Abstract
Study design
Hyperreflexia occurs after spinal cord injury (SCI) and can be assessed by measuring low frequency-dependent depression of the H-reflex. Previous studies showed the time course for the onset of hyperreflexia to occur between 6–28 days in the contusion model of SCI.
Objective
To determine the time course of the onset of hyperreflexia in the transection model of SCI and examine changes in Connexin-36 (Cx-36) protein levels in the lumbar enlargement of animals.
Setting
Spinal Cord Injury Mobilization Program of the Center for Translational Neuroscience, the research arm of the Jackson T. Stephens Neuroscience Institute, Little Rock, AR, USA.
Methods
Adult female rats underwent transection at T10 level. Low frequency-dependent depression of the H-reflex was tested at 7, 14, and 30 days post-transection. Lumbar enlargement tissue was harvested following reflex testing and western blots were performed after immunoprecipitation to compare Cx-36 protein levels.
Results
Significant decreases in low frequency-dependent depression of the H-reflex were observed in animals tested 14 and 30 days post-transection compared with control animals, but it was not different from control animals at 7 days. Significant decreases in Cx-36 protein levels were observed in animals 7 days post-transection compared with controls.
Conclusion
Rats transition to a state of hyperreflexia between 7 and 14 days post-transection. Cx-36 protein levels decreased at 7 days post- transection and gradually returned to control levels by 30 days post- transection. These data suggest there may be a relationship between changes in neuronal gap junction protein levels and the delayed onset of hyperreflexia.
Sponsors
NIH Grant RR020146 to the Center for Translational Neuroscience
Keywords: Connexin 36, H-reflex, spinal cord injury
Introduction
Hyperreflexia, a component of spasticity1 does not arise immediately after spinal cord injury (SCI), but emerges over time. The hyperreflexia that develops after injury has been suggested to limit functional recovery in patients with an incomplete injury2 and interferes with the activities of daily living in patients with a complete injury. The pathophysiology of hyperreflexia is unknown although many mechanisms have been postulated,2,3 including loss of presynaptic inhibition,3–5 increase in postsynaptic receptor excitability2, synapse growth2, and changes in intrinsic properties of motoneurons.6
The H-reflex is a compound electromyographic (EMG) response elicited by the synaptic activation of motoneurons by muscle afferents following stimulation of muscle nerves, and has been used to demonstrate changes in the excitability of the reflex pathway. Low frequency-dependent depression was described as the gradual decrease in H-reflex amplitude that occurs when a series of reflexes are elicited between 1 and 10 Hz7. Skinner et al.8 demonstrated significant changes in low frequency-dependent depression in the rat 90 days after complete transection. Thompson et al 9 found that there was no difference in low frequency-dependent depression between rats at 6 days postinjury and control animals, but reported a significant decrease in low frequency-dependent depression 28 and 60 days postinjury. This suggests a time course of transition to hyperreflexia occurring between 6 and 28 days that correlates with the findings of Malmsten 10 after chronic spinal cord hemisection in the rat using the monosynaptic reflex.
In the human, Leis et al.11 reported absent H-reflexes 24 hours after injury that recovered to normal amplitudes within several days postinjury. Calancie et al.4 showed normal low frequency-dependent depression in acute patients with SCI (defined as less than 2 weeks postinjury) and reduction in low frequency-dependent depression for patients with a chronic SCI (greater than 1 year), but the time course of the transition was not described. Schindler-Ivens and Shields5 observed H-reflex frequency-dependent depression in an acute patient over a time course of 44 weeks in addition to examining a group of acute and chronic patients. They found that the H-reflex of the acute group (within 6 weeks of the injury) showed a pattern of suppression similar to the able-bodied group. They also reported that the one patient examined over 6–44 weeks after injury, showed reduction in frequency-dependent depression between 6–18 weeks after injury, which continued to change until 44 weeks.
Such a delay implies that the decrease in habituation of reflexes occurs slowly. One potential mechanism that has not been proposed is a change in synchronized activity. Synchronized activity of neurons, including motoneurons, is prevalent during development. At birth, motoneurons in the rat spinal cord are dye coupled, electrically coupled and can produce synchronized motor rhythms in the absence of chemical synaptic activity.12–13 Electrical coupling is mediated between neurons by the gap junction protein Connexin-36 (Cx-36). During development, the number of motoneurons that are coupled decreases,12–13 leaving smaller functional motoneuron groups coupled, which is thought to enable fine-tuning of motor control in the adult animal.13 We hypothesize that after SCI and the loss of input from descending neurons, there are changes in electrical coupling of spinal neurons below the level of the lesion, resulting in changes in synchrony of motorneuronal firing that contributes to or results in, the hyperreflexia observed. Data on whether Cx-36 levels change after SCI is conflicting.14,15
In the present study, the time course of the transition to a state of hyperreflexia was investigated by measuring low frequency-dependent depression at 7, 14, and 30 days after complete transection. In addition, we investigated a potential mechanism by which hyperreflexia develops over time by analyzing the levels of Cx-36 protein at the same time points after transection.
Methods
Surgery
Adult female Sprague-Dawley rats (Harlan, 200–250 g, n = 42) underwent lower thoracic laminectomy under ketamine (60 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.) anaesthesia. A complete transection (Tx) of the spinal cord was made by aspiration and the transected ends of the cord retracted, producing a 2–3 mm cavity. Surgery and postsurgical care was performed as previously described.16 All procedures were approved by the Institutional Animal Care and Use Committee at UAMS. We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
One group of transected animals (Tx 7D, n=8) was tested for H-reflex frequency-dependent depression 7 days after complete Tx, a second group of transected rats (Tx 14D, n=8) was tested after 14 days, a third group of transected rats (Tx 30D, n =16) was tested after 30 days, and a final group served as non-transected controls (Control, n=10).
Reflex Testing
H-reflex testing was measured as previously described.16 Recordings were made using amplifier (Grass P511) filter settings of 3 Hz to 3 KHz with the 60 Hz notch filter in use. Responses to the stimulus were digitized and averaged using a GW Instruments (Somerville, MA) digitizer module and SuperScopeR software.
The reflex first was tested at 0.2 Hz to determine threshold and maximal response levels. After discarding the first 5 responses at each frequency in order to obtain an average of the stabilized reflex, averages of 10 responses were obtained. Averages were compiled following stimulation at 0.2, 1, 5, and 10 Hz. The change in the response at various frequencies was calculated as the percent of the response at 0.2 Hz in order to determine depression of the H-reflex as a function of stimulation frequency. Following the frequency series testing, the H-reflex amplitude was confirmed at 0.2 Hz for consistency. If the amplitude at recheck was less than 90% of the initial amplitude, the data was discarded.
At the end of the experiment, animals were euthanized with an overdose of barbiturate (Nembutal) and the Tx confirmed either visually or histologically following transcardial perfusion with paraformaldehyde (4%) and sucrose (20%).
Measurement and statistics
The amplitude of the H-wave was measured from peak to peak of the two components. For comparison of data between the different groups in each experiment, measures were tested using one factor, two factor or multifactor analysis of variance (ANOVA) to conclude whether any of the factors had a significant effect on the magnitude of the variable and also whether the interaction of the factors significantly affected the variable. Differences were considered significant at values of p<0.05. If statistical significance was present, posthoc tests were used to compare between groups.
Cx-36 protein analysis
At the end of recording, cores (3–4 cu mm, 300–600 mg) from the lumbar enlargement were removed using a 3–4 mm dermal biopsy punch after performing laminectomies in anesthetized rats. Tissue was homogenized in 600 μl ice cold RIPA buffer18 with HALT protease inhibitors (Pierce) and centrifuged to remove debris. Tissue and lysate was kept on ice at all times. 5 μg of anti-Cx-36 antibody (37-4600, Zymed, Invitrogen) per sample was covalently coupled to a gel support (Seize Primary, Pierce). 800 μg of protein from spinal cord lysates was mixed with antibody-coupled gel in a total of 1 ml RIPA (with protease inhibitors) and incubated 4°C, overnight, with gentle end-over-end mixing. Immunoprecipitates were washed twice with RIPA buffer and resolved by SDS-PAGE. The amount of Cx-36 immunoprecipitated was determined by western blot as previously described.18 Figure 1, left panel, shows the specificity of the antibodies for this procedure. Retina and liver are the positive and negative controls, respectively. The sensitivity of the procedure and the appropriate amount of antibody to quantify Cx-36 was determined by titration against different amounts of retina (left panel) and spinal cord (right panel) protein.
Figure 1.
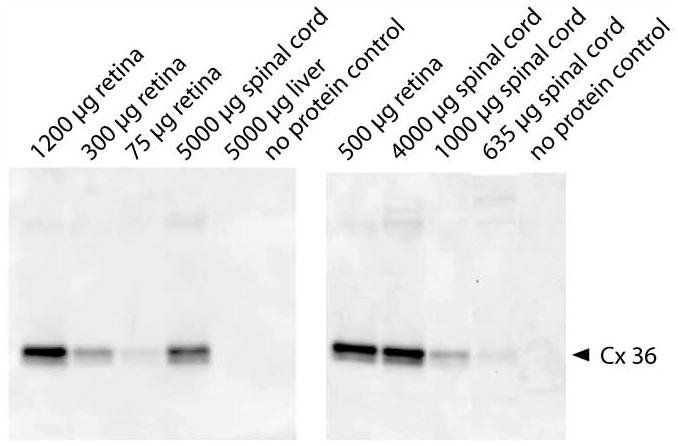
Immunoprecipitation followed by western blot was used to quantify the amount of Cx-36 in spinal cord. Left panel shows the specificity of the antibodies. A band was detected at the correct size in retina and spinal cord lysates but not liver lysates. Right panel shows the sensitivity of the procedure. A band was detected using 1000 μg protein but was less clear with 635 μg protein. 800 μg was chosen for the study. Left and right panels show titration of retina and spinal cord protein to verify that the amount of antibody used was within the linear range for this procedure.
Results
Figure 2A shows representative H-reflex recordings in animals transected and tested 7 days later. The responses shown are at 0.2 Hz (the 100% response), along with responses at 5.0 Hz and 10 Hz. Recordings of 1.0 Hz and repeat of 0.2 Hz are not shown. Note the decreased amplitude of the H-reflex at 5 and 10 Hz. Figure 2B shows H-reflex recordings in animals transected and tested at 14 days later. Note the lack of decrease in amplitude at 5 and 10 Hz. Figure 3 is a graph of the habituation of the H-reflex following stimulation at 0.2, 1, 5, and 10 Hz in control and Tx 7D, Tx 14D, Tx 30D groups. ANOVA of these groups showed statistically significant differences across experimental groups (df= 3, F= 8.75, p<0.0002). Post hoc comparisons showed statistically significant differences at 5 Hz in the Tx 7D vs Tx 14D, and vs Tx 30D (Scheffe p<0.05). Statistically significant differences were found at 10 Hz between the control group vs the Tx 14D (Scheffe p< 0.05) and the Tx 30D (Scheffe p<0.01) group.
Figure 2.
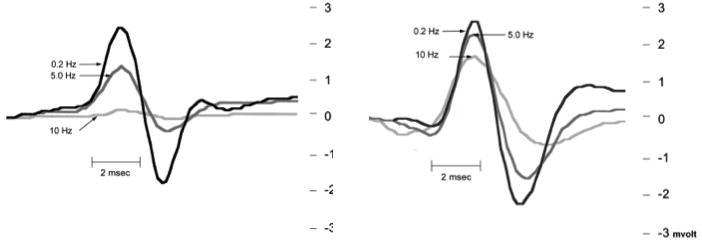
A. Representative H-reflex recordings of animals tested 7 days after Tx. The H-reflex was observed 8–10 ms after the stimulus. The H-reflex amplitudes at the different frequencies have been superimposed for comparison. The black line represents the H-reflex recorded at 0.2 Hz. The dark gray line represents recording at 5 Hz, and the light gray line represents recording at 10 Hz. Note the decrease in amplitude as frequency of stimulation was increased, indicative of low frequency-dependent depression present at 7 days posttransection. B. Representative H-reflex recording of animals tested 14 days after Tx. The H-reflex responses at the different frequencies have been superimposed for comparison. The black line represents H-reflex recorded at 0.2 Hz. The dark gray line represents recording at 5 Hz, and light gray line represents recording at 10 Hz. Note lack of decrement in amplitude as frequency of stimulation was increased, indicative of a decrease in frequency-dependent depression. The x-axis represents time in msec, the y-axis amplitude in mV.
Figure 3.
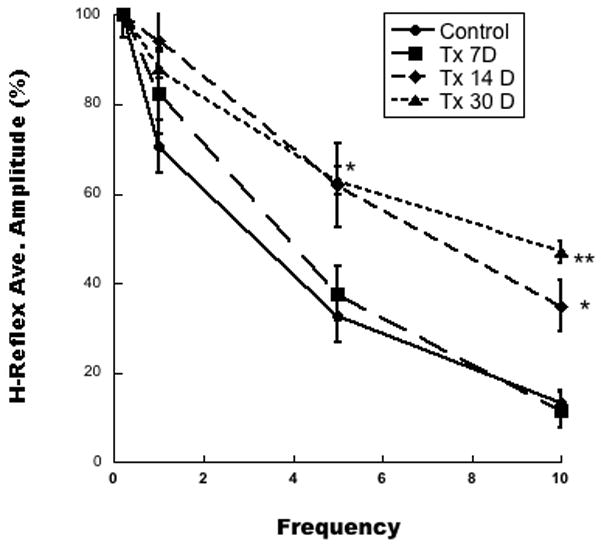
H-reflex amplitude at 0.2, 1, 5, and 10 Hz in intact (Control, filled circles), Tx 7D (squares), Tx 14 D (diamonds), and Tx 30D (triangles) groups. The Y axis shows amplitude expressed as a percent of the amplitude at 0.2 Hz designated as 100 percent, and all statistical comparisons in this figure were made against the Control group. At 5 Hz, the Tx 14D and Tx 30D groups differed from the Control group and the Tx7D (p< 0.05). At 10 Hz, the Tx 14 D group differed from the control group and Tx 7D (p<0.05), and the Tx 30 D group differed from the control and Tx7D group (p<0.01). * indicates p<0.05; ** indicates p<0.01. Note the lack of habituation of the H-reflex at 10 Hz in the Tx 14D and Tx 30D groups compared with the control group and the Tx 7D group.
Figure 4 shows that the levels of Cx-36 protein in the spinal cord decreased by about 30% 7 days after Tx (ANOVA df= 3, F= 8.57, p= 0.0002, and Scheffe post hoc comparison (p<0.01). No significant differences were observed at 14 days or 30 days after Tx (df= 2, F= 3.44, p=0.078).
Figure 4.
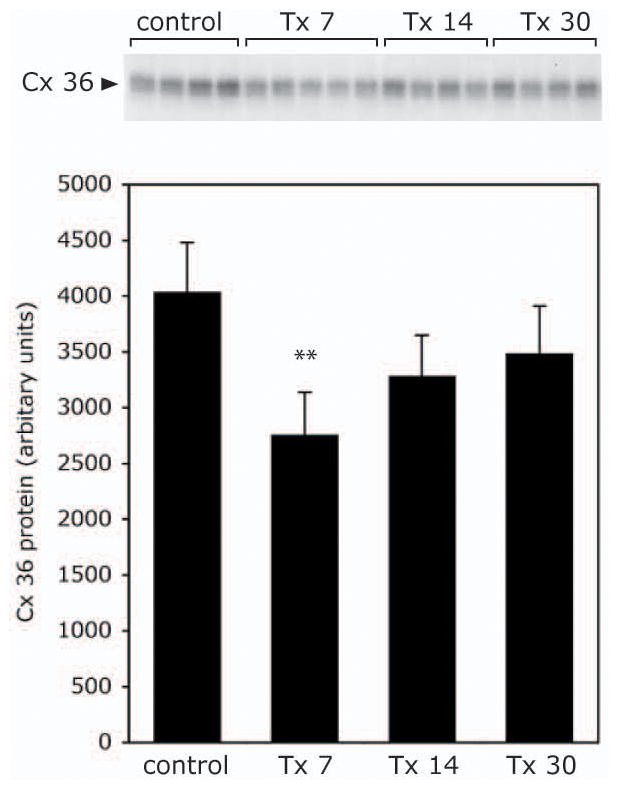
Transient decrease below the level of the lesion in Cx-36 protein levels after Tx. Upper panel, Cx-36 western blot following immunoprecipitation from spinal cord from control rats or 7, 14 or 30 days after Tx (Tx 7, Tx 14, or Tx 30). Lower panel, quantification of the data shown in the upper panel. Note that Cx-36 protein level decreased at 7 days, ** denotes p>0.01.
Discussion
Our results are consistent with our hypotheses that 1) acute (defined as 7 days post Tx) transected rats are not significantly different in frequency-dependent depression than intact rats, 2) the decrease in H-reflex frequency-dependent depression occurred between 7 and 14 days after Tx, 3) the time course observed in the Tx model is similar to that observed by others using the contusion model, and 4) that the onset of hyperreflexia follows a transient decrease in Cx-36 protein levels indicative of decreased electrical coupling.
The studies described provide evidence showing that the complete spinalized animal transitions to a state of hyperreflexia between 7 and 14 days post Tx. The H-reflex is a reliable measure of spinal circuitry that is altered after a SCI. The H-reflex has been a valuable tool to measure changes in circuitry in both contusion 9 and transection models 8,16 in rats. The H-reflex has also been used to assess changes in reflex pathways in the human. Schindler-Ivens & Shields 5 showed that 1) patients with chronic SCI showed loss of suppression of H-reflexes compared to acute subjects or control patients, and 2) there was significant loss of frequency-dependent depression in the chronic condition, which occurred ~6–18 weeks.
The finding that the spinalized animal transitions to a state of hyperreflexia between 7 and 14 days post Tx refines previous work that indicated that hyperreflexia develops 6–28 days after SCI, at least in the contusion model. The results described herein indicate that low frequency-dependent depression of the H-reflex was decreased in rats transected and assessed after 30 days, suggesting that hyperreflexia assumes a chronic level by as soon as 30 days following complete Tx. These results also indicate that the rat at 7 days post Tx does not present differently from a control animal, indicating that hyperreflexia is delayed, yet the reflex can be recorded, so that spinal circuitry does respond as in control animals. H-reflex testing results were observed in every animal in the 7 day Tx group indicating that the animal had transitioned from a state of spinal shock to the acute state by 7 days post injury. The Tx 14 D group was significantly different from the Tx 7D group, suggesting that this group had undergone changes in circuitry. However, the standard error was greater for the Tx 14D group than the other groups, suggesting that the time frame around 14 days is a transitional period with more variability.
Several mechanisms have been proposed to account for the development of hyperreflexia following SCI.3 One potential mechanism of interest is a change in synchronization of neurons, specifically, a change in electrical coupling and hence function of the gap junction protein Cx-36. The results presented in this study are consistent with those of Lee et al.15 where in situ hybridization was used to study Cx-36 mRNA expression after spinal cord transection. They reported no obvious changes of Cx-36 mRNA following spinal cord transection 4 weeks post injury, although they did report a down regulation during the first week post injury. Our study expands these results by showing that Cx-36 protein also decreased shortly after Tx. However, Lee et al. 15 concluded that Cx-36 mRNA expression only changed close to the site of injury, in contrast to our results showing a transient decrease in Cx-36 protein in the lumbar enlargement distant to the site of injury. The discrepancies between these reports may be due to the different methodologies, western blot, used here, measured Cx-36 in cored spinal cord tissue, whereas in situ hybridization used in the Lee et al. 15 study only detected Cx-36 mRNA in a thin cross section of the spinal cord.
Other studies have shown that, although there is an increase in gap junction activity (dye coupling) after nerve injury, this was not accompanied by changes in Cx-36 expression. 14 That study also used in situ hybridization to estimate Cx-36 mRNA levels. In addition, RT-PCR of lumbar spinal cord showed no obvious change in Cx-36 mRNA but this was investigated 1) 2 weeks after injury, when our results indicate that Cx-36 levels are returning to control levels in the Tx model, and 2) by PCR that is semi-quantitative and would not have detected a 30% decrease in Cx-36 mRNA. However, the major difference in that study compared to the present is that it involved transection of peripheral nerves.
The delay in the onset of hyperreflexia suggests that there are mechanisms involved other than the immediate loss of presynaptic inhibition. One potential additional mechanism is the switch in electrical coupling that may assume an abnormal state. The return of Cx-36 protein levels at 14–30 days coincides with the onset physiologic hyperreflexia observed. Perhaps the transient change observed reflects a reorganization of spinal cord circuitry as a result of the loss of descending control. It is not clear if this represents a change in the exteriorization, migration, alignment or opening/closing processes of Cx-36 hemichannels. Additional work will need to address the complex metabolic pathways involved in gap junction function.
At birth, motoneurons in the rat spinal cord are dye coupled, electrically coupled and can produce synchronized motor rhythms in the absence of chemical synaptic activity.13,19 Studies of the developing chick cord showed that spinal networks experience transient increases in synaptic activity, presumably arising from the coordinated firing of clusters of neurons, and that these events can trigger synchronized network activity.19 The ensemble activity of these networks allows the chick to synchronize motor activity (co-contractions) in order to break out of the shell, and then changes in organization allow the chick to manifest bipedal (alternating) locomotion while maintaining the ability to co-contract.17 Gap junctions are required for synchronizing the oscillatory responses of neurons and for clustering of coherent rhythmic activity. What functional significance can be accorded to electrical coupling in the spinal cord if Cx36 KO mouse, detailed analysis of motor patterns showed a10–20 ms degradation in coordination, 20 and a delay of more than 20 ms in the optokinetic reflex. 21 These studies taken together suggest that gap junctions confer an advantage in timing, probably due to their ability to promote coherence in brain rhythms. Studies have also shown that following midthoracic spinal cord Tx in the cat, electromyographic recordings following stimulation to spinal cord segments revealed decreased synchronization of muscle activity with reduced burst duration in transected animals compared with control animals. 22 This suggests that post Tx electromyographics suffer from synchronization. We speculate that Tx induces an initial loss of coupling at 7 days that is followed by resurgence of coupling at approximately 14 days but, perhaps, because descending modulation is lacking, then leads to inefficient or abnormal synchronization.
A recent landmark study described the presence of electrical coupling in locomotor-related interneurons in the spinal cord23, suggesting that this mechanism is not only present in motoneurons but also interneurons. Perhaps the changes in gap junction protein Cx-36 after SCI reflect changes observed in synchronization of firing of motoneurons and/or interneurons and lead to the hyperreflexia observed. This is further supported by the preliminary evidence showing that the stimulant modafinil, which has been shown to increase electrical coupling, 24 leads to the prevention of hyperreflexia in the transected rat if administered starting 7 days after Tx.25 These results suggest that increasing electrical coupling helps prevent hyperreflexia, although we do not know if such treatment can rescue from hyperreflexia once it has set in. Our results suggest that the role of gap junctions and electrical coupling in SCI should be explored further, especially during the transition to hyperreflexia, to define the locus and characteristics of these changes in coupling after SCI.
Acknowledgments
NIH Grant RR020146 to the Center for Translational Neuroscience
References
- 1.Dietz V. Locomotor recovery after spinal cord injury. Trends Neurosci. 1997;20:346–347. doi: 10.1016/s0166-2236(97)89934-1. [DOI] [PubMed] [Google Scholar]
- 2.Little JW, Ditunno JF, Stiens SA, Harris RM. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch Phys Med Rehab. 1999;80:587–99. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- 3.Hultborn H. Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Progress in Neurobiology. 2006;78:215–232. doi: 10.1016/j.pneurobio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence That Alterations in Presynaptic Inhibition Contribute to Segmental Hypo- and Hyperexcitability after Spinal Cord Injury in Man. Electroencephalogr Clin Neurophysiol. 1993;89:177–86. doi: 10.1016/0168-5597(93)90131-8. [DOI] [PubMed] [Google Scholar]
- 5.Schindler-Ivens S, Shields RK. Low Frequency Depression of H-Reflexes in Humans with Acute and Chronic Spinal-Cord Injury. Exp Brain Res. 2000;133:233–41. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd DP, Wilson VJ. Reflex Depression in Rhythmically Active Monosynaptic Reflex Pathways. J Gen Physiol. 1957;40:409–26. doi: 10.1085/jgp.40.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skinner RD, Houle JD, Reese NB, Berry CL, Garcia-Rill E. Effects of exercise and fetal spinal cord implants on the H-Reflex in chronically spinalized adult rats. Brain Res. 1996;729:127–31. [PubMed] [Google Scholar]
- 9.Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered Patterns of Reflex Excitability Subsequent to Contusion Injury of the Rat Spinal Cord. J Neurophysiol. 1992;68:1473–86. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- 10.Malmsten J. Time Course of Segmental Reflex Changes after Chronic Spinal Cord Hemisection in the Rat. Acta Physiol Scand. 1983;119:435–43. doi: 10.1111/j.1748-1716.1983.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 11.Leis AA, Zhou HH, Mehta M, Harkey HL, 3rd, Paske WC. Behavior of the H-Reflex in Humans Following Mechanical Perturbation or Injury to Rostral Spinal Cord. Muscle Nerve. 1996;19:1373–82. doi: 10.1002/(SICI)1097-4598(199611)19:11<1373::AID-MUS1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J Neurosci. 1999;19:10813–28. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton KD, Navarrete R. Postnatal Changes in Motoneurone Electronic Coupling Studied in the in Vitro Rat Lumbar Spinal Cord. J Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ. Nerve Injury Induces Gap Junctional Coupling among Axotomized Adult Motor Neurons. J Neurosci. 2000;20:674–84. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L. Glial and Neuronal Expression Patterns in the Rat Spinal Cord During Development and Following Injury. J Comp Neurol. 2005;489:1–10. doi: 10.1002/cne.20567. [DOI] [PubMed] [Google Scholar]
- 16.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, Garcia-Rill E. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord. 2006;44:28–34. doi: 10.1038/sj.sc.3101810. [DOI] [PubMed] [Google Scholar]
- 17.Bradley NS. Animal models offer the opportunity to acquire a new perspective on motor development. Phys Ther. 1990;70:776–787. doi: 10.1093/ptj/70.12.776. [DOI] [PubMed] [Google Scholar]
- 18.Heister D, Hayar A, Charlesworth A, Yates C, Zhou YH, Garcia-Rill E. Evidence for electrical coupling in the SubCoeruleus(SubC) nucleus. J of Neurophysiol. 2007 Apr;97(4):3142–7. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenner P, O’Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J of Neurophysiol. 2001 Sept;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- 20.Placatonakis DG, Bukovsky AA, Zeng ZH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Nat Acad Sci USA. 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kistler WM, De Jeu MT, Elgersma Y, Van Der Giesses RS, Hensbrock R, Lue C, et al. Analysis of Cx36 knockout does not support tenet that olivary gap junctions are required for complex spike synchronization and normal notor performance. Ann NY Acad Sci. 2002;978:391–404. doi: 10.1111/j.1749-6632.2002.tb07582.x. [DOI] [PubMed] [Google Scholar]
- 22.Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull. 1991;28:99–105. doi: 10.1016/0361-9230(92)90235-p. [DOI] [PubMed] [Google Scholar]
- 23.Hinckley CA, Ziskind-Conhaim L. Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J of Neurosci. 2006;26:8477–8483. doi: 10.1523/JNEUROSCI.0395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garica-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yates C, Reese N, Kiser T, Skinner RD, Garcia-Rill E. Modafinil (MOD) normalizes hyperreflexia induced by spinal cord transection in the rat. Neurosci Abst. 2007;33:405.21. [Google Scholar]


