlling for the other mediators, would mediate changes in PDSS. These results replicated those from the multimediator analysis, except that ASI/BSQ was found to be a significant mediator of PDSS in CART as well as in CT in the single mediator analysis (p < .05).4 To further explore this result, we performed two double mediator analyses, including ASI/BSQ in each and adding either PCO2 or ACQ as the second mediator. Results indicated that ASI/BSQ remained a significant mediator of PDSS in CART when combined with PCO2 (p < .05) but not when combined with ACQ. These findings suggest that the relation between ASI/BSQ and PDSS in CART is due to the relation between ASI/BSQ and ACQ.
Relations among the mediators
While the mediation model for PDSS establishes the direct relations between each mediator and PDSS, each mediator may also affect PDSS indirectly through its effect on the other mediators. The mediation model above did not explore the relations among the mediators. Thus, we examined the relations among the three mediators to determine if there was evidence for indirect effects of the mediators on panic symptom severity.
PCO2 and symptom appraisal
It was hypothesized that PCO2 would mediate changes in symptom appraisal in the CART condition. Mediation analyses indicated that PCO2 was a significant mediator of changes in symptom appraisal in CART, a*b = –.018, 95% CI [–.032, –.005], PM = .11, but not in CT, a*b = –.006, 95% CI [–.018, .003], PM = .04. Within-subject, cross-lag panel analyses supported this finding: Prior levels of PCO2 led to later levels of ASI/BSQ for CART but not for CT (see Figure 3a). There was no evidence that symptom appraisal mediated changes in PCO2 (reverse mediation), a*b = .004, 95% CI [–.009, .018], PM = .02 for CART; a*b = .004, 95% CI [–.009, .018], PM = .05 for CT, and cross-lag analyses showed that earlier symptom appraisal was not a significant predictor of later PCO2 for either condition (see Figure 3a).
PCO2 and perceived control
As hypothesized, PCO2 mediated changes in ACQ in the CART condition, a*b = .043, 95% CI [.014, .077], PM = .36, but not in the CT condition, a*b = –.001, 95% CI [–.011, .008], PM = –.01. Cross-lag panel analysis supported these findings: Earlier levels of PCO2 were related to later levels of ACQ in the CART condition but not in the CT condition (see Figure 3b). ACQ did not mediate changes in PCO2, a*b = .008, 95% CI [–.011, .031], PM = .04 for CART; a*b = –.006, 95% CI [–.025, .011], PM = –.07, for CT, nor did the cross lags indicate that earlier ACQ led to later PCO2 for either condition (see Figure 3b). Thus, the mediation and reverse mediation analyses, as well as the cross-lag analyses, support PCO2 as a unidirectional mediator of changes in perceived control.
Symptom appraisal and perceived control
Mediation analyses showed that ASI/BSQ mediated changes in ACQ regardless of condition, a*b = .075, 95% CI [.036, .123], PM = .62 for CART; a*b = .036, 95% CI [.016, .062], PM = .30 for CT, and that ACQ mediated changes in ASI/BSQ, a*b = –.049, 95% CI [–.088, –.017], PM = .31 for both conditions. However, cross-lag panel analyses showed only that earlier levels of ASI/BSQ were related to later levels of ACQ, but only in CART, and that earlier levels of ACQ were not related to later levels of ASI/BSQ in either condition (see Figure 3c). These findings suggest that symptom appraisal is generally bidirectionally related to perceived control; however, the lack of consistent cross-lag relations between the two suggests that they do not “cause” each other but are related in some other way (e.g., due to a mutual relation to a third variable or to construct overlap). The exception may be for CART, in which symptom appraisal preceded and affected perceived control. Although symptom appraisal and perceived control may have some construct overlap, the fact that ASI/BSQ mediated PDSS in CT when controlling for perceived control suggests that they also have some separate underlying components that differentially impact PDSS.
