Abstract
Background
Spontaneous or unexpected panic attacks, per definition, occur out-of-the blue, in absence of cues or triggers. Accordingly, physiological arousal or instability should occur at the onset of or during the attack, but not preceding it. To test this hypothesisweexaminedif points of significant autonomic changes preceded the onset of spontaneous panic attacks.
Methods
Forty-three panic disorder patients underwent repeated 24-hour ambulatory monitoring. Thirteen naturally panic attacks were recorded during 1,960 hours of monitoring. Minute-by-minute epochs beginning 60 minutes before, and continuing to 10 minutes after, the onset of individual attacks were examined for respiration, heart rate, and skin conductance level. Measures were controlled for physical activity and vocalization, and compared to time matched control periods within the same person.
Results
Significant patterns of instability across a numberof autonomic and respiratory variables were detected as early as 47 minutes before panic onset. The final minutes prior to onset were dominated by respiratory changes, with significant decreases in tidal volume followed by abrupt PCO2 increases. Panic attack onset was characterized by heart rate and tidal volume increases and a drop in PCO2. Symptom report was consistent with these changes. Skin conductance levels were generally elevated in the hour before and duringthe attacks. Changes in the matched control periods were largely absent.
Conclusions
Significant autonomic irregularities preceded the onset of attacks that were reported as abrupt and unexpected. The findings invite reconsideration of the current diagnostic distinction betweenuncuedand cued panic attacks.
Keywords: panic attacks, physiology, respiration, ambulatory, autonomic, diagnostic
Panic attacks (PAs) occur in a wide range of psychiatric disorders and are predictive of increased disease morbidity, higher rates of comorbidity and suicidality, and poorer treatment response (1-4). PAs are defined as discrete periods of intense fear and discomfort (5), that are paroxysmal, with a peak reached within 10 min. Unexpected PAs, as compared to cued (situational bound or predisposed) attacks, are thought to occur “spontaneously”, in absence of internal cues or situational triggers. (5-6). The “out of the blue” concept has deep historical roots (Freud; 1895, “anxiety attacks erupt suddenly into consciousness without being called further by any train of thought” p. 136, 7), but the apparent absence of preceding cognitive processes has stimulated a search for hidden triggers, both psychological and biological.
Two principal approaches can be used to investigate the question of hidden triggers. First, cued or laboratory-induced PA can be studied with the assumption that similar physiological mechanisms are involved in both cued and spontaneous PAs. Second, patients with spontaneous PAs can be continuously monitored, with hope that spontaneous PAs will be captured during the monitoring. A number of laboratory studies have investigated the biological markers of artificially induced PAs (e.g., sodium lactate or CO2enriched air). However, the expression of symptoms and physiological responding may differ from those of natural panic attacks depending on the specific substance administered, procedure, or setting. Further, patients may anticipate (be prepared for) adverse physical symptoms (8). Ambulatory monitoring constitutes one of the most valid approaches to assess what may happen physiologically prior to and during attacks. Ambulatory studies are limited in number as attacks fail to occur even in many hours of recording. Moreover, the gathered data is often confined to the attacks itself, but not the period preceding it, thus precluding the examination of proximal causes. Freedman and colleagues (9) reported descriptive data of eight PAs during 12-hr ambulatory assessments. In most patients, heart rate (HR) and skin temperature rose just prior to the attacks, compared to a matched 5-min high-anxiety control period. Increased HR or muscle tension were also observed during laboratory recordings in the minutes before and during both spontaneously occurring PAs (8, 10-11) and PAs induced by placebo sodium lactate infusions (12). Ambulatory studies by Taylor and colleagues (13-14) reported higher HR in the majority of reported attacks compared to non-panic periods. However, Gaffney and colleagues (15) saw HR changes in only eight of 31 attacks. Inspection of PAsin another study during 24-hr recordings of 8 patients on bed rest found only small HR increases in a small proportion of the reported attacks (16). Likewise, descriptive data of three panic attacks showed inconsistent patterns of change in HR and blood pressure from nonpanic to panic (17). In all of these studies, HR was measured only during brief periods around the reported attacks. Respiration, a physiological response system sometimes considered primary in attacks (18-19), has rarely been recorded in ambulatory studies. Reduced transcutaneous CO2 (PtcCO2) was found in one study (20), but not in another (21). The latter reported decreases in PtcCO2 in only one of 24 situationally provoked PAs and no evidence for level differences in PtcCO2 compared to an hour before the attacks. One study described tidal volume (VT) increases at the point of panic during ambulatory monitoring of three patients (22). PaCO2dropped and pH increased considerably in a patient experiencing an attack while undergoing dialysis (23).
