Abstract
The purpose of the study was to examine whether changes in pCO2 mediate changes in fear of bodily sensation (as indexed by anxiety sensitivity) in a bio-behavioral treatment for panic disorder that targets changes in end-tidal pCO2. Thirty-five panic patients underwent 4 weeks of capnometry-assisted breathing training targeting respiratory dysregulation. Longitudinal mediation analyses of the changes in fear of bodily symptoms over time demonstrated that pCO2, but not respiration rate, was a partial mediator of the changes in anxiety sensitivity. Results were supported by cross lag panel analyses, which indicated that earlier pCO2 levels predicted later levels of anxiety sensitivity, but not vice versa. PCO2 changes also led to changes in respiration rate, questioning the importance of respiration rate in breathing training.
The results provide little support for changes in fear of bodily sensations leading to changes in respiration, but rather suggest that breathing training targeting pCO2 reduced fear of bodily sensations in panic disorder.
Keywords: Carbon dioxide, Anxiety sensitivity, Breathing therapy, Mediation, Panic disorder, Respiratory physiology
1. Introduction
Abnormalities that may maintain panic disorder (PD) include low levels of carbon dioxide partial pressure (pCO2), which is the defining characteristic of hyperventilation, instabilities in blood gas regulation, and oversensitivities of chemoreceptors (oversensitive “suffocation alarm system”; Klein, 1993). There are several experimental studies that support this association: lowering pCO2 into the hypocapnic range or increasing pCO2 into the hypercapnic range often results in panic-like symptoms in PD patients (Dager et al., 1995; Maddock and Carter, 1991; Wilhelm et al., 2001; Gorman et al., 2004; but see Beck et al., 1999). Furthermore, sustained hypocapnic levels have been observed in PD (Papp et al., 1995; Salkovskis et al., 1986; Meuret et al., 2008; but see also, Garssen et al., 1996).
The nature of the pCO2 dysregulation has been outlined in two theories of PD. Klein (1993) postulated a hypersensitive suffocation alarm system that triggers panic attacks when pCO2 rises; consequently PD patients keep levels of pCO2 low. Ley (1985a) emphasized the panicogenic effects of acute hyperventilation by which a vicious circle of chronic and acute levels of hypocapnia (low pCO2 levels) causes feared bodily sensations (in particular, dyspnea), which leads to more hyperventilation. This cycle may ultimately culminate in full-blown panic (Ley, 1985a,b, 1987).
If respiratory dysregulation is a central feature of PD, then interventions specifically targeting respiratory dysregulation may be effective in treating it. We tested this idea by devising a capnometry-assisted breathing training therapy that uses immediate feedback to teach patients how to raise their pCO2 over a series of training and practice sessions. The treatment led to sustained increases in pCO2 levels and decreases in respiratory rate and was successful in substantially reducing panic severity and frequency (Meuret et al., 2008). However, the mechanism by which this treatment worked remains unknown.
The purpose of this study was to examine the causal and temporal relationships between pCO2, respiration rate, and self-reported changes in fearful symptom interpretation in a respiratory treatment for PD. We chose anxiety sensitivity (AS) as a measure of fear of bodily symptoms. This construct is conceptualized as a disposition that determines the tendency to respond fearfully to anxiety symptoms such as heart racing or shortness of breath (Reiss and McNally, 1985). These (feared) physical symptoms are closely related to symptoms triggered by hypocapnia.
We predicted that by reducing hypocapnia, a reduction of fear of bodily sensations, and thereby the fearful interpretation of those symptoms, would be achieved. Thus, changes in pCO2 would mediate and precede changes in fearful interpretation of symptoms measured by the anxiety sensitivity index (ASI).
2. Method
2.1. Study design
The data set for the present analyses was part of a randomized controlled trial investigating the efficacy of capnometry-assisted breathing training for treatment of panic disorder. While the overall outcome of the treatment was reported elsewhere (Meuret et al., 2008), we here analyze the temporal relationships of pCO2 and anxiety sensitivity changes across the course of treatment. A more detailed description of the methods is provided in our prior report.
2.2. Participants
The sample consisted of 35 patients with a principle DSM-IV (American Psychiatric Association, 1994) Axis I diagnosis of PD with or without agoraphobia. Patients met the following inclusion criteria: (a) ages 18–60, (b) if taking psychotropic medication, on a stable dose for at least 3 months prior to treatment with an agreement not to change dosage until after the 2-month follow-up, unless necessary, and (c) no evidence of organic mental disorder, suicide intention, schizophrenia, alcohol or drug dependence, cardiovascular disease, pulmonary disease, epilepsy or pregnancy.
The final sample consisted of 22 females and 13 males. Thirty-one were White, one African–American, two Hispanic and two were Asian. On average, participants were 41 years old (SD: 8.6), with a duration of the PD averaging 9 years (range 0.5–32). Fifty-four percent reported some degree of agoraphobic avoidance. Forty-nine percent had at least one secondary current Axis I diagnosis. Of these, all were diagnosed with at least one additional anxiety disorder (48% specific phobia, 30% social anxiety disorder, 22% generalized anxiety disorder). Fourteen percent were diagnosed with an additional current diagnosis of major depressive disorder and 14% were diagnosed with both anxiety and mood disorder. Thirty-one percent of the patients were taking a stable dose of psychotropic medications (benzodiazepines (n = 6), antidepressants (n = 4), beta-blockers (n = 1) and other anxiolytics (n = 1)). Diagnosis was obtained using the structured clinical interview for DSM-IV patient edition (First et al., 1994). Interrater reliability was high for all Axis I diagnoses and PD (κ = .83, κ = 1.00).
This study was approved by the Institutional Review Board at Stanford University and all subjects signed written informed consent before entering the study.
2.3. Treatment
The treatment was designed to increase self-monitored end-tidal pCO2 and to reduce respiration rate (RR) by means of breathing exercises. It consisted of five weekly 1 h treatment sessions. During these sessions, patients were educated about the effects of hyperventilation and respiratory dysregulation on panic symptoms. They were taught how to increase self-monitored end-tidal pCO2, and to reduce RR and respiratory instability with the goal being to change hypocapnic levels of pCO2 (hypocapnic range as defined by pCO2 < 35 mm Hg; Oakes, 1996), thereby changing symptoms and fear of these symptoms associated with PD. No other instructions or techniques were discussed or used (e.g., cognitive restructuring and in vivo exposure).
Highly structured home exercises, using a handheld capnometry device in addition to pacing tones, were a central component of this treatment. The instrument analyses and displays breath-by-breath end-tidal pCO2 (in mm Hg) and RR (in breaths/min), and records them along with the time and date (Meuret et al., 2005a,b). In addition to the visual feedback of the capnometer, patients were provided audio tapes to guide their pace of breathing during the exercises. For more details see Meuret et al. (2001, 2004, 2008).
2.4. Assessments
Fear of bodily symptoms and physiological data (pCO2 and RR) were collected at five time points: pretreatment, sessions two, three and four, and at posttreatment. The measures of interest were assessed as described below.
Fear of bodily symptoms was assessed with the anxiety sensitivity index (ASI). The 16 items comprising the ASI (Reiss et al., 1986) assess a set of beliefs that unexplained somatic sensations are dangerous and may cause deleterious physical, psychological, or social consequences that go beyond any immediate physical discomfort. Items are rated on a scale from 0 (very little) to 4 (very much). Anxiety sensitivity is conceptualized as a dispositional and dimensional construct that determines the tendency to respond fearfully to anxiety symptoms (McNally, 1994; Reiss, 1991; Reiss and McNally, 1985; Taylor et al., 1992)1 The ASI was administered at pretreatment and posttreatment, and at the beginning of each treatment session. Mean ASI scores were 30.7 at pretreatment and 17.0 at posttreatment.
Baseline end-tidal pCO2 (pCO2)
End-tidal pCO2 (mm Hg) was collected and stored by the capnometry device to measure treatment compliance and progress. Patients completed an average of 47.6 (91.3%) of the 52 homework exercises assigned over the course of the four week treatment. Baseline pCO2 levels were obtained at pretreatment and from the first homework exercise after each treatment session, except posttreatment, for which we used the last exercise before the posttreatment assessment (there were no exercises after posttreatment assessment). This portable capnometer technique has been demonstrated to meet international accuracy standards (Biedler et al., 2003). Baseline pCO2 changed from hypocapnic levels at pretreatment (32.3 mm Hg) to normocapnic levels are posttreatment (38.2 mm Hg).
Baseline respiration rate (RR)
Respiration rate was computed and recorded at the same assessment times as pCO2. While the treatment goal for pCO2 was to achieve and maintain normocapnic levels, the goal for RR was to gradually reduce the rate to lower levels. The pacing tones were set to correspond to an RR of 13 breaths per minute in the first week, and rates of 11, 9 and 6 breaths per minute in successive following weeks. Baseline RR was 12.4 breath/minute at pretreatment and 11.4 breath/minute at posttreatment.
3. Data analysis
The longitudinal nature of our design produced a multilevel, or nested, data structure. The lower level, or level-1, data consisted of the repeated measures that were collected at each treatment session (i.e., ASI, pCO2 level, RR). The level-1 data were nested within upper level, or level-2, units (i.e., participants). Thus, our data structure was comprised of repeated measures (level-1 data) nested within individuals (level-2 data).
The focus of this longitudinal approach to the data analysis is to examine how an individual’s score on a variable changes as a function of time. Thus, growth curves over time were calculated for each variable (ASI, pCO2, and RR), for each participant. We employed HLM 6.06 (Raudenbush et al., 2004) to calculate these growth curves using a multilevel random coefficients regression framework. An advantage of this framework is that it handles unbalanced designs efficiently, allowing the number of observations to vary across participants.
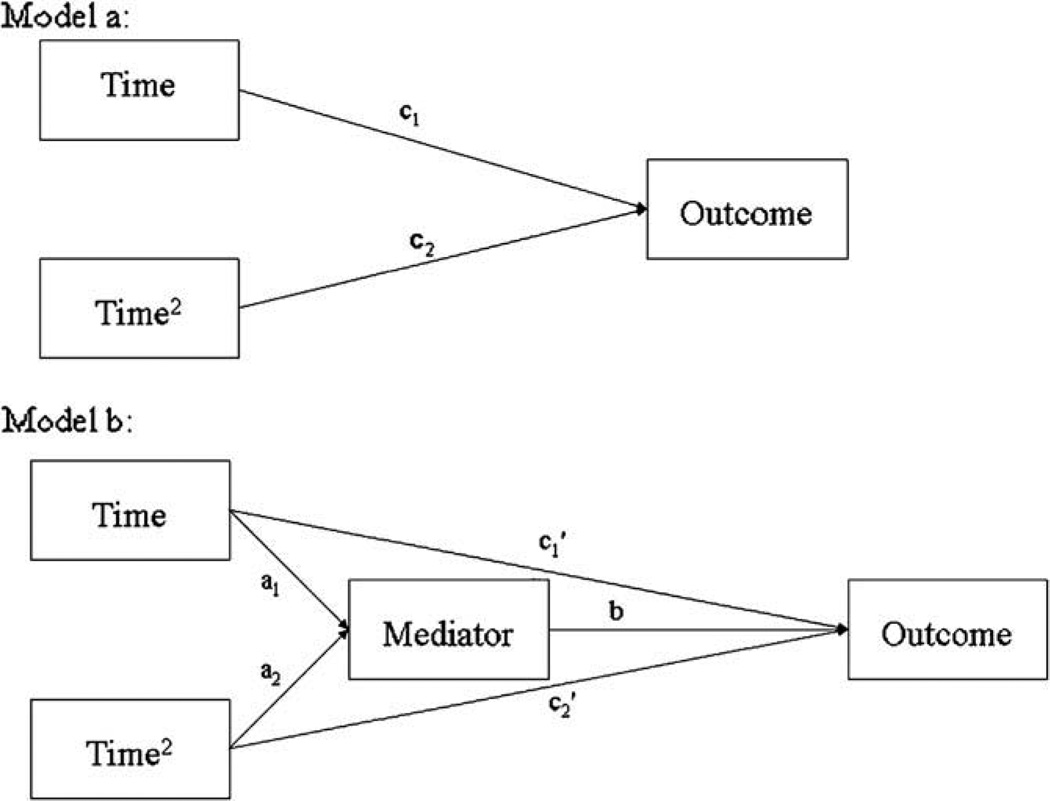
We first calculated the growth curves for the unmediated effect of Time on Outcome (Fig. 1a). Since the growth curve for the variables of interest might be curvilinear (e.g., the increase in pCO2 may flatten out once it reaches normal levels), the model allowed for both linear and quadratic growth over time. We next examined whether changes in pCO2 during treatment (i.e., over time) partially mediated changes in ASI during that treatment. This mediation model is shown in Fig. 1b.
Fig. 1.
Path diagrams for the longitudinal mediation analysis. Model “a” represents the non mediated effect of Time on Outcome. Model “b” shows the mediated effect of Time on Outcome.
Recently, Maxwell and Cole (2007) demonstrated that traditional mediation analyses (i.e., examining mediation of between subjects, or between condition, differences) rarely result in findings that reflect factors driving change within subjects over time. They suggest examining mediation of the change in outcome over time, longitudinally, within individuals. Thus, we employed the HLM analytical framework used in recent studies of the mediation of change within subjects over time (Moscovitch et al., 2005; Smits et al., 2006). This framework is referred to as “lower level mediation of a lower level effect” (Bauer et al., 2006; MacKinnon, 2008) because the mediator (e.g., pCO2) and the outcome (e.g., ASI) are both level-1 (i.e., lower level) variables that are measured repeatedly over time. The HLM equations for the mediation model shown in Fig. 1b are:
- Level-1 equation for mediator:
- Level-1 equation for outcome:
where i represents individual i and j represents the assessment (coded 0–4, representing assessments 1–5). Thus a1i and a2i represent the linear and quadratic slope of the mediator over time (treatments) for individual i (i.e., these regression coefficients represent the effect of the independent variable on the mediator), represent the slope of the outcome over time for individual i, controlling for the mediator, and bi is the relation between the mediator and the outcome for individual i.
HLM also includes a level-2 equation for every level-1 regression coefficient (slope). These level-2 equations can include characteristics of the individuals that may impact the level-1 regression coefficients (such as gender). However, since we did not hypothesize any individual level determinants of the level-1 regression coefficients, the level-2 equations just included a term which represented the “average” level-1 regression coefficient, plus a term representing the individual differences in the level-1 regression coefficient (following the model in Bauer et al. (2006)).2 These average regression coefficients then become the path coefficients in Fig. 1b. Thus, although HLM does calculate regression coefficients within subjects for each individual, the mediation analysis is performed using the “average” regression coefficients pooled across all the participants. Therefore, similar to more typical tests of mediation of a treatment effect between conditions, we obtain the average effect of the treatment on the mediator (a1 and a2, representing the linear and quadratic effects of treatment on the mediator) and we obtain the effect of the mediator on the outcome (b). If the product of the two segments of the joint, mediated pathways is significant (e.g., ), then we have a significant mediated effect (MacKinnon et al., 2004; Bauer et al., 2006).
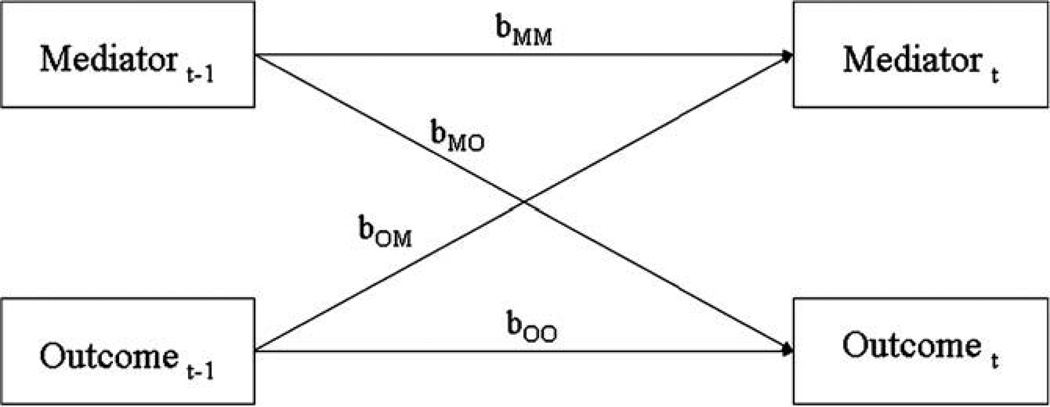
However, demonstrating a statistically significant mediated pathway, even if it is longitudinal, is not sufficient to conclude mediation. The size and significance of path coefficients in a path model (such as our mediation model in Fig. 1b) are only correct if the model is correct. And a significant mediated pathway does not, in itself, conclusively demonstrate that the directional relations in the path model are correct. For example, even if we found a significant path “b” in Fig. 1b, that does not mean that the mediator caused the outcome. A significant path “b” could result from “reverse mediation,” and the significant relation between the mediator and outcome in Fig. 1b could actually be the result of earlier levels of the outcome determining later levels of both the mediator and the outcome. To provide additional support for the proposed mediation model and to discount the possibility of reverse mediation, one must also establish: (1) that changes in the mediator precede changes in the outcome (Kraemer et al., 2001), and (2) that the relation between the mediator and the outcome is not due to reverse mediation (changes in the outcome causing changes in the mediator). Therefore, we performed cross lag panel analyses to determine the temporal precedence of the variables and to establish that earlier levels of the mediator predicted later levels of the outcome, and not vice versa (see Fig. 2). Following the technique used in Smits et al. (2006), we performed this analysis within subjects using HLM, thereby deriving cross lag coefficients for the relations within each participant over time. In addition to providing evidence for causal precedence, this analysis can indicate if the outcome causes the mediator to change, and hence whether reverse mediation may be occurring. We also performed a formal reverse mediation analysis following the procedure employed by Moscovitch et al. (2005), in which the “outcome” and the “mediator” in Fig. 1b were reversed, to insure that reverse mediation was not responsible for the obtained mediation results.
Fig. 2.
Path diagram representing the cross lag panel analysis within subjects over time.
Since we were investigating the mechanisms of change for patients undergoing treatment, we followed the approach used by previous researchers examining mechanisms of change in individuals receiving treatment.(e.g., Moscovitch et al., 2005; Smits et al., 2006) and only investigated those individuals receiving treatment and not those in the control condition.
4. Results
Initial analyses indicated that changes in pCO2 and respiration rate (RR) were nonlinear over time. Therefore, we included both linear and quadratic terms to represent the curvilinear effect of treatment over time. Examining the overall effect of the treatment over time (Fig. 1a) on the three variables of interest (ASI, pCO2, and RR), we found that for ASI, only the linear trend was significant (see the columns of Table 1labeled “Predictors of the Outcome”), indicating that ASI decreased linearly over time. For pCO2, the linear trend was positive while the quadratic trend was negative (Table 1), indicating that pCO2 initially increased rapidly, with that increase leveling off as time passed (the rate of change in pCO2 was relatively flat for treatments 4 and 5). For RR, the linear trend was positive and the quadratic trend was negative. In this case, RR initially increased, but between sessions 2 and 3 it leveled off, and by the final treatment RR was about 1.5 breath per minute slower that it was initially. Since both the linear and quadratic trends for two of the three variables were significant, we tested for mediation of both the linear and the quadratic effect of treatment (see Fig. 1b).
Table 1.
Regression coefficients for the mediation models
| Model | Predictors of the mediator (Fig. 1b) | Predictors of the outcome (Fig. 1a) | Predictors of the outcome in mediated model (Fig. 1b) | |||||
|---|---|---|---|---|---|---|---|---|
| Mediator | Outcome | Time (a1) | Time2 (a2) | Time (c1) | Time2 (c2) | Time (c1’) | Time2 (c2’) | Mediator (b) |
| pCO2 | ASI | 3.29** | −0.53** | −4.90** | 0.40 | −3.91* | 0.24 | −0.30* |
| ASI | pCO2 | −4.90** | 0.40 | 3.29** | −0.53** | 3.07** | −0.51** | −0.05 |
| RR | ASI | 1.65** | −0.51** | −4.90** | 0.40 | −5.42** | −0.50 | 0.01 |
| ASI | RR | −4.90** | 0.40 | 1.65** | −0.51** | 1.71** | −0.52** | 0.01 |
| pCO2 | RR | 3.29** | −0.53** | 1.65** | −0.51** | 2.28** | −0.63** | −0.19* |
| RR | pCO2 | 1.65** | −0.51** | 3.29** | −.053** | 3.54** | −0.61** | −0.15 |
Note:
p < .05,
p < .01.
Instead of using the causal steps approach to mediation (e.g., Baron and Kenny, 1986) which has low power and an overly conservative Type 1 error rate (MacKinnon et al., 2002), we directly tested the significance of the mediated pathway using the distribution of products test (MacKinnon et al., 2004). To perform this test, the “size” of the joint, mediated pathway is calculated by multiplying the regression coefficients of the two segments of the mediated pathway (e.g., in Fig. 1b), and then a 95% confidence interval for this product is calculated. We used the program PRODCLIN (MacKinnon et al., 2007) to calculate the 95% confidence intervals for the joint, mediated pathways. Confidence intervals that did not include 0 indicated a significant mediated pathway (MacKinnon et al., 2004). Since this product represents the magnitude of the mediated effect of Time on Outcome through the mediator, the distribution of products test tells us whether the mediated effect is significant. When we report a mediated effect, we also report the proportion mediated (PM) as a measure of effect size (Shrout and Bolger, 2002). PM is the proportion of the total effect of Time on the Outcome (coefficients c1 and c2 in Fig. 1a) that is mediated by the mediator. For example, PM for the linear effect of Time on Outcome is .
4.1. The relation between pCO2 and ASI over time
Fig. 1 shows the mediation model for the effect of treatments over time on outcome, both with and without a mediator. We first examined whether pCO2 mediated the changes in ASI over time. Testing this mediation model, we found that pCO2 was significantly related to ASI, b = −.30, t (171) = −1.99, p < .05 (Table 1). Directly testing the mediated pathway from the linear and quadratic effects of treatment over time to ASI through the mediator, pCO2, we found that both mediated pathways were significant, , 95% CI: −.04 to −2.23, p < .05, PM (proportion mediated) = .20, for mediation of the linear trend, and , 95% CI: .01–.36, p < .05, PM = .40, for mediation of the quadratic trend.
Testing the reverse mediation model, results indicated that ASI was not a significant determinant of pCO2, b = .05, t (171) = 1.86, p = .07 (Table 1). Directly testing the significance of the mediated pathways for both the linear and quadratic effect of treatment over time on pCO2 through ASI, we found that neither pathway was significant, ) = .223, 95% CI: −.004 to .54, p < .08, PM = .07, for the linear trend, and , 95% CI: −.02 to .06, n.s., PM = .03, for the quadratic trend.
Power analyses indicated that failure to find significant evidence for ASI mediating pCO2 is unlikely due to lack of power. Post hoc power analyses of these particular HLM analyses using the HLM power analysis program pINT (and using the covariance structure from these data as inputs into pINT) indicated sufficient power to detect a significant relation between the mediator and outcome (>.95 for a medium effect size). The power to detect a significant mediated pathway, using the conservative criterion that both segments of the mediated pathway must be significant, was greater than 0.85.
We next performed a cross lag panel analysis to examine the temporal relation between pCO2 and ASI (see Fig. 2). Not only does cross lag analysis provide information about the timeline between the variables, an important criteria for establishing mediation (Kazdin, 2007), but it also is one of the most effective analytical techniques to investigate the causal relations between variables which are not experimentally manipulated (Greenberg, 2008). A significant cross lag coefficient from a mediator to an outcome demonstrates that earlier levels of the mediator predict later changes in the outcome. It is important to note that these cross lag coefficients control for earlier levels of the outcome. If the cross lag analysis had not controlled for earlier levels of the outcome, then a relation between earlier levels of the mediator and later levels of the outcome could merely be due to earlier levels of the outcome causing both earlier levels of the mediator and later levels of the outcome.
Following the technique employed by Smits et al. (2006), we used HLM to calculate the path coefficients in Fig. 2 within subjects, over time, thus determining whether earlier levels of pCO2 were related to later levels of ASI, and vice versa, within subjects. The level 1 models included both ASI at time t − 1 and pCO2 at time t − 1 as time varying predictors of ASI and pCO2 at time t. Thus, for each individual, there were four sets of data points: ASI and pCO2 at time 2 being predicted by both ASI and pCO2 at time 1, ASI and pCO2 at time 3 predicted by both ASI and pCO2 at time 2, ASI and pCO2 at time 4 predicted by both ASI and pCO2 at time 3, and ASI and pCO2 at time 5 being predicted by both ASI and pCO2 at time 4. The model yielded regression coefficients which measured the effect of prior levels of both ASI and pCO2 on later levels of ASI and pCO2 within subjects, over time. Thus, a significant cross lag regression coefficient from pCO2 at time t − 1 to ASI at time t would indicate that an individual’s pCO2 at one assessment would impact their ASI at the next assessment, implying that changes in their pCO2 would lead to later changes in their ASI. Although cross lag analysis does not establish mediation, it can support and verify the causal ordering of the variables in a mediation analysis.
The results from these cross lag HLM analyses are shown in Table 2. First examining the stability of the measures over time, it can be seen that prior levels of pCO2 were related to later levels of pCO2, b = .16, t (137) = 2.44, p < .05, and prior ASI was related to later ASI, b = .41, t (34) = 4.64, p < .001. Turning to the cross lag relationships, higher levels of pCO2 were related to later reductions in ASI, b = −.02, t (137) = −2.31, p < .05, but earlier levels of ASI were not a determinant of later levels of pCO2, b = .19, t (137) = .43, n.s. (power to detect a medium effect size was greater than .90 in this analysis). These data, along with the results of the mediation analyses, support the hypothesis that pCO2 partially mediates changes in ASI, and that these mediation findings are not due to “reverse” mediation.3
Table 2.
Regression coefficients for the cross lag panel analyses
| Variables | Predictors of mediator at time t |
Predictors of outcome at time t |
|||
|---|---|---|---|---|---|
| Mediator | Outcome | Mediator t−1 (bMM) |
Outcome t−1 (bOM) |
Mediator t−1 (bMO) |
Outcome t−1 (bOO) |
| pCO2 | ASI | 0.16* | 0.19 | −.02* | 0.41** |
| RR | ASI | 0.25** | 0.94 | 0.01 | 0.48** |
| pCO2 | RR | 0.15* | 0.05 | −0.15* | 0.25** |
Note:
p < .05,
p < .01.
4.2. Respiration rate and ASI over Time
The mediation and cross lag analyses were repeated to examine the relationship between RR and ASI. Although both RR and ASI were significantly changed by the treatment (see Table 1), neither variable was significantly related to the other in the mediation analyses (Table 1). In addition, none of the mediated pathways were significant and none of the cross lag coefficients were significant.
4.3. pCO2. and respiration rate over Time
The mediation and cross lag analyses were also conducted to investigate the relation between pCO2 and RR. We first examined the model in which pCO2 was hypothesized to mediate changes in RR over time. The mediation analysis showed that higher levels of pCO2 were related to lower RR, b = −.19, t (34) = 2.16, p < .05 (see Table 1). In addition, the tests of the significance of the mediated pathways indicated that pCO2 was a significant mediator of both the linear and quadratic effects of Time on RR, , 95% CI: −.032 to −1.30, p < .05, and , 95% CI: .010–.218, p < .05, respectively.
Unfortunately, we could not compute the PM for either trend because pCO2 acted as a suppressor of the linear and quadratic effects of Time on RR, and the formula for PM is not appropriate for suppressor relationships (Shrout and Bolger, 2002). However, one way to calculate an approximation of PM in this situation is to examine the overall relation between time and RR over all the trials in the study (i.e., the combined effects of the linear and quadratic time effects), both with and without pCO2 as a mediator. The overall effect of Time on RR (according to the model in Fig. 1a and using the regression coefficients c1 and c2 for RR as the outcome, found in Table 1) over the five trials of the study was: 1.65 * 4 + (−.51) * 42 = −1.56.4 The effect of Time on RR using the mediation model which controls for the effect of pCO2 (Fig. 1b and regression coefficients for RR in Table 1), was 2.28 * 4 + (−.63) * 42 = −.96. Thus, the direct effect of Time on RR was decreased 38% (from −1.56 to −.96) by adding pCO2 as a mediator, implying that the mediated pathways account for approximately 38% of the total effect of Time on RR.
Analyzing the “reverse” mediation model, we found that RR was not significantly related to pCO2, b = −.15, t (171) = 1.78, p < .08 (Table 1). The test of the mediated pathways indicated that RR was not a significant mediator of either the linear or quadratic effects of treatment on pCO2, , 95% CI: .01 to −.62, p < .08, for the linear trend, and , 95% CI: .01 to −.18, p < .08, for the quadratic trend. Again, PM could not be computed because RR acted as a suppressor of both effects of Time on pCO2. However, calculating the overall effect of Time on pCO2 (the combined linear and quadratic effect) over the five trials of the study, both with and without RR as a mediator can indicate the total impact of RR as a mediator (an approximation to PM). We found that the total effect of Time on pCO2 (without controlling for RR as a mediator) was 3.29 * 4 + (−.53) * 42 = 4.7. The overall effect of Time on pCO2, controlling for RR in the mediation model, was 3.54 * 4 + (−.61) * 42 = 4.4. Thus, the direct effect of Time on pCO2 was decreased by about 7% (from 4.7 to 4.4) by controlling for RR, indicating that only 7% of the total effect of Time on pCO2 was accounted for by the indirect effects through RR.
Table 2 presents the results from the within subjects cross lag panel analysis for the effects of pCO2 on RR and vice versa. Both RR and pCO2 had significant auto regressive relationships, b = .25, t (137) = 2.88, p < .01, and b = .15, t (137) = 2.66, p < .01, respectively, but the only significant cross lag relation was that higher levels of pCO2 predicted lower levels of RR at the next assessment point, b = −.15, t (137) = −1.95, p = .05. Earlier levels of RR were not related to later levels of pCO2, b = .05, p > .50. Since earlier levels of pCO2 led to later levels of RR, this cross lag result is consistent with the mediation analyses indicating that pCO2 partially mediates changes in RR, and it supports the “direction” of the effect specified in the mediation model (Fig. 1b). Since earlier levels of RR did not lead to later levels of pCO2, the cross lag analysis also supports the results of the mediation analysis indicating that there was no evidence for “reverse” mediation.
5. Discussion
The results of our mediation analyses showed that pCO2 partially mediated the impact of capnometry-assisted breathing training on fear of bodily symptoms over time. The proportion of the change in fear of bodily sensations (measured by the ASI) that was mediated by pCO2 ranged from 20–40%, a proportion similar to that reported in other longitudinal HLM analyses of treatment outcome studies examining cognitions as mediators of treatment change (e.g., Smits et al., 2006; Hofmann et al., 2007). In addition, cross lag panel analysis indicated that higher pCO2 levels were related to lower levels of ASI at the next assessment, but not vice versa. Together with the fact that there was little evidence for reverse mediation accounting for the relation between pCO2 and ASI, these results suggest that pCO2 mediates a substantial portion of the effect of the treatment on ASI. To our knowledge, this is the first mediation analysis assessing psychological and physiological changes in PD treatment.
Hyperventilation causes CO2 concentration of the blood to drop below normal, leading to respiratory alkalosis that elicits a whole range of feared bodily sensations, such as heart racing, shortness of breath, dizziness, tingling, chest pain, numbness or lightheadedness. Shortness of breath, together with palpitations and faintness, are the most commonly reported symptoms experienced during panic attacks (Meuret et al., 2006). Why did reductions in hyperventilation (i.e., increases in pCO2 levels) lead to decreases in fear of bodily sensations? One possibility is that raising pCO2 reduces the occurrence of bodily sensations, and thus reduces the fear associated with them (e.g., Ley, 1985a,b). Alternatively, alteration of hypocapnic levels over time by means of repeated elevation of pCO2 during homework sessions may have desensitized a hypersensitive suffocation alarm system leading to a reduction in catastrophic cognitions (e.g., “I will suffocate when I become short of breath”) (Klein, 1993). According to Klein’s theory, hyperventilation serves as a protection against feelings of dyspnea by driving pCO2 far below the threshold of feeling short of breath. Successful elevation of pCO2 by means of the direct feedback of pCO2 received during the repeated exercises may have altered the hypersensitivity of the suffocation alarm system. In other words, increased resting pCO2 may have resulted in an increased threshold for suffocation sensations, thereby reducing respiration-related interoceptive cues that trigger panic attacks. Previous studies have shown the air hunger threshold to lie within a narrow range of pCO2 above an individual’s baseline pCO2, and that this threshold re-sets after chronic change in an individual’s baseline pCO2 (Banzett et al., 1996; Bloch-Salisbury et al., 1996). As a consequence of this desensitization, the fear of bodily sensations, or anxiety sensitivity, may have been reduced.
Another study exploring mechanism of therapy change in panic (Hofmann et al., 2007) uncovered a mediating role of cognitions for patients receiving CBT but not for those receiving pharmacotherapy (imipramine). This interesting disparity in mechanisms may reflect some of the insights gained from our study. While a reduction in catastrophic interpretations of bodily symptoms is closely related to treatment success, change in this central characteristic of panic disorder may be achieved in a number of ways. CBT protocols target the interpretations of uncomfortable bodily sensations by cognitive restructuring exercises, but changes could also be driven by targeting other aspects of the psychophysiology of PD, such as pCO2 levels. For instance, imipramine, the drug used in the above mentioned study, may exert its effect by increasing the overall serotonergic and noradrenergic neurotransmission and thereby also reduce anxiety sensitivity of panic patients in the course of treatment (Mavissakalian et al., 1998). These neurotransmitter systems are known to play a role in the control of respiration (Lundberg et al., 1980) and may thus be effective at least in part by reducing respiratory hyperactivity in PD (Gorman et al., 1997; Perna et al., 2002). Whether effects of our treatment are mediated through respiration changes, or whether respiration plays the role of a peripheral marker of corrective changes in these neurotransmitter systems, remains to be determined.
Another finding of our analysis was that higher levels of pCO2 were causally associated with lower RR. We found that pCO2 accounted for approximately 38% of the change in RR over time. RR changes, on the other hand, did not seem to drive the increases in pCO2 observed in the current treatment. Thus, we found that lowering respiration rate is unlikely to lead to meaningful changes in pCO2, contrary to what is sometimes assumed. Changes in RR contributed minimally to changes in pCO2. Furthermore, they followed rather than preceded changes in pCO2. In addition, several studies report basal respiration rates in PD to be lower or equal to healthy controls (Gorman et al., 2004; Wilhelm et al., 2001; Carr et al., 1996), questioning the focus on changing in RR in traditional breathing retraining.
What mechanism may have accounted for these findings? Perhaps increases in pCO2 over time triggered symptoms of dyspnea. This is turn could have lead to slower basal respiration rate as more efficient way to ventilate, with less work of breathing per unit time. Indeed, when resistive loads are added to a respiratory device, subjects tend to switch to slower and deeper breathing (e.g., Hirsch and Bishop, 1982; Calabrese et al., 1998). However, in our patients pCO2 increases were associated with decreases in fear of symptoms (ASI), which would have been counteracted by an increase in dyspnea. More research is needed to investigate the temporal relationship between pCO2, dyspnea and respiration rate in panic disorder treatment.
Under conditions of stable physical activity, decreases in minute ventilation lead to increases in pCO2, and minute ventilation can be decreased by lower respiration rate, lower tidal volume, or a combination of both. Thus, with no apparent change in respiration rate, reduced breath volume was the most likely mechanism behind pCO2 increases. This interpretation fits well with studies investigating breathing pattern changes that contribute to hypocapnia in anxiety disorders: Some studies have found that fear-related hyperventilation is linked mainly to increases in tidal volume rather than to increases in respiration rate (Gorman et al., 1988; Papp et al., 1993; Abelson et al., 2001; Ritz et al., in press). A possible central role for volume has important theoretical and practical implications. CBT protocols that included traditional breathing training (Schmidt et al., 2000) may have put greater emphasis on slowing respiratory rate as the target of treatment without measuring pCO2, thus failing to target hyperventilation efficiently (Meuret et al., 2005a,b, 2003). Slow breathing is likely to lead to compensatory deeper breathing, which will perpetuate or exacerbate hyperventilation in PD (Ley, 1991; Meuret et al., 2003; Conrad et al., 2007). In testing the efficacy of breathing training in PD treatment, a focus on both respiration rate and volume is needed, ideally supported by information on actual pCO2 levels.
Our findings demonstrate that physiological changes (namely increases in pCO2) mediate changes in a psychological construct (AS) that has been associated with the maintenance of panic disorder. Even though the treatment was aimed at changing respiratory dysregulation in accordance with respiratory theories of PD, the goal of this study was not to provide evidence for a particular theory of PD but to examine a possible mediator of treatment change. It cannot be ruled out that changes in respiration mediate changes in emotional distress in general or in other common/nonspecific variables discussed in the psychotherapy literature. Future studies should therefore examine the specificity of the observed mediation effect by including a control condition (e.g., cognitive therapy). The present study only included a wait-list control group.3 At this point, we can only state that our conclusions regarding the temporal sequence of mediators and outcomes must be restricted to this therapy and to these mediators (ASI and CO2). A further limitation of our study is that the cross lag panel analysis is sensitive to the time lag in the analysis. Perhaps earlier levels of ASI were not related to later levels of pCO2 because the lag time in our study (approximately 1 week) was either too short for the effect to appear, or so long that the effect had already decayed. Finally, all of the results reported here are correlational, and although they suggest temporal precedence, they cannot prove causality or exclude the possibility that an unmeasured third variable is responsible for the observed relations.
In sum, the results presented here emphasize the important role for respiratory factors in PD and their temporal relationship to changes in fear of bodily sensations in a treatment targeting respiration. They provide little support for the assumption that breathing training exerts its influence by primarily changing fear of bodily sensations rather than respiration. In general, they demonstrate that changing breathing patterns can be a powerful tool in treating panic disorder.
Acknowledgement
We acknowledge the helpful comments of Dr. Thomas Ritz on an earlier draft of this article.
Drs. Meuret and Rosenfield are supported by NIH Grant HL089761. Dr. Hofmann is supported by NIMH Grant MH079236. He is also a paid consultant of Organon (Schering-Plough).
Role of funding source
This study was supported in part by the Russell and Beth Siegelman Charitable Fund.
Footnotes
Some psychometric studies of the ASI support a multidimensional and hierarchical factor structure consisting of 3 lower order and 1 higher order factor (e.g., Zinbarg et al., 1997), whereas other studies support a taxonic latent class structure composed of 2 dimensional types of anxiety sensitivity (e.g., Bernstein et al., 2007). To be consistent with the majority of experimental studies that utilized the ASI (see Taylor, 1999, for a review), and given the conceptual definition of the construct, we decided to examine anxiety sensitivity as a unidimensional construct.
The mediation analyses reported herein were repeated adding gender, age, duration of PD, and baseline activity level as level 2 predictors of the intercepts and slopes in those analyses. None of these variables was found to significantly moderate the mediation relations reported herein. These mediation analyses were also conducted using the “physical symptoms” subscale of the ASI instead of the full scale, with results identical to those reported for the full scale.
We also performed a more typical between-subjects mediation analysis, testing pCO2 and RR as mediators of difference in ASI between the treatment and control conditions (wait-list control group). In these between subjects mediation analyses, treatment condition was the independent variable, the mediator was either pCO2 or RR (measured at post treatment), and the outcome was post treatment ASI. Neither pCO2 nor RR was a significant mediator of the between-conditions differences. This result is consistent with Maxwell and Cole (2007), who showed that results for within subjects mediation are rarely similar to results for between subjects mediation.
Since the regression coefficients represented the linear and quadratic change per week, and since there were 4 weeks between the beginning (week 1) and end of treatment (week 5), the total change in the outcome over the 4 weeks is the sum of the linear regression coefficient times 4 plus the quadratic regression coefficient times 4 squared.
Conflict of interest statement
All other authors declared that they have no conflicts of interest.
Contributors
Alicia E. Meuret, Ph.D. designed study, assisted in the statistical analysis, and wrote manuscript. David Rosenfield, Ph.D. undertook the statistical analysis and contributed to the writing of the manuscript. Stefan G. Hofmann, Ph.D. contributed to the writing and editing of the manuscript. Michael K. Suvak, M.A. assisted in the statistical analysis. Walton T. Roth, M.D. contributed to the writing and editing of the manuscript.
References
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biological Psychiatry. 2001;49:588–595. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus–response characteristics of CO2 -induced air hunger in normal subjects. Respiratory Physiology. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Preacher KJ, Gil KM. Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations. Psychological Methods. 2006;11:142–163. doi: 10.1037/1082-989X.11.2.142. [DOI] [PubMed] [Google Scholar]
- Beck JG, Ohtake PJ, Shipherd JC. Exaggerated anxiety is not unique to CO2 in panic disorder: a comparison of hypercapnic and hypoxic challenges. Journal of Abnormal Psychology. 1999;108:473–482. doi: 10.1037//0021-843x.108.3.473. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ, Norton PJ, Schmidt NB, Taylor S, Forsyth JP, et al. Taxometric and factor analytic models of anxiety sensitivity: integrating approaches to latent structural research. Psychological Assessment. 2007;19:74–87. doi: 10.1037/1040-3590.19.1.74. [DOI] [PubMed] [Google Scholar]
- Biedler AE, Wilhelm W, Kreuer S, Soltesz S, Bach F, Mertzlufft FO, et al. Accuracy of portable quantitative capnometers and capnographs under prehospital conditions. The American Journal of Emergency Medicine. 2003;21:520–524. doi: 10.1016/j.ajem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Bloch-Salisbury E, Shea SA, Brown R, Evans K, Banzett RB. Air hunger induced by acute increase in pCO2 adapts to chronic elevation of pCO2 in ventilated humans. Journal of Applied Physiology. 1996;81:949–956. doi: 10.1152/jappl.1996.81.2.949. [DOI] [PubMed] [Google Scholar]
- Calabrese P, Dinh TP, Eberhard A, Bachy JP, Benchetrit G. Effects of resistive loading on the pattern of breathing. Respiratory Physiology. 1998;113:167–179. doi: 10.1016/s0034-5687(98)00063-2. [DOI] [PubMed] [Google Scholar]
- Carr RE, Lehrer PM, Hochron SM, Jackson A. Effects of psychological stress on airways impedance in individuals with asthma and panic disorder. Journal of Abnormal Psychology. 1996;105:137–141. doi: 10.1037//0021-843x.105.1.137. [DOI] [PubMed] [Google Scholar]
- Conrad A, Müller A, Doberenz S, Kim S, Meuret AE, Wollburg E, et al. Psychophysiological effects of breathing instructions for stress management. Applied Psychophysiology and Biofeedback. 2007;32:89–98. doi: 10.1007/s10484-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Dager SR, Strauss WL, Marro KI, Richards TL, et al. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. American Journal of Psychiatry. 1995;152:666–672. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview of DSM-IV: patient edition (SCID-I/P), Version 2.0. New York: Biometrics Research Department; 1994. [Google Scholar]
- Garssen B, Buikhuisen M, van Dyck R. Hyperventilation and panic attacks. American Journal of Psychiatry. 1996;153:513–518. doi: 10.1176/ajp.153.4.513. [DOI] [PubMed] [Google Scholar]
- Greenberg DF. Causal analysis with nonexperimental panel data. In: Menard S, editor. Handbook of longitudinal research: design, measurement and analysis. New York: Academic Press; 2008. pp. 259–278. [Google Scholar]
- Gorman JM, Browne ST, Papp LA, Martinez J, Welkowitz L, Coplan JD, et al. Effect of antipanic treatment on response to carbon dioxide. Biological Psychiatry. 1997;42:982–991. doi: 10.1016/s0006-3223(97)00160-1. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, et al. Ventilatory physiology of patients with panic disorder. Archives of General Psychiatry. 1988;45:31–39. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biological Psychiatry. 2004;56:862–867. doi: 10.1016/j.biopsych.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Rosenfield D, Suvak MK, Barlow DH, Gorman JM, et al. Preliminary evidence for cognitive mediation during cognitive-behavioral therapy of panic disorder. Journal of Consulting and Clinical Psychology. 2007;75:374–379. doi: 10.1037/0022-006X.75.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Bishop BJ. Human breathing patterns on mouthpiece or face mask during air, CO2, or low O2. Applied Physiology. 1982;53:1281–1290. doi: 10.1152/jappl.1982.53.5.1281. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics and related conditions: an integrative hypothesis. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators and independent, overlapping and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Ley R. Blood, breath and fears: a hyperventilation theory of panic attacks and agoraphobia. Clinical Psychology Review. 1985a;5:271–285. [Google Scholar]
- Ley R. Agoraphobia, the panic attack and the hyperventilation syndrome. Behaviour Research and Therapy. 1985b;23:79–81. doi: 10.1016/0005-7967(85)90145-7. [DOI] [PubMed] [Google Scholar]
- Ley R. The efficacy of breathing retraining and the centrality of hyperventilation in panic disorder: a reinterpretation of experimental findings. Behaviour Research and Therapy. 1991;29:301–304. doi: 10.1016/0005-7967(91)90121-i. [DOI] [PubMed] [Google Scholar]
- Ley R. Panic disorder: a hyperventilation interpretation. In: Michaelson L, Ascher M, editors. Cognitive-behavioral assessments and treatment of anxiety disorders. New York: Guilford Press; 1987. pp. 191–212. [Google Scholar]
- Lundberg DBA, Mueller RA, Breese GR. An evaluation of the mechanism by which serotonergic activation depresses respiration. Journal of Pharmacological and Experimental Therapy. 1980;212:397–404. [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODLIN. Behavior Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence intervals for the indirect effect: distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Carter CS. Hyperventilation-induced panic attacks in panic disorder with agoraphobia. Biological Psychiatry. 1991;29:843–854. doi: 10.1016/0006-3223(91)90051-m. [DOI] [PubMed] [Google Scholar]
- Mavissakalian MR, Perel JM, Talbott-Green M, Sloan C. Gauging the effectiveness of extended imipramine treatment for panic disorder with agoraphobia. Biological Psychiatry. 1998;43:848–854. doi: 10.1016/s0006-3223(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Panic disorder: a critical analysis. New York: Guilford Press; 1994. [Google Scholar]
- Meuret AE, Ritz T, Dahme B, Roth WT. Therapeutic use of ambulatory capnometry. In: Gravenstein JS, Jaffe M, Paulus D, editors. Capnography. Clinical application. Camridge, MA: Cambridge University Press; 2005a. [Google Scholar]
- Meuret AE, Ritz T, Wilhelm FH, Roth WT. Voluntary hyperventilation in the treatment of panic disorder-functions of hyperventilation, their implications for breathing training, and recommendations for standardization. Clinical Psychology Review. 2005b;25:285–306. doi: 10.1016/j.cpr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Meuret AE, White KS, Ritz T, Roth WT, Hofmann SG, Brown TA. Panic attack symptom dimensions and their relationship to illness characteristics in panic disorder. Journal of Psychiatric Research. 2006;40:520–527. doi: 10.1016/j.jpsychires.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder: useful intervention or impediment? Behavior Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory biofeedback-assisted therapy in panic disorder. Behavior Modification. 2001;25:584–605. doi: 10.1177/0145445501254006. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Roth WT. Respiratory feedback for treating panic disorder. Journal of Clinical Psychology. 2004;60:197–207. doi: 10.1002/jclp.10245. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, Hofmann SG, Suvak MK, In-Albon T. Mediation of changes in anxiety and depression during treatment of social phobia. Journal of Consulting and Clinical Psychology. 2005;73:945–952. doi: 10.1037/0022-006X.73.5.945. [DOI] [PubMed] [Google Scholar]
- Oakes DF. Clinical practitioner’s pocket guide to respiratory care. 4th ed. Old Towne, ME: Health Educator Publications Inc; 1996. [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation and panic disorder. American Journal of Psychiatry. 1993;150:1149–1157. [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, et al. Rebreathing tests in panic disorder. Biological Psychiatry. 1995;38:240–245. doi: 10.1016/0006-3223(94)00296-F. [DOI] [PubMed] [Google Scholar]
- Perna G, Bertani A, Caldirola D, Gabriele A, Cocchi S, Bellodi L. Antipanic drug modulation of 35% CO2 hyperreactivity and short-term treatment outcome. Journal of Clinical Psychopharmacology. 2002;22:300–308. doi: 10.1097/00004714-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Reiss S. Expectancy model of fear, anxiety and panic. Clinical Psychology Review. 1991;11:141–153. [Google Scholar]
- Reiss S, McNally RJ. Expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. San Diego: Academic Press; 1985. pp. 107–121. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach A, Roth WT. Do blood phobia patients hyperventilate during exposure by breathing faster, deeper or both? Depression and Anxiety. doi: 10.1002/da.20466. [in press] [DOI] [PubMed] [Google Scholar]
- Salkovskis PM, Jones DR, Clark DM. Respiratory control in the treatment of panic attacks: replication and extension with concurrent measurement of behaviour and pCO2. British Journal of Psychiatry. 1986;148:526–532. doi: 10.1192/bjp.148.5.526. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, et al. Dismantling cognitive-behavioral treatment for panic disorder: questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Smits JAJ, Rosenfield D, McDonald R, Telch MJ. Cognitive mechanisms of social anxiety reduction: an examination of specificity and temporality. Journal of Consulting and Clinical Psychology. 2006;74:1203–1212. doi: 10.1037/0022-006X.74.6.1203. [DOI] [PubMed] [Google Scholar]
- Taylor S. Anxiety sensitivity: theory, research and treatment of the fear of anxiety. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. [Google Scholar]
- Taylor S, Koch WJ, McNally RJ, Crockett DJ. Conceptualizations of anxiety sensitivity. Psychological Assessment. 1992;4:245–250. [Google Scholar]
- Wilhelm FH, Gerlach AL, Roth WT. Slow recovery from voluntary hyperventilation in panic disorder. Psychosomatic Medicine. 2001;63:638–649. doi: 10.1097/00006842-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Barlow DH, Brown TA. Hierarchical structure and general factor saturation of the anxiety sensitivity index: evidence and implications. Psychological Assessment. 1997;9:277–284. [Google Scholar]