Discussion
The goal in this study was to examine modality-specific mediators of change in panic symptoms and physiology using longitudinal, moderated mediation analyses. Both cognitive and respiratory training led to significant and comparable improvements in panic symptom severity, symptom appraisal, and perceived control. CART, but not CT, led to corrections of initial hypocapnic to normocapnic levels and to lower RR. Results of the multimediator model were consistent with a modality-specific relation between each mediator and panic symptom severity, while controlling for the other mediators. Although symptom appraisal and PCO2 were both moderated mediators of changes in panic severity, the paths that were moderated were different for these two mediators. For symptom appraisal, only its relation to panic severity (path b) was moderated by treatment condition. For PCO2, only its change over time (path a) was moderated by treatment condition. Overall, the proportion of the change in panic symptom severity that was accounted for by each mediator ranged from 15% to 27%. These proportions are comparable to recently published longitudinal MLM analyses of anxiety intervention studies that examined cognitions (typically 10%–30%; cf. Hofmann et al., 2007; Smits et al., 2006) and physiology (20%–40%; Meuret et al., 2009) as mediators of treatment change. In the following, the findings for each proposed mediator are discussed in detail. Diagrams summarizing all the significant mediating relations, separately for CART and CT, can be found in Figures 2b and 2c.
Mediational Role of PCO2
PCO2 emerged as a powerful mediator of change in panic symptom severity for patients undergoing respiratory training (CART) but not for patients undergoing CT. The facts that PCO2 mediated changes in panic symptom severity only in CART and that panic symptom severity did not mediate changes in PCO2 suggest that PCO2 is directly responsible for some of the changes in panic symptom severity that occur in CART. PCO2 was also a modality-specific mediator (CART only) for changes in symptom appraisal and perceived control. The specificity of PCO2 as a mediator in CART was further strengthened by the temporal precedence analysis (cross lags) indicating that changes in PCO2 were unidirectionally related to changes in symptom appraisal and perceived control. These results replicated findings from a previous study demonstrating that PCO2 mediated and drove changes in symptom appraisal (anxiety sensitivity) in CART (Meuret et al., 2009) and refute prior notions that breathing techniques “are likely to sustain rather than modify catastrophic cognitions” (Salkovskis, Clark, & Gelder, 1996, p. 457).
The findings are consistent with cross-sectional research highlighting the importance of respiratory pathways, particularly hypocapnia, in PD (Gorman et al., 2004; Klein, 1993; Ley, 1985). The longitudinal design of the current study demonstrated that correction of sustained hypocapnia by therapeutically increasing PCO2 levels not only was associated with reductions in panic symptom severity, but also drove changes in symptom appraisal and perceived control. Several explanations for the detected pathways are plausible: Hypocapnia, caused by increased minute ventilation, has been linked to physical symptoms (e.g., dizziness, shortness of breath) that are feared by patients with PD (Meuret, in press). Normalization of these aberrantly low levels of PCO2 should thus ameliorate bodily symptoms and, consequently, their catastrophic interpretation (i.e., less sensations of shortness of breath should lead to less fear engendered by shortness of breath).
This temporal pathway is consistent with the idea that successful alteration of one system (i.e., physiology) induces changes in another system (i.e., cognition; see, e.g., Borkovec, Newmann, Pincus, & Lytle, 2002). As treatment progresses, patients may become more successful in counteracting the onset of physical symptoms by changing their respiratory patterns. Alternatively, repeated exposure to increasing levels of PCO2 during homework exercises may have led to a desensitization of a hypersensitive suffocation alarm system (Klein, 1993). Klein's theory proposes that rising CO2 falsely heralds impending suffocation. CART would thus reduce panic vulnerability by building tolerance toward feared respiratory-related sensations and by the absence of compensatory hyperventilatory episodes. Recent research has demonstrated chemosensitivity of cells in the amygdala (Ziemann et al., 2009), with increases in PCO2 directly leading to an intense fear responses. It could be speculated that attenuations in fear responses are linked to alterations in these chemosensory properties. Arguably, although respiratory symptoms are among the most frequent and distressing panic symptoms (e.g., Meuret et al., 2006), they reflect only a subset of PD symptoms. However, due to the interconnected autonomic system, ameliorations in respiratory functioning could influence symptom production in other panic-related systems (e.g., cardiac activity). Whether the correction in hypocapnia is associated with improvements in long-term prevention of panic relapse and better general health outcomes (e.g., Laffey & Kavanagh, 2002) requires further research.
Treatment gains in traditional breathing training have previously been interpreted as a function of an improved sense of control, rather than a result of physiological change. Our unidirectional findings on the mediational and temporal relation of PCO2 and perceived control argue against PCO2 functioning merely as a “rationale placebo” (Garssen, de Ruiter, & Van Dyck, 1992). Notwithstanding, one cannot rule out that the feedback about the self-induced change in PCO2 may be a driving mechanism of change (e.g., Holroyd et al., 1984), as opposed to the actual biological change. However, the fact that PCO2 was related to panic symptom severity over and above its effect on perceived control argues against this interpretation. Furthermore, the fact that PCO2 mediated panic symptom severity in CT speaks against a simple expectancy effect, because these patients were unaware of their PCO2 levels. Finally, CART-trained patients were instructed to gradually regulate their RR down to six breaths/min in the fourth week of training, a breathing rate that has been shown to stimulate and exercise the baroreflex (Lehrer et al., 2003). However, as we found in a previous investigation (Meuret et al., 2009), there was little evidence that RR was related to changes in symptom appraisal or panic symptom severity, despite its successful reduction in CART.
Mediational Role of Symptom Appraisal
In CT (but not in CART), changes in symptom appraisal mediated changes in panic symptom severity and vice versa. This bidirectional relation generally parallels other bidirectional findings on reduction in cognitive misappraisal and panic symptom severity in patients undergoing cognitive– behavior therapy (Teachman et al., 2008). Furthermore, analyses indicated that symptom appraisal appeared to be a mediator of PDSS in the CART condition except when perceived control was included as an additional mediator. These findings indicate that, in the present study, symptom appraisal had no significant effect on PDSS in the CART condition over and above its relation with perceived control. Coupled with the cross-lag finding that symptom appraisal impacted perceived control in CART, these results suggest this relation is primarily due to the effect of symptom appraisal on perceived control. The differing results between the single- and multimediator analyses highlight the importance of controlling for multiple potential mediators in mediation analyses. Failing to control for important third mediators in single mediator analyses can result in overestimation of regression coefficients (Tabachnick & Fidell, 2007), and Type I error.
The fact that symptom appraisal was related to reductions in PDSS in CT, but not in CART, is an intriguing finding that suggests additional processes are at play. Among several plausible reasons for this difference is one directly relating to the proposed tenets of CART: If hypocapnia is indeed responsible for the generation of panic-like symptoms (e.g., dizziness), reducing or reversing hypocapnia should lower the likelihood of experiencing such aversive physiological symptoms. Consequently, as symptom occurrence fades, the relation between appraisal of these symptoms and panic severity becomes insignificant. Another explanation may relate to demand characteristics and/or expectations: CT patients are told that correction of maladaptive cognitions should decrease panic severity. Thus, when they learn to master the restructuring of maladaptive thoughts, they may expect (and thereby report) fewer symptoms. Future studies should assess and manipulate the role of expectancy as a nonspecific moderator.
Another interesting finding was the modality-independent bidirectional relation between symptom appraisal and perceived control. One explanation of this mutual relation could be similarities in measurement methodologies: Although physiological measures, such as PCO2, are recorded in real time, most current measures of cognitions fail to reflect ongoing, moment to moment thinking (Jarrett, Vittengl, Doyle, & Clark, 2007). Rather, they partly echo retrospective experiences that can be subject to responder biases, demand characteristics, and retrospective judgments (Davison, Vogel, & Coffman, 1997). Future studies should explore multidimensional measures, such as the implicit association tests used in the study by Teachman et al. (2008) or on-line recording of verbalizations (think-aloud paradigm; Davison et al., 1997).
Mediational Role of Perceived Control
Perceived control appeared to be a powerful modality-nonspecific mediator of changes in PDSS, even when controlling for the effects of PCO2 and/or symptom appraisal. To our knowledge, this is the first study to demonstrate the unique contribution of perceived control to the improvement in panic symptoms severity in disparate interventions for PD and independent of modality specific mediators. Focusing on strengthening patients’ perception of control over external and internal experiences is a likely candidate for enhancing panic symptom reduction. Further research is needed to verify the contributing role for this and other modality nonspecific mediators.
Limitations and Summary
Despite the intriguing findings, a number of limitations require mention. Temporal analysis (cross lag) is sensitive to the time lag in the analysis and may fail to find a relation because the lag is either too short for the effect to appear or so long that the effect has already decayed. The majority of our results are correlational, and although they suggest temporal precedence, they cannot prove causality or exclude the possibility that an unmeasured third variable is responsible for the observed relations. In particular, the multimediator analysis of PDSS included only two time points, so temporal precedence remains unknown. Another limitation is that ADIS–IV–L interrater reliability specific for the portion of the sample recruited at CARD was not available. Finally, future researchers should consider using a placebo control group to test for other nonspecific treatment factors, assess reductions in panic symptom severity on a session-by-session basis to allow for temporal analyses and to obtain a more representative posttreatment measure, and explore the relations of anxiety sensitivity with the other measures incorporating its taxonic structure (e.g., Bernstein et al., 2007).
In sum, the degree of relatedness, temporality, and specificity of these pathways of change varied widely between the investigated interventions. Both respiratory and cognitive variables were intervention-specific and model-consistent mediators of treatment change in PD. Thus, these findings provide substantial support for two influential but distinct panic models. Moreover, our findings extend the current literature on treatment mechanisms. Some theorists have hypothesized that any effective treatment modality might be mediated through cognitions (e.g., Clark et al., 1994). In contrast, the findings of our study suggest that different treatment modalities are associated with different treatment mechanisms. This notion is consistent with more recent findings suggesting that, although cognitive–behavioral therapy and pharmacotherapy both effectively change cognitions and panic symptom severity, they work by different mechanisms (Hofmann et al., 2007).
The practical implications of our findings are that both cognitive and respiratory interventions are effective for treating PD but that they operate via different mechanisms. This information contributes to our knowledge of core essential processes that cut across treatment modalities and emphasizes the importance of identifying mediators of treatments to optimize treatment outcome. Furthermore, the successful reductions in panic symptom severity achieved in our study seem remarkable, given the brevity of our interventions, and speak for the operating mediators that were pursued with tightly controlled homework assignments. Future studies should investigate additive effects of both techniques as well as the effects of pretreatment moderators on treatment response.
Supplementary Material
Acknowledgments
Stefan G. Hofmann is a paid consultant of Merck Pharmaceuticals. The writing of this article was partly facilitated by National Institute of Mental Health Grant R01HL089761-01A1 (Alicia E. Meuret, David Rosenfield) and the generous support of the Beth and Russell Siegelman Foundation (Alicia E. Meuret). Stefan G. Hofmann is supported by National Institute of Mental Health Grant MH079236. We gratefully acknowledge Michelle G. Craske and Thomas Ritz for their helpful feedback on an earlier draft of this paper and James Abelson for his valuable suggestions in designing this study. We further acknowledge the therapists, clinical interviewers, and independent assessors who were involved in this study, in particular David Moscovitch, Hyo-Jin Kim, Stefan Schultz, Erica Ayala, Tina Boisseau, and Amy Lawrence.
Footnotes
The sample size was considered adequate because a priori power analyses, employing the multilevel power analysis program PinT and using the data from Meuret et al. (2009), confirmed sufficient power (>.80) to detect a medium effect size for treatment differences in slopes. Also, prior research has shown that estimates of regression coefficients and their standard errors are accurate in sample sizes as low as 30 in multilevel models (Maas & Hox, 2005, pp. 89–90).
CT is often combined with other techniques (e.g., behavioral experiments), and this complicates the direct testing of the pathways of its proposed mediator. Notwithstanding, there is some evidence that training in cognitive procedures is efficacious in reducing aspects of panic in full isolation from exposure and behavioral procedures (Salkovskis, Clark, & Hackmann, 1991; Van den Hout, Arntz, & Hoekstra, 1994). The cognitive skills training we used in the present study should not be equated with cognitive therapy, which includes additional treatment components, such as behavioral techniques. The manual for the cognitive intervention used in this study can be obtained from the first author.
The amount of missing data in this study was acceptable (109 out of a possible 902 data points were missing [12.1%], including the missing data from noncompleters, who made up 12.2% [5 of 41] of those included in the data analysis). Analyses in which missing data patterns were included as predictors of outcomes and slopes showed no effects of missing data patterns, indicating “ignorable” missingness (Hall et al., 2001).
Additional information and analyses are located in the journal's online supplemental materials.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2nd ed. Guilford Press; New York, NY: 2002. [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive– behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2573–2574. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Norton PJ, Schmidt NB, Taylor S, Forsyth JP, Cox B. Taxometric and factor analytic models of anxiety sensitivity: Integrating approaches to latent structural research. Psychological Assessessment. 2007;19:74–87. doi: 10.1037/1040-3590.19.1.74. [DOI] [PubMed] [Google Scholar]
- Biedler AE, Wilhelm W, Kreuer S, Soltesz S, Bach F, Mertzlufft FO, Molter GP. Accuracy of portable quantitative capnometers and capnographs under prehospital conditions. American Journal of Emergency Medicine. 2003;21:520–524. doi: 10.1016/j.ajem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Borden J, Clum G, Salmon P. Mechanisms of change in the treatment of panic. Cognitive Therapy and Research. 1991;15:257–272. [Google Scholar]
- Borkovec TD, Newman MG, Pincus AL, Lytle R. A component analysis of cognitive–behavioral therapy for generalized anxiety disorder and the role of interpersonal problems. Journal of Consulting and Clinical Psychology. 2002;70:288–298. [PubMed] [Google Scholar]
- Bouchard S, Gauthier J, Nouwen A, Ivers H, Vallières A, Simard S, Fournier T. Temporal relationship between dysfunctional beliefs, self-efficacy and panic apprehension in the treatment of panic disorder with agoraphobia. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:275–292. doi: 10.1016/j.jbtep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM–IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110:49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Carr RE, Lehrer PM, Hochron SM. Panic symptoms in asthma and panic disorder: A preliminary test of the dyspnea–fear theory. Behaviour Research and Therapy. 1992;30:251–261. doi: 10.1016/0005-7967(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear in agoraphobics: The Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Clark DM. A cognitive approach to panic. Behavior Research and Therapy. 1986;24:461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Hackman A, Midleton H, Asastasiades P, Gelder M. A comparison of cognitive therapy, applied relaxation and imipramine in the treatment of panic disorder. British Journal of Psychiatry. 1994;164:759–769. doi: 10.1192/bjp.164.6.759. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Hackmann A, Wells A, Ludgate J, Gelder MG. Brief cognitive therapy for panic disorder: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 1999;67:583–589. doi: 10.1037//0022-006x.67.4.583. [DOI] [PubMed] [Google Scholar]
- Conrad A, Müller A, Doberenz S, Kim S, Meuret AE, Wollburg E, Roth WT. Psychophysiological effects of breathing instructions for stress management. Applied Psychophysiology and Biofeedback. 2007;32:89–98. doi: 10.1007/s10484-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Craske MG, Barlow DH, Meadows E. Mastery of your anxiety and panic: Therapist guide for anxiety, panic, and agoraphobia (MAP-3) Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Craske MG, Hazlett-Stevens H. Facilitating symptom reduction and behavior change in GAD: The issue of control. Clinical Psychology: Science and Practice. 2002;9:69–75. [Google Scholar]
- Craske MG, Rowe M, Lewin M, Noriega-Dimitri R. Interoceptive exposure versus breathing retraining within cognitive–behavioural therapy for panic disorder with agoraphobia. British Journal of Clinical Psychology. 1997;36:85–99. doi: 10.1111/j.2044-8260.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Davison GC, Vogel RS, Coffman SG. Think-aloud approaches to cognitive assessment and the articulated thoughts in simulated situations paradigm. Journal of Consulting and Clinical Psychology. 1997;65:950–958. doi: 10.1037//0022-006x.65.6.950. [DOI] [PubMed] [Google Scholar]
- Devilly G, Borkovec T. Psychometric properties of the Credibility/Expectancy Questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Di Nardo SC, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM–IV: Lifetime version (ADIS–IV–L) Psychological Corporation/Graywind; San Antonio, TX: 1994. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV, Patient Edition (SCID–I/P Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Garssen B, de Ruiter C, Van Dyck R. Breathing retraining: A rational placebo? Clinical Psychology Review. 1992;12:141–153. [Google Scholar]
- Gelder MG, Clark DM, Salkovskis P. Cognitive treatment for panic disorder. Journal of Psychiatric Research. 1993;27:171–178. doi: 10.1016/0022-3956(93)90026-x. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: Application to the NIMH treatment of depression collaborative research program dataset. Archives of General Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biological Psychiatry. 2004;56:862–867. doi: 10.1016/j.biopsych.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hall SM, Delucchi KL, Vellicre WF, Kahler CW, Ranger-Moore J, Hedeker D, Niaura R. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hegel MT, Ferguson RJ. Psychophysiological assessment of respiratory function in panic disorder: Evidence for a hyperventilation subtype. Psychosomatic Medicine. 1997;59:224–230. doi: 10.1097/00006842-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Hoffart A, Sexton H, Hedley LM, Martinsen EW. Mechanisms of change in cognitive therapy for panic disorder with agoraphobia. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:262–275. doi: 10.1016/j.jbtep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Rosenfield D, Suvak MK, Barlow DH, Gorman J, Woods SW. Preliminary evidence for cognitive mediation during cognitive–behavioral therapy of panic disorder. Journal of Consulting and Clinical Psychology. 2007;75:374–379. doi: 10.1037/0022-006X.75.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive–behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd KA, Penzien DB, Hursey KG, Tobin DL, Rogers L, Holm JE, Chila AG. Change mechanisms in EMG biofeedback training: Cognitive changes underlying improvements in tension headache. Journal of Consulting and Clinical Psychology. 1984;52:1039–1053. doi: 10.1037//0022-006x.52.6.1039. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Doyle K, Clark LA. Changes in cognitive content during and following cognitive therapy for recurrent depression: Substantial and enduring, but not predictive of change in depressive symptoms. Journal of Consulting and Clinical Psychology. 2007;75:432–446. doi: 10.1037/0022-006X.75.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Methodology: What is it and why is it important. In: Kazdin AE, editor. Methodological issues and strategies in clinical research. American Psychological Association; Washington, DC: 2002. pp. 5–22. [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Kavanagh BP. Hypocapnia. New England Journal of Medicine. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Hamer RM. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65:796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Ley RA. Blood, breath and fears: A hyperventilation theory of panic attacks and agoraphobia. Clinical Psychology Review. 1985;5:271–285. [Google Scholar]
- Maas CJM, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences. 2005;1:85–91. [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Erlbaum; New York, NY: 2008. [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavioral Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Lorenz M. Anxiety sensitivity in agoraphobics. Journal of Behavior Therapy and Experimental Psychiatry. 1987;18:3–l1. doi: 10.1016/0005-7916(87)90065-6. [DOI] [PubMed] [Google Scholar]
- Meuret AE. Therapeutic use of ambulatory capnography. In: Gravenstein J, Jaffe M, Paulus D, editors. Capnography: Clinical aspects. 2nd ed. Cambridge University Press; Cambridge, England: in press. [Google Scholar]
- Meuret AE, Rosenfield D, Suvak MK, Hofmann SG, Roth WT. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. Journal of Psychiatric Research. 2009;43:634–641. doi: 10.1016/j.jpsychires.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, White KS, Ritz T, Roth WT, Hofmann SG, Brown TA. Panic attack symptom dimensions and their relationship to illness characteristics in panic disorder. Journal of Psychiatric Research. 2006;40:520–527. doi: 10.1016/j.jpsychires.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training in panic disorder treatment: Useful intervention or impediment to therapy? Behavior Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. Journal of Clinical Psychology. 2004;60:197–207. doi: 10.1002/jclp.10245. [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. American Journal of Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Craske MG, Brown TA, Barlow DH. Measurement of perceived control over anxiety-related events. Behavior Therapy. 1996;27:279–293. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincolnwood, IL: 2004. [Google Scholar]
- Raudenbush SW, Liu X. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods. 2001;6:387–401. [PubMed] [Google Scholar]
- Reiss S, Peterson R, Gursky D, McNally R. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behavior Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Pettit D. Are current theories of panic falsifiable? Psychological Bulletin. 2005;131:171–192. doi: 10.1037/0033-2909.131.2.171. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Clark DM, Gelder MG. Cognition–behaviour links in the persistence of panic. Behaviour Research and Therapy. 1996;34:453–458. doi: 10.1016/0005-7967(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Clark DM, Hackmann A. Treatment of panic attacks using cognitive therapy without exposure or breathing retraining. Behavior Research and Therapy. 1991;29:161–166. doi: 10.1016/0005-7967(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J. Dismantling cognitive–behavioral treatment for panic disorder: Questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Papp LA. Multicenter Collaborative Panic Disorder Severity Scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Shear MK, Rucci P, Williams J, Frank E, Grochocinski V, Vander Bilt J, Wang T. Reliability and validity of the Panic Disorder Severity Scale: Replication and extension. Journal of Psychiatric Research. 2001;35:293–296. doi: 10.1016/s0022-3956(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, McDonald R, Telch MJ. Cognitive mechanisms of social anxiety reduction: An examination of specificity and temporality. Journal of Consulting and Clinical Psychology. 2006;74:1203–1212. doi: 10.1037/0022-006X.74.6.1203. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Standard errors and sample sizes for two-level research. Journal of Educational Statistics. 1993;18:237–259. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Pearson Education; Boston, MA: 2007. [Google Scholar]
- Teachman BA, Smith-Janik SB, Marker CD. Automatic associations and panic disorder: Trajectories of change over the course of treatment. Journal of Consulting and Clinical Psychology. 2008;76:988–1002. doi: 10.1037/a0013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein JY, Sandler IN, MacKinnon DP, Wolchik SA. How did it work? Who did it work for? Mediation in the context of a moderated effect for children of divorce. Journal of Consulting and Clinical Psychology. 2004;72:617–624. doi: 10.1037/0022-006X.72.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch MJ, Shermis MD, Lucas JA. Anxiety sensitivity: Unitary personality trait or domain-specific appraisals? Journal of Anxiety Disorders. 1989;3:25–32. [Google Scholar]
- Van den Hout M, Arntz A, Hoekstra R. Exposure reduced agoraphobia but not panic, and cognitive therapy reduced panic but not agoraphobia. Behaviour Research and Therapy. 1994;32:447–451. doi: 10.1016/0005-7967(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Wemmie JA. The amygdala is a chemo-sensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.