The aim of this study was to examine whether and whendetectable physiological changes occurred in the sequence of the panic event. We assessed minute-by-minute data of 8 respiratory and autonomic indices, all continuously monitored for speech, physical activity, and skin temperature starting 60 minutes before PA onset and continuing until10 min after. Non-panic periods of the same duration in the same individual were used for comparison to verify that detected changes were not due to random fluctuations.
METHODS
PARTICIPANTS
Forty-three patients with Panic Disorder (PD) with or without agoraphobia were recruited throughadvertisements to participate in a treatment study (24). Patients’ mean age was 41.6 years (range 23-61). The majority were Caucasians (81.8%), 9.1% were Hispanic, and 9.1% were Asian. Patients met the following inclusion criteria: DSM-IV principal diagnosis of PD, age 18 to 65, with following exclusion criteria: no evidence of an organic mental disorder, suicidality, schizophrenia, alcohol or drug abuse or dependence, cardiovascular or pulmonary disease, epilepsy, or pregnancy. The Structured Clinical Interview for DSM-IV criteria (25) confirmed diagnoses, with high inter-rater reliability for comorbid diagnoses ( = .83) and PD ( = 1.00). 55% of the sample had at least one additional DSM-IV Axis I diagnosis. Of these, all had an additional anxiety diagnosis and 27% had an additional mood disorder. 55% met criteria for panic disorder with agoraphobia. On average, patients had suffered from PD for three years (range 0.5-16). Patients’ PD severity was in a moderate to severe range (26). Three patients were on psychotropic medications (2 on a 0.5 mg dose of lorazepam and one on a stable dose of 20 mg of citalopram). One patient took 0.5 mg of lorazepam 40 min after the onset of his PA.
84 ambulatory monitoring periods of 24 hours each were collected from 43 patients, both pre- and post-treatment (n=24) or twice before treatment (wait-list controls, n=19), two periods were lost due to drop-outs after wait period.A total of 13 PAs were reported by 11 patients (4 men, 7 women).1All patients signed informed consent forms as approved by Stanford University Institutional Review Board.
PROCEDURES
Patients reported to the laboratory in the morning of the recording and received detailed instructions about how to wear the ambulatory deviceand problems that might arise (i.e., loosening of electrodes). Patients were told to continue with their normal daily routine, but to refrain from vigorous physical activity, sexual activity, alcohol, drug, or caffeine use during monitoring. Deliberately seeking out feared situations was discouraged.
SELF-REPORT MEASURES
Patients were instructed to press an event marker when a PA occurred and to fill out a panic attack log immediately afterwards. Patients rated their peak symptoms and emotions (anxiety and worry) on a scale of 0 (none) to 10 (extreme), plus the time of onset and duration of their PA. They also rated whether the attack was expected or unexpected. Only PAs meeting a minimum of four out of the 13 DSM-IV panic attack symptoms (5) and accompanied by at least moderate levels of anxiety, were analyzed. At recording outset, patients rated the likelihood of experiencing a PA during the following 24 hours.
PHYSIOLOGICAL MEASURES
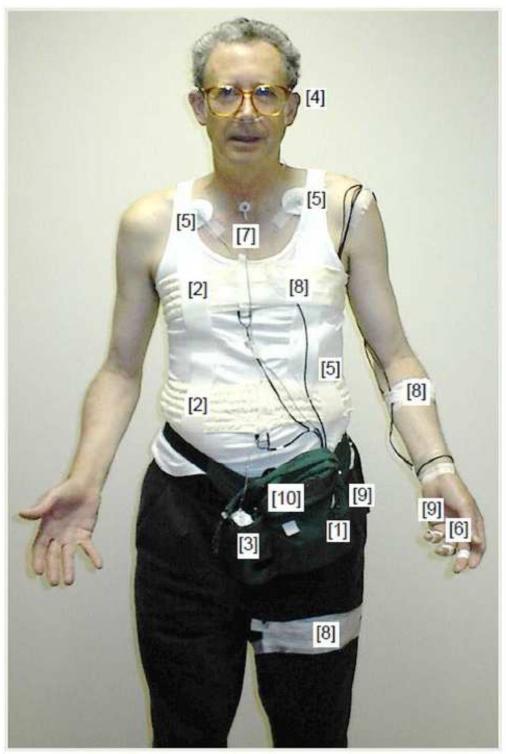
Data surrounding the PAs were extracted from the continuous recordings of a multichannel ambulatory monitoring device (Vitaport-II, Becker Meditec, Germany) with an additional input from a portable capnometer (Capnocount Mini, Weinmann, Germany). Both devices were carried in a waist bag, which also contained the event marker button (Figure 1). A laboratory technician attached the electrodes and calibrated the ambulatory device.
Figure 1.
Illustration of the ambulatory set-up for 24-h monitoring. Ambulatory recorder [1], thoracic and abdominal plethysmography bands [2], capnometry device [3] with attached nasal cannula [4], EKG electrodes [5], electrodermal activity [6],sound sensor [7], accelerometers [8], external and finger temperature sensors [9], and event marker button [10].
Respiratory variables
Respiration was monitored with inductive plethysmography using 2 elasticbands in which wires were embedded (Respitrace Corporation, NY) and sampled at 25 Hz. Band expansion was calibrated for volume using a fixed volume (800 mL) plastic bag (Respibag, Ambulatory Monitoring, Inc., NY) that the subject inflated and deflated completely several times and in different postures. Calibrated tidal volume (VT) for each breath for the two bands was weighted and added (27). VT was calculated as the volume difference between peaks and valleys of valid breaths. In addition, respiration rate (RR) was extracted as the number of breaths per minute. Minute ventilation (V’E)was calculated as the product of VT and RR. The root mean square of successive differences of RR and VT were computed to obtain indices of breath-by-breath RR instability (RRRMSSD) and VTvariability(VTRMSSD), respectively. End-tidal PCO2was measured continuously at 25 Hz using a portable capnometer with a 1.2-mm diameter disposable plastic tube ending in dual nostril prongs. PCO2was determined from the raw signal as the level at which PCO2stopped rising at the end of expiration.
Autonomic variables
The electrocardiogram was sampled at 400 Hz from three electrodes in a Lead-II configuration. Heart rate (HR) was calculated by automatic detection of R-waves and calculation of successive heart periods in addition to visual inspection of the electrocardiogram to insure detection of artifacts.Skin conductance level(SCL)was measured from two electrodes filled with isotonic electrode gel attached to the volar surfaces of the medial index and middle fingers of the patients’ non-dominant hand.
Control variables
Body movement and vocalizationwere recorded to control for physical activity and the activating effects of talking (28). Accelerometers were taped to the upper left leg, lower arm, and thorax. A voice and light sensor was attached below the larynx. Patients’ skin temperature served as control variable for skin conductance. The sensor was placed on digit 5 of the non-dominant hand. These channels were sampled at 25 Hz. Data were edited off-line and averaged for 1-min periods using an integrated set of analysis programs (for more details see 29).
Data selection for panic attacks and matched control periods
Data beginning 60 min prior to a reported PA and ending 10 min after its onset were extracted from the 24-hr data set2.The time of the attack was determined by a marker in the physiological data stream and was verified by the times the patient entered in the PA log. PA periods were matched with non-PA 70-min control periods (NPA), selected at random from the remainder of the patient’s 24-hr recording, but at least 3hr before or after the PA and at the same time of day or night as the PA.
STATISTICAL ANALYSIS
Change point analysis (30-31) was used to detect points at which the 70 min PA and NPA time series displays a significant change. If a change point (CP) was detected, the search continued, starting at the next time point, to determine the presence of subsequent CPs. Permutation analysis determined the significance level of the CPs. The minimum duration of time for each CP was set at 5min. We judged this interval to be long enough to rule out random, transient changes, but short enough to capture changes that might be important to the PA. Change Point analysis for continuous physiologic data is described in detail elsewhere (31).
We sequentially analyzed the entire 70 min time series (including the 60-min pre-panic period), expecting to identify CPs near the reported panic onsets. Because of the exploratory nature of the CP analysis, we adopted a conservative Bonferroni correction to establish significance (p ≤ .0001).3 To remove the effects of the control variables, mixed-effects regression models (MRMs) were applied, using the residuals from the MRM as the data with the effects of the controls removed (see (31) for details). Finally, to verify that the CPs found in the PA analyses were significantly different from random variations in the NPA time series, MRM was used to compare the PA time series to the NPA time series at CPs close to the reported PAs. Movement and speech (and skin temperature for SCL) were included as control variables in these MRMs. A significantly larger pre-post CP difference in the PA vs. NPA time series would further indicate that the change in the PA time series was not due to the random fluctuations. Traditional analytical approaches to this data are available upon request.
Finally, we examined whether the significant physiological changes that occurred at PA onset were related to symptoms reported during the PAs. For each PA, we calculated the mean for each physiological variable during the PA and subtracted the corresponding mean during the NPA control period. This provided us with a measure of the difference between each variable’s normal level and the level experienced during the PA, which was then correlated with symptom self-reports.
RESULTS
PANIC ATTACK SELF REPORTS
The median length of the 13 PAs was 8 min (range=1-30, M=10.6). 38.4% of the PAs occurred when the patient was alone, 23.1% in the presence of friends, and 38.5% with family. One PA occurred during sleep. 69.2% of PAs were reported as “unexpected”. Among the expected attacks, 2 occurred at home (no trigger listed), 1 “on the freeway”, and 1 “during an argument with friend”. With the exception of “driving”, none of the situations were previously listed by the patients as PA triggers. Anxiety during the attacks was moderate to high(M=8.0, SD=1.22; range 5-10). Severity ratings on the 13 DSM-IV symptoms (Table 1) were highest for shortness of breath and heart racing (both M=6.2, SD=2.9) and lowest for fear of dying (M=1.0, SD=2.3). Patients who had predicted that they would have a PA during the recording period were no more likely to experience an attack than patients who had predicted no attack (χ2(1,41)=0.46, p=.70).
Table 1.
DSM-IV panic symtpom and emotions reported during panic attacks and rest*
| Variable | Panic Attacks | Rest |
|---|---|---|
| Anxiety | 8.00±1.22 | 3.90±3.14 |
| Worry | 6.00±2.77 | 3.09±2.63 |
| Shortness of breath | 6.15±2.88 | 2.36±2.98 |
| Racing/pounding heart | 6.15±2.88 | 1.81±2.40 |
| Unsteadiness/dizziness/faintness | 4.08±3.45 | 0.45±0.82 |
| Chest pain/discomfort | 3.77±4.07 | 1.82±2.63 |
| Feelings of unreality | 3.62±3.38 | 1.45±1.98 |
| Sweating | 2.77±3.54 | 1.00±0.89 |
| Hot flashes/cold flashes | 2.62±3.64 | 0.45±0.82 |
| Fear of losing control/going crazy | 2.31±2.81 | 0.18±0.40 |
| Trembling/shaking | 1.85±2.03 | 0.90±2.39 |
| Chocking sensations | 1.77±3.14 | 0.00±0.00 |
| Nausea/abdominal distress | 1.77±2.77 | 0.91±2.42 |
| Numbness/tingling | 1.23±2.39 | 0.18±0.40 |
| Fear of dying | 1.00±2.27 | 0.18±0.60 |
Data are given as means ± SD.
Ratings based on following instruction: “Please rate the maximum severity of the symptoms and emotions you experienced as soon as possible after a panic attack occurred (for PA)” or “Please rate the maximum severity of the symptoms and emotions you experienced during the last 2 hours (for resting).” [0 for “none”, 2 - “mild, 5 - “moderate”, 8 - “strong”, and 10 - “extreme”].
PHYSIOLOGICAL CHANGES IN THE HOUR BEFORE PA ONSET
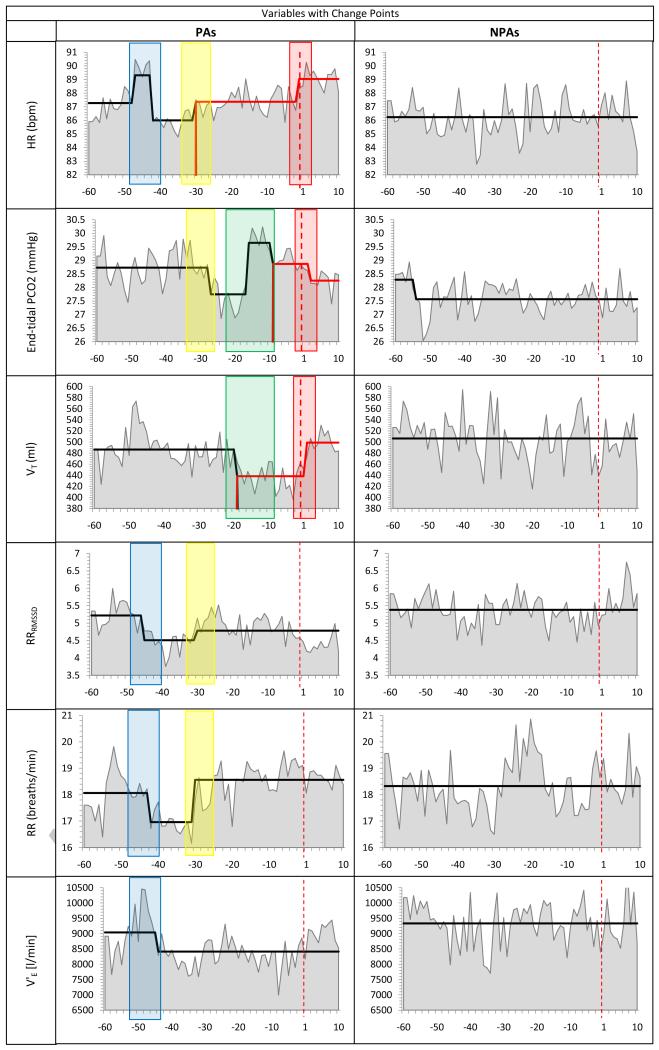
The hour preceding the onset of the PAs was marked by significant cardio-respiratory changes. Figure 2 illustrates the countdown to the onset of the PAs starting at the hour prior to onset (min −60) to the last min before PA onset. The changes cluster in three time periods: (i) min 47-42 before PA onset (blue box in Fig. 2): an initial increase in HR at min −47 (all change pointsps ≤.0001)was followed by a significant decrease in RR and RRRMSSD (min −45). This drop was followed by a decrease in V’E (min −44). HR decreased 2 min later (min −42). (ii) min 30-27 (yellow box in Fig 2): a significant increase in HR occurred simultaneously with significant increases in RR and RRRMSSD (at min −30), followed by a drop in PCO2 three min later (min −27). (iii) Starting 19 min before PA onset (green box in Fig 2), a significant decrease in VToccurred, followed by a sharp increase in PCO2 at min −16. Then PCO2 decreased about 9 min before PA onset.
Figure 2.
Bold black and red lines reflect average levels of the measure and significant changes in those average levels. The time of the reported PAs (or corresponding NPA time periods) is marked as a vertical dashed red line at time point 1. The red box encloses CPs occurring at PA onset, the blue box CPs during min −47 to −42, the yellow box CPs during min −30 to −27, and the green box CPs during min −19 to −9.
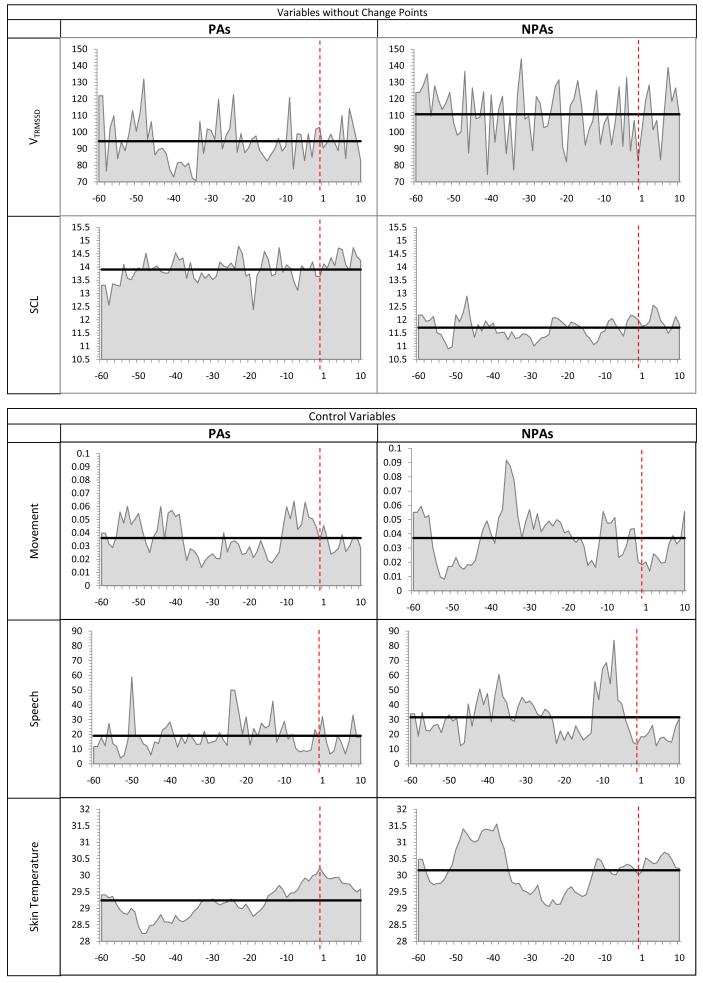
The only physiological variables with no detected CPs were, VTRMSSDand SCL (Figure 3). However, higher levels of SCL were found in the PA than in the NPA series (t(1469)=5.87, p<.001), throughout the time series. No CPs were detected in the control channels. Only one CP was detected for the eight NPA time series: PCO2 decreased at min 5 of the recording of the NPA time series. PCO2 levels for both PA and NPA time series were in a hypocapnic range.
Figure 3.
Variables with no CPs and control variables
Changes in the PA time series were more frequent than in the NPA time series. The total numberof CPs across all physiological variables was 15 in the PA time series vs.1 in the NPA series (t(7)=3.56, p<.01). This relative lack of change in the NPA time series, coupled with the very conservative alpha level, indicates that the changes detected were likely directly related to the emerging PAs.
PHYSIOLOGICAL CHANGES AT PANIC ATTACK ONSET
As illustrated in Figure 2 (enclosed by the red box), the minute prior to PA onset was marked by a significant increase in HR followed by a significant increase in VT at min 1 following the PA onset,and a significant drop in PCO2at min 2. No further CPs were detected. No significant changes were detected at the same times in the NPA series.
HR changes from pre to during-PA were significantly greater than those in the NPA series (t(954)=5.23, p<.001). Furthermore, absolute levels of HR were significantly higher during the PAs (min −1 through +10) than during the same time-matched NPA control period (t(954)=2.17, p<.05). Similarly, the pre-to during-PA changes for both VT and PCO2 were significantly greater in the PA series vs. the NPA series (ps<.05). However, the level of these measures during the PAs did not differ significantly from the NPA series. Only for HR did levels during the PAs (after the CPs) exceed NPA control periods.
The conventional approach of comparing the level of the physiological variables for the 5-min (or 10-min) periods before and after PA onset (rather than using change point analysis to detect substantial activity changes in the available time series) did not yield significant differences. Similarly, trend analyses centered at the onset of the reported PAs revealed no significant differences. However, a significant quadratic trend for HR (increases before PA onset, decreases after)was found under very restrictive conditions when analyses were centered at minute 1 of the PA.
RELATIONS BETWEEN PHYSIOLOGICAL MEASURES AND SYMPTOMS
Higher HR during PAs (compared to NPAs) was related to reports of more faintness (r=.77, p<.01) and to greater fear of losing control (r=.66, p<.05). Similarly, higher PCO2 was related to higher levels of anxiety (r=.70, p<.05), chest pain (r=.76, p<.05), and fear of dying (r=-.76, p<.05). Finally, higher VT was related to more faintness (r=.62, p< .05).
DISCUSSION
Using a novel analysis to detect points of significant change in continuous physiological time series, we found that the hour preceding the onset of naturally occurring panic attacks was marked by significant cardio-respiratory instability. These changes were largely absent in the control periods. The physiological instabilities occurred in repeated “bouts” often initiated by HR accelerations. The period surrounding panic onset was dominated by respiratory changes. Before panic onset, VTdecreased and PCO2increased, plateaued, and then decreased. At panic onset, HR and VTrose and then PCO2dropped. Panic onset was followed by a surprising absence of significant physiological change. The findings imply that the prototypical PA described by Barlow and colleagues (32) as “an instantaneous alarm reaction, both subjectively and physiologically peaking within 3-5 min […]” (p.556) may be an incomplete picture. The unreported cardio-pulmonary instabilities beginning as early as 47 minutes before the initiation of “spontaneous” panic attacks, albeit undetected in the patients’ perception, invites reconsideration of the nosological distinction between cued and spontaneous PAs.
The unique physiological up- and down-regulations recorded long before panic onset may be manifestations of attacks below the sufferer’s perception threshold. Perhaps they were being triggered by the same underlying mechanisms that later led to a full-blown attack, but were attenuated and/or incomplete. The HR spike at −47 min was not accompanied by decreases in PCO2 or by increases in VT and RR that would have been consistent with hyperventilation. Only the changes around the attack itself approximated a hyperventilation pattern.Thus, the earlier physiological changes may represent partial attacks, which various respiratory adjustments managed to weaken. The repeated bouts of instability may have a cumulative effect, sensitizing interoceptive awareness until one set of instabilities is experienced as an abrupt attack coming “out-of-the-blue.”
The decreases in PCO2 near to and following panic onset are consistent with previous reports (20-21), and have been interpreted as contributory causes of panic (19), compensatory reactions to a pathological suffocation alarm (18), or simply manifestations of emotional reactivity (33). The timing of our respiratory and HR changes resembles observations of Goetz and colleagues (12) of PAs during a lactate-challenge placebo condition, in which VT increased sharply 1.5 min prior to PA onset, peaked at min 1 of the PAs, and subsided afterwards. The peak level of VT had the same timing in our study. The authors speculated that the increase in ventilatory drive, manifested in increased V’E, had lowered carbon dioxide. The nature of these physiological events lends credence to theories postulating a central role for cardio-respiratory disturbances in panic. The timing of respiratory change in our patients is supportive of Klein’s idea that hyperventilation is an effort to avoid stimulation of an overly sensitive suffocation alarm system triggered by rises in PCO2 levels (18). Levels of PCO2 in our study were generally in a somewhat hypocapnic range, both for the PA and NPA time series, but rose and then dropped before PA onset. Perhaps these rises, although absolute levels were not high, triggered sensations of suffocation. In our data, higher levels of PCO2were correlated with greater self-reported anxiety, chest pain, and fear of dying.
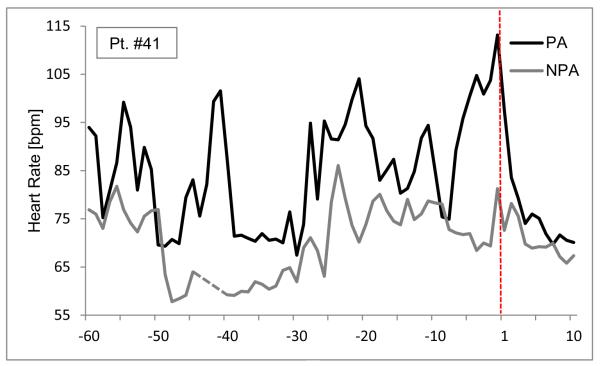
Only a few of our patients (see Figure 4) showed the dramatic increases in HR at PA onset as were reported in case examples by Cohen et al. (10).Our general finding was that a series of bouts of subtle physiological changes mark the hour before the attack in which HR and respiratory measures rise and fall in a complex pattern. It is unclear whether patients sensed these events as an “aura” of the impending panic, giving them a premonition that a full-blown attack will follow. Retrospective and prospective studies (34-35) assessing symptoms in the hour preceding unexpected PAs did not find that panickers felt different from usual. Our results show that internal cues of impending PAs may have been present, but were not perceived.Thus, whether an attack is reported to be expected or unexpected may not be an essential nosological characteristic but dependent on the panicker’s sensitivity to bodily change, as was suggested by Craske et al. (1).
Figure 4.
Illustration of single case (Pt #41) example of heart rate in the hour prior to, during, and the first 10 min following the PA.The time of the reported PAs (or corresponding NPA time periods) is marked as a vertical dashed red line at time point 1. The dotted line reflects missing data which was interpolated in the analysis.
A limitation of our statistical method is that it detects changes in a time series synchronized across subjects and may not accurately reflect the timing of changes in individual subjects. The CP pattern for measures near PA onset is consistent with previous and with theoretical accounts, but the CP pattern for variables in the hour before the attack was less expected. Possibly elevated pre-attack physiological variability in individual subjects was detected as CPs even when fluctuations were not perfectly synchronized across subjects. