Abstract
Purpose
Too investigate asymmetry in eyelid movements with blinking, the stability of the asymmetry, and its modifiability in normal humans.
Methods
Differences in the start time and amplitude between the two eyelids were assessed for voluntary blinks and reflex blinks evoked by supraorbital trigeminal nerve stimulation. These variables were also measured before and up to 18 months after 2 hours of unilateral upper lid restraint.
Results
With voluntary blinks, one eyelid consistently began to close earlier and made a larger eyelid movement than the other eyelid. Stimulation of the supraorbital branch of the trigeminal nerve evoked relatively larger amplitude blinks in one eyelid that correlated with the asymmetries of voluntary blinks. There was a continuum of eyelid asymmetry across all subjects that was stable and independent of other biological asymmetries, such as handedness. Briefly reducing eyelid mobility created a long-lasting change in eyelid asymmetry with blinking.
Conclusions
Eyelid asymmetry results from differences in the excitability of motoneurons in the left and right facial motor nuclei and does not appear to involve asymmetries in cortical inputs to the brain stem. Because adaptive processes modify the motoneuron excitability that creates eyelid asymmetry, these processes may underlie changes in blinking associated with facial palsy and may play a role in the development of disorders that affect one side of the face, such as hemifacial spasm.
Humans exhibit several motor, sensory, and functional asymmetries in which one side of the body is dominant or more responsive than the other. The most evident motor asymmetry is handedness, with people typically being either right or left handed. In addition to this well-known asymmetry, human facial expressions of happiness and sadness produce larger movements of the left than the right side of the face.1,2 The accepted explanation of this facial motor asymmetry is specialization of the right cerebral hemisphere for emotional expression.3 These observations suggest that right cortical dominance over facial muscles should produce larger movements of the left than of the right eyelid with voluntary blinking.
Clinical studies suggest that differences in excitability between motoneurons in the left and right facial motor nuclei may create asymmetries for reflex blinking. Facial palsy patients exhibit increased motoneuron excitability in the facial nucleus on the affected relative to the unaffected side of the face.4–6 One explanation for this increased excitability in Bell’s palsy is changes in the motoneuron membrane properties caused by axotomy. It is also possible that the increased excitability involves an adaptive increase in excitatory presynaptic drive to facial motoneurons,7 to compensate for muscle weakness. Consistent with this explanation, mimicking facial palsy with unilateral eyelid restraint in normal humans appears to increase motoneuron excitability in the restrained eyelid in the absence of axotomy.8
Our study determined whether eyelid asymmetry exists in normal individuals and investigated whether eyelid asymmetries result from cortical or brain stem mechanisms. Assessing the timing and amplitude of left and right eyelid movement with cortically controlled voluntary blinks and brain stem–generated trigeminal reflex blinks demonstrates the presence of functional eyelid asymmetry. Comparison of the eyelid asymmetry present in voluntary and reflex blinks showed that a difference in motoneuron excitability is the primary source of eyelid asymmetry in both voluntary and reflex blinks. We also showed that eyelid asymmetry is a modifiable property of the eyelid motor system.
Methods
Subjects
All experiments adhered to the tenets of the Declaration of Helsinki. Informed consent from the participants and prior institutional review board approval were obtained. The 18 subjects, 10 men and 8 women, ranging from 22 to 59 (mean, 29 ± 10) years of age, did not have any history of medications or neurologic, eye, or eyelid disorders that would affect blinking.
Procedures
Upper eyelid position was monitored bilaterally with the magnetic search coil technique using a 30-turn, 2-mm diameter coil attached to the middle of the upper eyelid as close as possible to its margin.9 The system reliably measured eyelid rotations of less than 0.5°, equivalent to 100 µm of linear movement.9
Unilateral electrical stimulation of the supraorbital branch of the trigeminal nerve (SO) evoked bilateral trigeminal reflex blinks. To elicit these reflex blinks, a pair of 9-mm diameter gold-plated electrodes (Grass-Telefactor, West Warwick, RI) was placed over both the left and right SO. One electrode of the pair was placed directly over the supraorbital notch and the second approximately 2.5 cm above the first. The SO stimulus was a 170-µs constant current delivered at twice the threshold current (2T) necessary to evoke a blink consistently when stimuli occurred with at least a 20-second interstimulus interval. Across all subjects, 2T current intensity ranged from 2.8 to 8 mA. In four of the eighteen subjects, the effect of changing SO stimulus intensity on eyelid asymmetry was also tested. For these subjects, the perception threshold (PT), the lowest SO stimulus intensity at which the subject consistently reported perceiving SO stimulation, was determined. The subjects then received SO stimuli ranging from twice to six times perception threshold (2PT–6PT). Four times perception threshold is roughly equivalent to the twice blink threshold stimulus intensity used for all the other subjects.10 We independently determined threshold for the left and right SO. In addition to reflex blinks, all subjects were instructed to make voluntary blinks when they heard a tone. All subjects participated in experiments using SO stimuli, but only a subset of the subjects made voluntary blinks in addition to the reflex blinks.
Experimental Design
The experiments tested whether one eyelid began closing before the other and whether one eyelid made larger amplitude blinks than the other. The experiments were conducted as follows: (1) Eleven subjects were asked to make voluntary blinks when they heard a 1-kHz tone. Tones were presented every 4 ± 2 seconds. To identify asymmetries in the initiation of eyelid closure, the latency of the onset of right eyelid closure was subtracted from the latency of right eyelid closure for every blink. If the left eyelid began closing before the right eyelid, this value was negative. A difference in the amplitudes of the voluntary blinks between the two eyelids was quantified by subtracting the amplitude of the right eyelid’s blink from the amplitude of the left eyelid’s blink. Because we defined eyelid lowering as a negative value, when the left eyelid made a larger blink than the right, the value was negative. (2) SO-evoked blinks were elicited in 18 subjects, 11 of whom also participated in the voluntary blink experiment, by stimulating the right and left SO. SO stimuli were presented every 25 ± 5 seconds and alternated between the left and right SO electrodes. To quantify asymmetry of reflex blink amplitude, we calculated the ratio of eyelid amplitude with the eyelid contralateral to the stimulus as the numerator and the ipsilateral eyelid as the denominator (e.g., left SO-evoked blinks were quantified as right eyelid amplitude/left eyelid amplitude). If the blink made by the left eyelid was relatively larger than the lid movement generated by the right eyelid, then the value of left SO stimulation right eyelid/left eyelid ratio (R/LLSO) minus the right SO left eyelid/right eyelid ratio (L/RRSO) was negative. (3) To four of the 18 subjects, we presented the SO stimulus at intensities from 2PT to 6PT to test the effect of SO stimulus intensity on eyelid amplitude asymmetry. (4) To identify asymmetries caused by experimental error, 4 of the 18 subjects were tested two or more times by different experimenters using different experimental setups. (5) We reanalyzed the data of Schicatano et al.,8 to determine whether unilateral eyelid restraint modified eyelid asymmetry. In addition, we determined reflex blink amplitude in four of the five subjects in that study, to characterize the duration of the changes in eyelid asymmetry produced by 2 hours of eyelid restraint. (6) We established other asymmetries in these subjects, such as handedness, eye dominance, and the ability to wink one or both eyelids.
Data Collection and Analysis
Eyelid position of both eyelids was digitized at 2 kHz/channel (data translation, 12-bit accuracy) and stored for off-line analysis. Laboratory-written software was used to calculate blink duration, amplitude, and peak velocity. The computer program established the start of the blink as the time at which eyelid velocity achieved 7.5% of the Vmax attained during eyelid closure. Blink duration was defined as the time from blink start until the eyelid reached maximum eyelid closure. Blink amplitude was the difference between eyelid position at blink start and maximum eyelid closure. Peak velocity was the Vmax achieved during eyelid lowering. All results are presented as the mean ± SD. Statistical significance was determined with paired samples t-test, accepting P ≤ 0.05 as significant.
Results
Characteristics of Eyelid Asymmetry
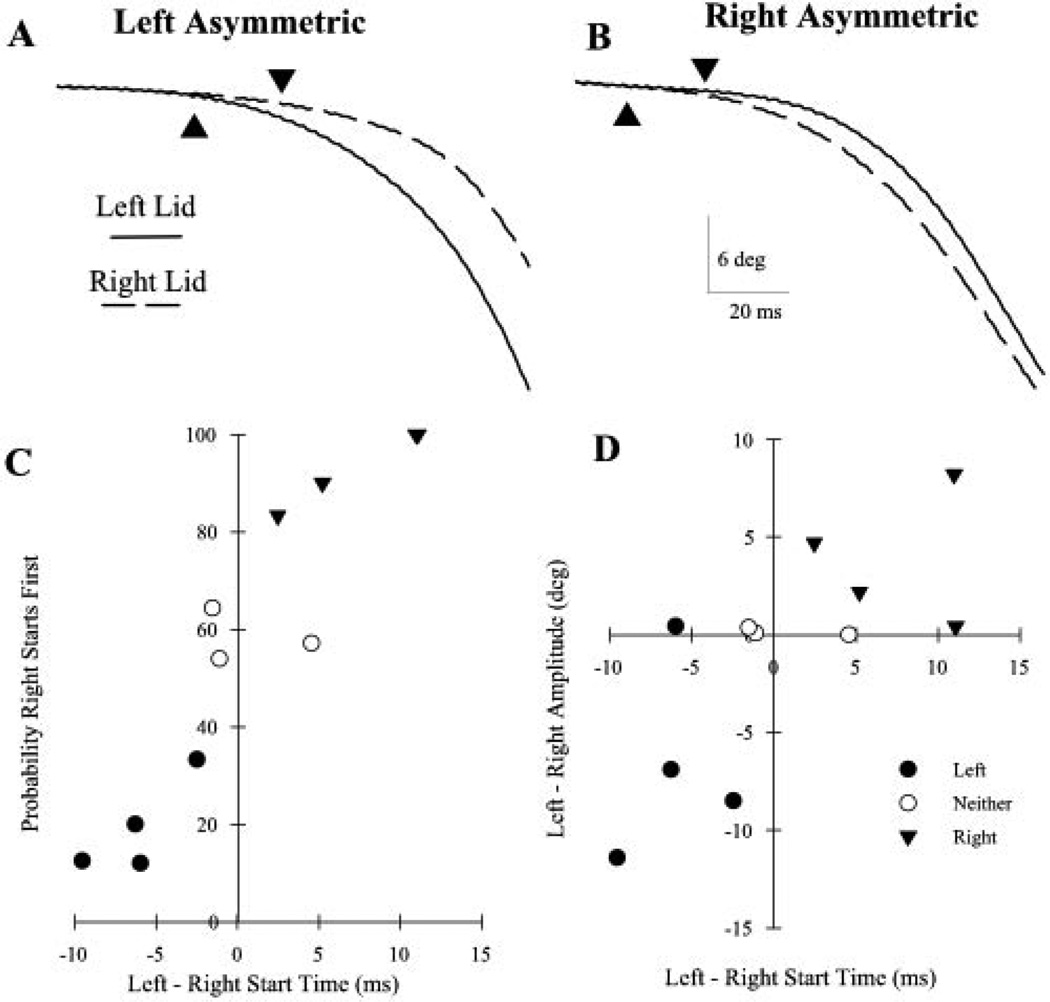
If one pool of orbicularis oculi (OO) facial motoneurons was more excitable than the other, then the eyelid innervated by this pool of motoneurons should begin closing first because its motoneurons would reach threshold before those in the other pool. As predicted, one eyelid consistently had a shorter latency for the initiation of voluntary blinks in most of the 11 subjects (Fig. 1). In four (36%), the left eyelid began closing significantly earlier than the right (Fig. 1A; 5.7 ms). The right eyelid began closing before the left in only 19.5% of the trials (Fig. 1C; filled circles). We called these subjects “left asymmetric.” In four (36%), the right eyelid began closing before the left (Fig. 1B; 5.3 ms; “right asymmetric”) in a significant (93.3%) number of the trials (Fig. 1C; inverted filled triangle). In the remaining three (23%), the difference between the blink start times for the two eyelids was not significantly different (0.63 ms). In these subjects, the right eye began closing before the left in approximately half of the trials (58.4%; Fig. 1C; open circles). In all subjects, the difference between mean left and right eyelid start times correlated with the probability of the left eyelid’s starting to blink before the right eyelid (Fig. 1C; Spearman rank correlation, r = 0.93, P < 0.01).
Figure 1.
Voluntary blinks exhibit asymmetrical start times and amplitudes. (A) The left eyelid (solid line) began closing before the right eyelid (dashed line) when a left asymmetric subject made a voluntary blink. (B) The right eyelid began closing before the left eyelid when a right asymmetric subject made a voluntary blink. Each pair of traces in (A) and (B) is a single voluntary blink. Triangles: start of the blink, as determined by the computer program. (C) The probability that the right eyelid began moving before the left eyelid plotted as a function of the difference in the start times for the two eyelids in 11 subjects. Each point is the mean result of at least six trials. There are two subjects with an 11-ms difference in start times for the two eyelids. (D) The mean difference in voluntary blink amplitude of the two eyelids plotted as a function of the difference in the start times for the two eyelids for the same 11 subjects in (C). If the left eyelid made a larger amplitude blink than the right eyelid, the difference was negative. Each point is the average of results in at least six trials.
If one pool of OO motoneurons is more excitable than the other, then voluntary blinking should also produce unequal-amplitude blinks (Fig. 1D). As predicted, three of the four left asymmetric subjects made larger blinks with their left than with their right eyelids (−6.6°, filled circles). In the four right asymmetric subjects, the right eyelid made larger blinks than the left eyelid (3.9°, inverted filled triangles). In contrast, there was no relationship between blink amplitude and eyelid start times in the subjects who did not show a significant difference in the start times of the two eyelids (−0.15°, open circles). Unlike the left bias of facial asymmetry in expression,1,2 there was a continuum of eyelid asymmetries based on the differences in start time and amplitude between the two eyelids with voluntary blinks.
The interpretation that these data reflect differences in motoneuron excitability rests on the assumption of equal cortical drive to the left and right facial nuclei, to initiate voluntary blinks. The differences in start time and blink amplitude of voluntary blinks could have resulted from an asymmetry in the cortical inputs to the left and right pools of OO motoneurons. We further tested the existence of unequal facial motoneuron excitability by using the brain stem–driven trigeminal reflex blink, which does not engage cortical inputs to facial motoneurons. If one pool of OO motoneurons is more excitable than the other, then the trigeminal input should recruit more OO motoneurons in the more excitable facial motor nucleus than in the contralateral nucleus. Activation of more motoneurons should produce a larger blink in the eyelid innervated by the more excitable motoneurons, and this amplitude difference should be independent of any asymmetries in the trigeminal input to the left and right pools of motoneurons.
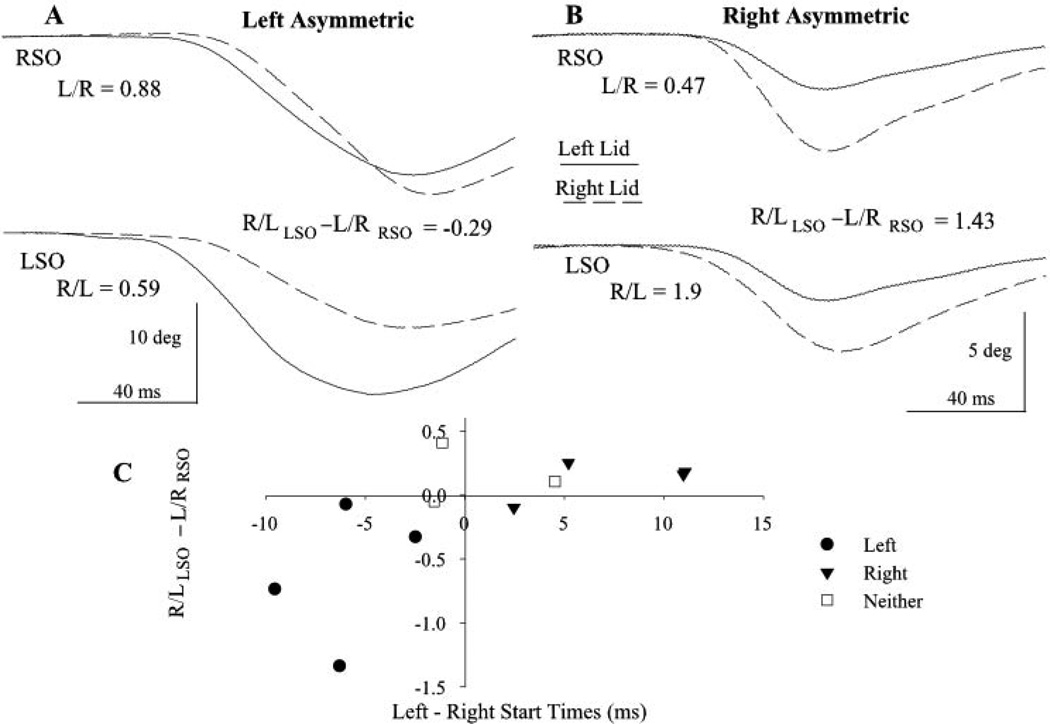
Similar to voluntary blinks, SO-evoked blinks also exhibited eyelid asymmetries (Fig. 2). In some subjects, one eyelid made a larger eyelid closure regardless of which side was presented the SO stimulus (Fig. 2B). In these subjects, one pool of OO motoneurons was clearly more excitable than the other. In most subjects, however, the asymmetry was more subtle because an SO stimulus typically evoked a larger movement in the eyelid ipsilateral to the stimulus than in the contralateral eyelid (Fig. 2A). Nevertheless, in individuals in which the blink in the eyelid ipsilateral to SO stimulation was larger than the blink in the contralateral lid, one eyelid consistently exhibited a relatively larger blink. To quantify eyelid asymmetry from reflex blink amplitude, we calculated the ratio of eyelid amplitude with the eyelid contralateral to the stimulus as the numerator and the ipsilateral eyelid as the denominator (e.g., left SO-evoked blinks were quantified as right eyelid amplitude/left eyelid amplitude; Fig. 2A; R/L). If the blink made by the right eyelid was relatively larger than that evoked in the left eyelid, then the value of left SO stimulation right eyelid/left eyelid ratio minus the right SO left eyelid/right eyelid ratio (R/LLSO − L/RRSO) was positive (Fig. 2B). As occurred with voluntary blinks, comparing relative trigeminal reflex blink amplitude for the two eyelids revealed a continuum of eyelid asymmetry. In four subjects, the SO evoked a relatively larger amplitude blink in the left than in the right eyelid (−0.74 ± 0.42). Eight subjects generated a relatively larger right than left eyelid movement (0.36 ± 0.21), and six subjects did not exhibit a significant amplitude difference between the eyelids (0.001 ± 0.09).
Figure 2.
Movement of the two eyelids with trigeminal reflex blinks. (A) The left eyelid movement (solid line) was relatively larger than right eyelid movement (dashed line) in response to left (LSO) and right (RSO) supraorbital nerve stimulation in a left asymmetric subject. (B) The right eyelid movement was always larger than that of the left eyelid in response to SO stimulation for a right asymmetric subject. Each pair of traces is a single blink evoked by left SO (top traces) or right SO (bottom traces) for two subjects. The R/LLSO − L/RRSO values are for the illustrated trials. (C) Relative blink amplitude (R/LLSO − L/RRSO) for each subject plotted as a function of the mean difference in start time of voluntary blinks between the two eyelids. R/L and L/R are the ratios of blink amplitude for the left (L) and right (R) upper eyelid evoked by LSO and RSO stimuli, respectively. There are two subjects with an 11-ms difference in start times for the two eyelids. Each point is the mean of at least 20 trials.
The degree of eyelid asymmetry evinced by differences in voluntary blink start times correlated with the relative SO reflex blink amplitude for the 11 subjects tested in both paradigms (Fig. 2C; Spearman rank correlation r = 0.74, P < 0.01). The larger difference between the onsets of eyelid movement of the two eyelids with voluntary blinks was associated with a similar difference in relative blink magnitude with SO stimulation.
Overall, eyelid asymmetry was independent of other motor and sensory asymmetries. Three of the eight subjects who were right handed exhibited larger and shorter latency left eyelid movements. One of the two left-handed subjects made larger right eyelid movements. The eye used for visual sighting, a measure of eye dominance,11 also failed to predict eyelid asymmetry. The more excitable pool of OO motoneurons was opposite the dominant eye in 6 of 12 subjects tested. The ability of people to wink only one eyelid also failed to correlate with eyelid asymmetry. In 5 of 14 subjects who could wink only one eyelid, blinks were larger in the eyelid opposite to the eyelid they could wink.
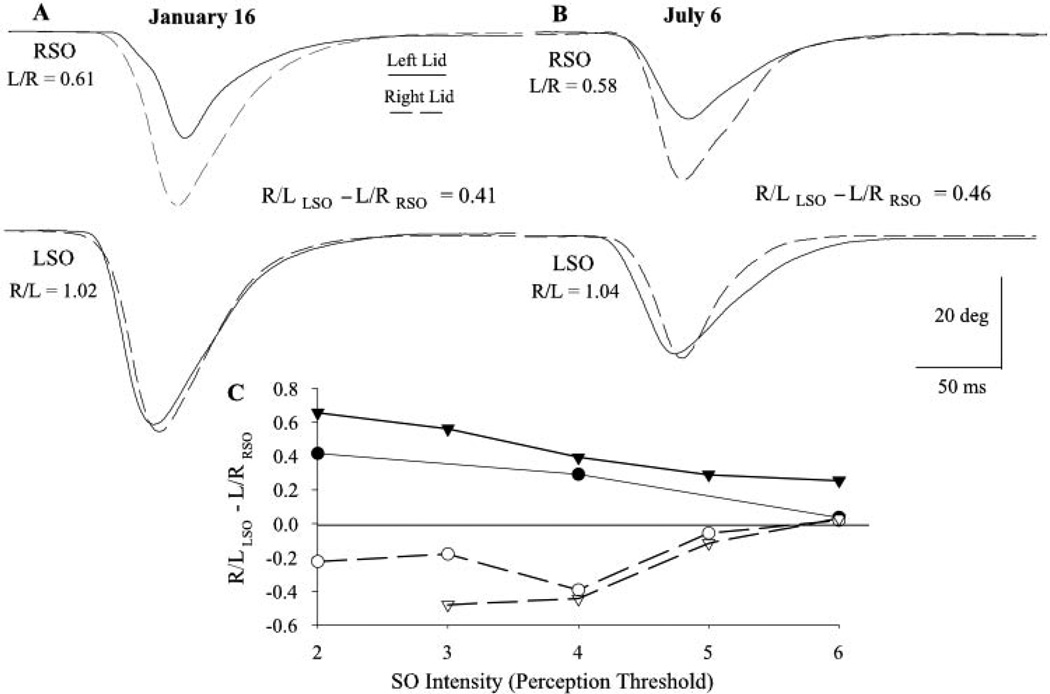
The lack of correlation between eyelid asymmetry and other motor and sensory asymmetries may have indicated that eyelid asymmetry results from unintended irregularities in the experimental setup. To assess this possibility, different experimenters tested four subjects repeatedly in different laboratory setups over a 15-month period. Eyelid asymmetry was stable in all four subjects. There were no significant changes in eyelid asymmetry across experiments determined from the start times of voluntary blinks and the relative blink amplitude of SO-evoked blinks. The change in the start time of the left and right eyelid with voluntary blinks was less than 3 ms for each subject and averaged only 0.64 ± 2.23 ms for all subjects and experiments. The average change in relative SO reflex blink amplitude between experiments was 0.11 ± 0.37 for all subjects and experiments. For example, the R/LLSO − L/RRSO relative amplitude value in the subject illustrated in Figures 3A and 3B changed by only 0.05 over a 6-month period.
Figure 3.
Eyelid asymmetry is stable over time and stimulus conditions. The relative eyelid amplitude of blinks evoked by stimulation of the right SO (RSO, top traces) and left SO (LSO, bottom traces) for this subject is the same when measured on January 16 (A) and 6 months later, on July 6 (B). Each pair of traces represents the left eyelid (solid line) and right eyelid (dashed line) movement evoked on the same trial. R/L and L/R are the mean ratios of blink amplitude evoked by left and right SO stimulation, respectively. (C) Relative blink amplitude (R/LLSO − L/RRSO) for two right asymmetric subjects (filled symbols) and two left asymmetric subjects (open symbols) plotted as a function of SO stimulus intensity relative to perception threshold. Each point is the mean result of at least five trials.
In the four subjects tested, eyelid asymmetry did not reverse with increasing SO stimulation intensities (Fig. 3C). Consistent with the hypothesis that differences in OO motoneuron excitability created eyelid asymmetry, increasing stimulus intensity reduced the degree of asymmetry. The lowest stimulus intensity should have produced the highest level of eyelid asymmetry because the more excitable pool of OO motoneurons should have had more active motoneurons than the less responsive contralateral nucleus. At the highest intensity stimulus, however, most of the OO motoneurons in both nuclei should have been recruited, thereby masking any asymmetry in OO excitability created by differences in motoneuron excitability between the left and right facial nuclei. The stability of eyelid asymmetry implied that the nervous system maintained a stable difference in motoneuron excitability between the left and right OO motoneurons.
Modifying Eyelid Asymmetry
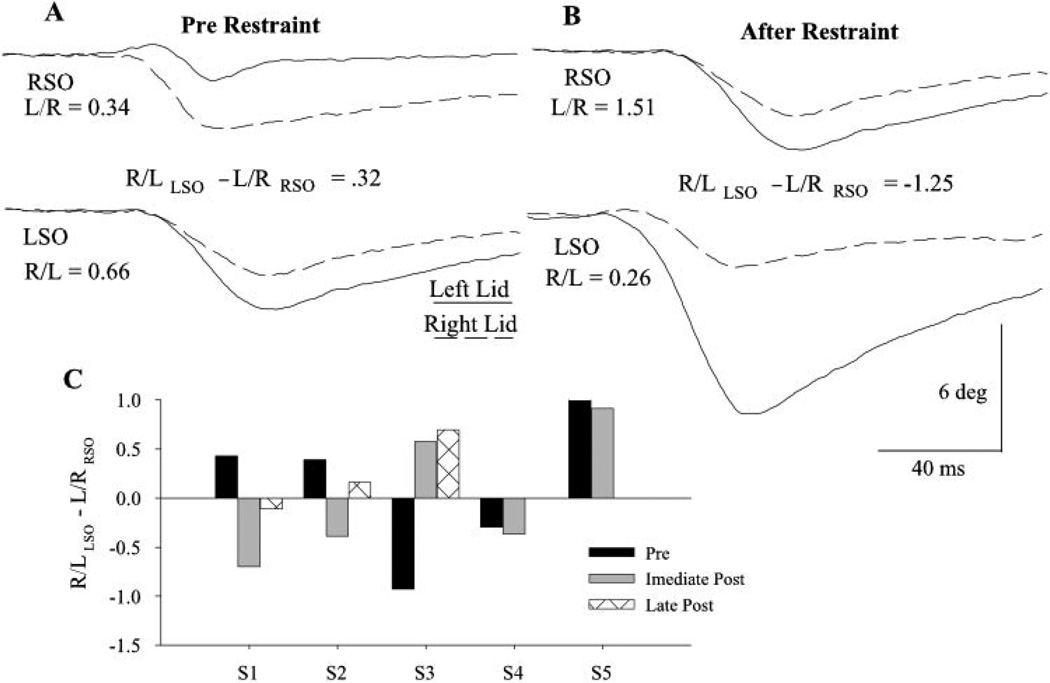
In a previous study, Schicatano et al.8 demonstrated that restraining one eyelid for only 2 hours increased the excitability of OO motoneurons and trigeminal blink circuits ipsilateral to the restrained eyelid in normal subjects. If an excitability difference between the left and right OO motoneurons was the basis of eyelid asymmetry, then eyelid restraint should have modified this asymmetry. We reanalyzed the Schicatano et al.8 data using our relative blink amplitude measure to determine whether upper eyelid restraint modified eyelid asymmetry. Before eyelid restraint, SO stimulation revealed that three of the five subjects exhibited right eyelid asymmetry (Figs. 4A, 4C; S1, S2, S5) and the other two showed left asymmetry (Fig. 4C; S3, S4). Restraining the upper eyelid innervated by the less excitable OO motoneurons for 2 hours modified eyelid asymmetry. For example, before left eyelid restraint, subject 1 exhibited a mean eyelid amplitude ratio of 0.43 (Fig. 4A; 4C, S1) indicating a right eyelid asymmetry. After left eyelid restraint, the mean SO eyelid amplitude ratio converted to −0.7, a left eyelid asymmetry (Fig. 4B, 4C, S1). Similarly, subject 2 switched from right eyelid to left eyelid asymmetry after left eyelid restraint (Fig. 4C, S2). Extremely right eyelid asymmetric subject 5 exhibited reduced right eyelid asymmetry after left eyelid restraint (Fig. 4C, S5). Reversing eyelid asymmetry was not specific to left eyelid restraint. After right eyelid restraint, subject 3 converted from left eyelid to right eyelid asymmetry (Fig. 4C, S3). Also consistent with the hypothesis that the nervous system adaptively increased the excitability of the motoneurons innervating the restrained eyelid, restraint of the left eyelid slightly increased left eyelid asymmetry in subject 4 (Fig. 4C, S4).
Figure 4.
Unilateral upper eyelid restraint produced long-lasting changes in eyelid asymmetry. The relative eyelid amplitude of blinks evoked by stimulation of the right SO (RSO, top traces) and left SO (LSO, bottom traces) before (A) and immediately after 2 hours of left upper eyelid restraint (B) converted subject 1 from right to left asymmetric. Each pair of traces is the left (solid line) and right eyelid (dashed line) movement evoked on the same trial. The R/LLSO − L/RRSO values are for the illustrated trials. (C) The relative blink amplitude of five subjects (S1–5) before and immediately after 2 hours of unilateral eyelid restraint and the relative eyelid amplitude measured 18 months (S1), 8 days (S2), and 13 months (S3) after the eyelid restraint experiment are shown. For subjects 1, 2, 4, and 5, the left eyelid was restrained. Subject 3s right eyelid was restrained. Each bar is the mean of at least nine trials.
Because the lid asymmetry calculation was a ratio of eyelid amplitudes, an increase in the blink amplitude of the previously restrained eyelid, a decrease in blink amplitude by the unrestrained eyelid, or a combination of the two factors could have reversed eyelid asymmetry. Over all subjects, blink amplitude evoked in the previously restrained eyelid increased an average of 52%, whereas the unrestrained eyelid exhibited only an 8% increase relative to blink amplitude before restraint.8 Thus, the data revealed increased excitability only for the motoneurons innervating the previously restrained eyelid. This result demonstrated that the excitability of facial motoneurons created eyelid asymmetry and that decreased eyelid motility modified this property.
Modification of eyelid asymmetry produced by 2 hours of eyelid restraint was long-lasting. Subject 1 remained left eyelid asymmetric 18 months after restraint (Fig. 4C, S1). Subject 2 returned to being right asymmetric 8 days after restraint, but was less so than before restraint (Fig. 4C, S2). In subject 3, right eyelid asymmetry continued for 13 months after restraint, but when tested 3 years later, the subject had returned to left eyelid asymmetry (Fig. 4C, S3). Thus, only 2 hours of eyelid restraint produced long-lasting excitability changes between the left and right pools of OO motoneurons.
Discussion
Eyelid Asymmetry and OO Motoneuron Excitability
A disparity in the excitability of the two pools of OO motoneurons appears to create an asymmetry in blinking independent of cortical influences. Normal humans exhibit differences in the start times of voluntary blinks, when one eyelid consistently starts closing before the other (Fig. 1) and display correlated amplitude differences in which eyelid makes a relatively larger eyelid movement for voluntary (Fig. 1D) and reflex blinks (Fig. 2). Although interpretations other than differences in motoneuron excitability could explain some of these data, a disparity in the responsiveness of motoneurons in the left and right facial nuclei to blink-evoking inputs emerges as the simplest interpretation of all these data.
An alternate explanation is that the cortical input to the left and right facial nuclei is asymmetric.1–3 Although different strengths of descending cortical inputs to facial motoneurons can explain the voluntary blink data, an unequal phasic cortical input cannot explain the disparity in relative SO-evoked blink amplitude. Because the trigeminal reflex blink pathway is a brain stem reflex that does not rely on cortical inputs, disparities in phasic cortical inputs cannot play a role in reflex blink amplitude differences. Thus, the correlation between voluntary blink start time and relative SO-evoked blink amplitude (Fig. 2C) implies that these eyelid asymmetries share a common basis independent of the cortex. The decrease in eyelid asymmetry with increasing trigeminal stimulation intensity is also consistent with disparities in motoneuron excitability between the left and right facial nuclei (Fig. 3C) rather than asymmetries in cortical inputs. As increased synaptic input to the facial nucleus recruits more and more of the motoneuron pool, differences in blink amplitude created by unequal excitability of OO motoneuron pools disappears. Therefore, a difference in OO motoneuron excitability between the two facial motor nuclei is a better explanation of the data than phasic asymmetries in cortical drive.
Another possible explanation of the eyelid asymmetries observed in our study is interexperimental variation. If asymmetry causes such variation, then repeated testing of the same subjects by different experimenters using different equipment should produce different degrees or directions of eyelid asymmetry in each experiment. The data do not support this possibility. There was almost no quantitative change in eyelid asymmetry over several months (Figs. 3A, 3B). Likewise, differing estimates of SO threshold intensities between sides or experiments are not a likely cause of eyelid asymmetry, because large changes in stimulus intensity do not reverse eyelid asymmetry. Thus, a stable difference in OO motoneuron excitability between the left and right motor nuclei is a better explanation of these data than is discrepancy in experimental setup.
Neural Mechanisms for Modifying OO Motoneuron Excitability
As an essential component of adaptation to upper eyelid restraint,12 the cerebellum is a candidate to create eyelid asymmetry. Considerable evidence indicates that the interpositus nucleus modulates trigeminal reflex blinks13–17 by increasing facial motoneuron activity18 via the red nucleus.19–21 Consistent with a role for the cerebellum in altering motoneuron excitability, blink-related interpositus neurons increase their tonic firing frequency in response to upper eyelid restraint.22 Thus, the interpositus can elevate the excitability of OO motoneurons by increasing tonic input from the red nucleus to the OO motoneurons in one facial nucleus.
The long duration of eyelid asymmetry modifications (Fig. 4C) may result from a change in the expression profile of channels or receptors between the OO motoneurons in the two facial nuclei. For example, facial motoneurons upregulate the sodium channel Nav1.3/brain type III after axotomy.23–25 This upregulation may be a response to axotomy or to a perceived muscle weakness such as occurs with temporary eyelid restraint. Because the cerebellum is essential for the adaptation initiated by upper eyelid restraint,12 it is possible that the modified cerebellar output with eyelid restraint initiates changes in the channel expression of OO motoneurons that extends the duration of this adaptive change.
Adaptive Modification of OO Motoneuron Excitability
Despite the stability of eyelid asymmetry under normal circumstances, a brief reduction in the motility of the eyelid innervated by the less excitable population of OO motoneurons causes a long-lasting shift in eyelid asymmetry (Fig. 4C). After only 2 hours of restraint, the previously restrained eyelid makes relatively larger blinks than the contralateral eyelid for periods lasting from 8 days to 18 months. This observation suggests that the nervous system adaptively adjusts motoneuron excitability in response to perceived muscle weakness to maintain blink amplitude in the face of changes in OO muscle strength. This adaptive modification is unlikely to result from tonic asymmetric cortical inputs, because eyelid restraint produces these changes in decerebrate animals.26
The present study reveals that adaptive modification to eyelid restraint involves two mechanisms with distinct temporal characteristics. As measured with the paired stimulus paradigm, trigeminal nucleus excitability increases during restraint and returns to normal within less than 1 hour after returning normal motility to the eyelid. This change in trigeminal excitability appears to follow the rise and fall of cornea irritation with upper eyelid restraint.8 In contrast to trigeminal excitability, the increased excitability of the OO motoneurons in one facial nucleus lasts from days to months (Fig. 4C), even though normal eyelid motility returns immediately with elimination of eyelid restraint. The difference in the duration of increased trigeminal and motoneuron excitability may reflect the stimuli bringing forth the two forms of adaptation. Cornea irritation, the error stimulus driving the increase in trigeminal excitability, can resolve quickly. For example, cornea irritation is no longer perceptible within minutes of removing a foreign object from the eye. Thus, there is no reason to maintain elevated trigeminal excitability in the absence of cornea irritation. In contrast to the rapid reduction in cornea irritation, the error signal initiating adaptive increases in motoneuron excitability, OO weakness, typically results from more long-lasting damage such as muscle or nerve injury. It is useful to alter the channel and receptor profiles so as to maintain elevated motoneuron excitability for long periods in the absence of an error signal. This long-term modification matches the normal recovery period for muscle or nerve damage, such as occurs in Bell’s palsy.
Eyelid Asymmetry and Eyelid Disorders
Many of the blink modifications that occur in disease states result from nervous system adaptations attempting to reestablish the integrity of the blink system. Patients with Bell’s palsy, in which facial nerve damage weakens or paralyzes the facial muscles, exhibit increased motoneuron excitability ipsilateral to the nerve damage.5,7,27–29 Our data indicate that the increased motoneuron excitability is a compensation for muscle weakness. Eyelid asymmetry is an appropriate and effective compensatory adaptation to the reduced blink amplitude experienced by patients with facial nerve palsy.
Eyelid asymmetry may also play a crucial role in the development of hemifacial spasm (HFS). This disorder is characterized by unilateral spasms of eyelid closure and concomitant contraction of other facial muscles. The arterial compression of one facial nerve at its root entry zone that causes HFS4,30–32 reduces the number of functional facial nerve axons, effectively weakening the facial muscles. We propose that the OO muscle weakness created by arterial compression in HFS33 unilaterally increases facial motoneuron excitability as shown in the present study. The prolonged eyelid weakness in HFS may exaggerate these increases in the motoneuron excitability to a level that motoneurons become hyperexcitable. Trigeminal hyperexcitability such as occurs in HFS34,35 causes oscillatory inputs to OO motoneurons.36 A trigeminal signal that is insufficient to evoke spasms in the normal facial nucleus may strongly activate hyperexcitable facial motoneurons and create spasms of eyelid closure. Thus, the intense spasms of eyelid closure in HFS may result from an interaction of abnormal motoneuron excitability and a hyperexcitable oscillating trigeminal input.37
Acknowledgments
The authors thank Donna Schmidt for technical support.
Supported by Grant EY07391 from the National Eye Institute (CE) and Grant 5F30NS4467303 from the National Institute of Neurologic Disease and Stroke (ISK).
Footnotes
Disclosure: I.S. Kassem, None; C. Evinger, None
References
- 1.Nicholls ME, Ellis BE, Clement JG, Yoshino M. Detecting hemifacial asymmetries in emotional expression with three-dimensional computerized image analysis. Proc R Soc Lond B Biol Sci. 2004;271:663–668. doi: 10.1098/rspb.2003.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimberg U, Petterson M. Facial reactions to happy and angry facial expressions: evidence for right hemisphere dominance. Psychophysiology. 2000;37:693–696. [PubMed] [Google Scholar]
- 3.Borod JC, Koff E, Yecker S, Santschi C, Schmidt JM. Facial asymmetry during emotional expression: gender, valence, and measurement technique. Neuropsychologia. 1998;36:1209–1215. doi: 10.1016/s0028-3932(97)00166-8. [DOI] [PubMed] [Google Scholar]
- 4.Valls-Sole J. Facial palsy, postparalytic facial syndrome, and hemi-facial spasm. Mov Disord. 2002;17(suppl 2):S49–S52. doi: 10.1002/mds.10059. [DOI] [PubMed] [Google Scholar]
- 5.Cossu G, et al. Reflex excitability of facial motoneurons at onset of muscle reinnervation after facial nerve palsy. Muscle Nerve. 1999;22:614–620. doi: 10.1002/(sici)1097-4598(199905)22:5<614::aid-mus10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Valls-Sole J, Montero J. Movement disorders in patients with peripheral facial palsy. Mov Disord. 2003;18:1424–1435. doi: 10.1002/mds.10605. [DOI] [PubMed] [Google Scholar]
- 7.Manca D, Munoz E, Pastor P, Valldeoriola F, Valls-Sole J. Enhanced gain of blink reflex responses to ipsilateral supraorbital nerve afferent inputs in patients with facial nerve palsy. Clin Neurophysiol. 2001;112:153–156. doi: 10.1016/s1388-2457(00)00516-2. [DOI] [PubMed] [Google Scholar]
- 8.Schicatano EJ, Mantzouranis J, Peshori KR, Partin J, Evinger C. Lid restraint evokes two types of motor adaptation. J Neurosci. 2002;22:569–576. doi: 10.1523/JNEUROSCI.22-02-00569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evinger C, Manning KA, Sibony PA. Eyelid movements: mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387–400. [PubMed] [Google Scholar]
- 10.Ellrich J, Katsarava Z, Przywara S, Kaube H. Is the R3 component of the human blink reflex nociceptive in origin? Pain. 2001;91:389–395. doi: 10.1016/S0304-3959(00)00465-6. [DOI] [PubMed] [Google Scholar]
- 11.Ibi K. Characteristics of dynamic accommodation responses: comparison between the dominant and non-dominant eyes. Ophthalmic Physiol Opt. 1997;17:44–54. [PubMed] [Google Scholar]
- 12.Pellegrini JJ, Evinger C. Role of cerebellum in adaptive modification of reflex blinks. Learn Mem. 1997;4:77–87. doi: 10.1101/lm.4.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Welsh JP, Harvey JA. Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J Neurosci. 1989;9:299–311. doi: 10.1523/JNEUROSCI.09-01-00299.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh JP. Changes in the motor pattern of learned and unlearned responses following cerebellar lesions: a kinematic analysis of the nictitating membrane reflex. Neuroscience. 1992;47:1–19. doi: 10.1016/0306-4522(92)90116-j. [DOI] [PubMed] [Google Scholar]
- 15.Wikgren J, Korhonen T. Interpositus nucleus inactivation reduces unconditioned response amplitude after paired but not explicitly unpaired treatment in rabbit eyeblink conditioning. Neurosci Lett. 2001;308:181–184. doi: 10.1016/s0304-3940(01)02000-6. [DOI] [PubMed] [Google Scholar]
- 16.Wikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus) Behav Neurosci. 2002;116:1052–1058. doi: 10.1037//0735-7044.116.6.1052. [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Garcia JM, Gruart A. The role of interpositus nucleus in eyelid conditioned responses. Cerebellum. 2002;1:289–308. doi: 10.1080/147342202320883597. [DOI] [PubMed] [Google Scholar]
- 18.Fanardjian VV, Manvelyan LR. Peculiarities of cerebellar excitation of facial nucleus motoneurons. Neurosci Lett. 1984;49:265–270. doi: 10.1016/0304-3940(84)90300-8. [DOI] [PubMed] [Google Scholar]
- 19.Holstege G, van Ham JJ, Tan J. Afferent projections to the orbicularis oculi motoneuronal cell group: an autoradiographical tracing study in the cat. Brain Res. 1986;374:306–320. doi: 10.1016/0006-8993(86)90425-7. [DOI] [PubMed] [Google Scholar]
- 20.Morcuende S, Delgado-Garcia JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci. 2002;22:8808–8818. doi: 10.1523/JNEUROSCI.22-20-08808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takada M, Itoh K, Yasui Y, Mitani A, Nomura S, Mizuno N. Distribution of premotor neurons for orbicularis oculi motoneurons in the cat, with particular reference to possible pathways for blink reflex. Neurosci Lett. 1984;50:251–255. doi: 10.1016/0304-3940(84)90494-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen F-P, Evinger C. Two functions of blink-related interpositus neurons. Soc Neurosci Abstracts. 2003;29:391–398. [Google Scholar]
- 23.Patko T, Vassias I, Vidal PP, De Waele C. Modulation of the voltage-gated sodium- and calcium-dependent potassium channels in rat vestibular and facial nuclei after unilateral labyrinthectomy and facial nerve transsection: an in situ hybridization study. Neuroscience. 2003;117:265–280. doi: 10.1016/s0306-4522(02)00829-1. [DOI] [PubMed] [Google Scholar]
- 24.Vassias I, Patko T, Vidal PP, de Waele C. Modulation of the beta1–3 voltage-gated sodium channels in rat vestibular and facial nuclei after unilateral labyrinthectomy and facial nerve section: an in situ hybridization study. Brain Res Mol Brain Res. 2003;120:73–78. doi: 10.1016/j.molbrainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Iwahashi Y, Furuyama T, Inagaki S, Morita Y, Takagi H. Distinct regulation of sodium channel types I, II and III following nerve transection. Brain Res Mol Brain Res. 1994;22:341–345. doi: 10.1016/0169-328x(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 26.Evinger C, Pellegrini JJ, Manning KA. Adaptive gain modification of the blink reflex: a model system for investigating the physiologic bases of motor learning. Ann NY Acad Sci. 1989;563:87–100. doi: 10.1111/j.1749-6632.1989.tb42192.x. [DOI] [PubMed] [Google Scholar]
- 27.Nacimiento W, Podoll K, Graeber MB, et al. Contralateral early blink reflex in patients with facial nerve palsy: indication for synaptic reorganization in the facial nucleus during regeneration. J Neurol Sci. 1992;109:148–155. doi: 10.1016/0022-510x(92)90161-d. [DOI] [PubMed] [Google Scholar]
- 28.Klostermann W, Wessel K. Crossed R1 response of the blink reflex in peripheral facial palsy. Electromyogr Clin Neurophysiol. 1995;35:69–71. [PubMed] [Google Scholar]
- 29.Valls-Sole J, Tolosa ES, Pujol M. Myokymic discharges and enhanced facial nerve reflex responses after recovery from idiopathic facial palsy. Muscle Nerve. 1992;15:37–42. doi: 10.1002/mus.880150107. [DOI] [PubMed] [Google Scholar]
- 30.Tan NC, Chan LL, Tan EK. Hemifacial spasm and involuntary facial movements. Qjm. 2002;95:493–500. doi: 10.1093/qjmed/95.8.493. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins RH. Hemifacial spasm: a review. Surg Neurol. 1991;36:251–277. doi: 10.1016/0090-3019(91)90087-p. [DOI] [PubMed] [Google Scholar]
- 32.Jannetta PJ. Outcome after microvascular decompression for typical trigeminal neuralgia, hemifacial spasm, tinnitus, disabling positional vertigo, and glossopharyngeal neuralgia (honored guest lecture) Clin Neurosurg. 1997;44:331–383. [PubMed] [Google Scholar]
- 33.Manning KA, Evinger C, Sibony PA. Eyelid movements before and after botulinum therapy in patients with lid spasm. Ann Neurol. 1990;28:653–660. doi: 10.1002/ana.410280509. [DOI] [PubMed] [Google Scholar]
- 34.Ogawara K, Kuwabara S, Kamitsukasa I, Mizobuchi K, Misawa S, Hatton T. Trigeminal afferent input alters the excitability of facial motoneurons in hemifacial spasm. Neurology. 2004;62:1749–1752. doi: 10.1212/01.wnl.0000125183.81555.e4. [DOI] [PubMed] [Google Scholar]
- 35.Pavesi G, Cattaneo L, Chierici E, Mancia D. Trigemino-facial inhibitory reflexes in idiopathic hemifacial spasm. Mov Disord. 2003;18:587–592. doi: 10.1002/mds.10405. [DOI] [PubMed] [Google Scholar]
- 36.Evinger C, Mao JB, Powers AS, et al. Dry, eye, blinking, and blepharospasm. Mov Disord. 2002;17(suppl 2):S75–S78. doi: 10.1002/mds.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evinger C, Kassem IS. In: Animal Models of Movement Disorders. Ledoux M, editor. Amsterdam: Elsevier Academic Press; 2005. pp. 253–264. [Google Scholar]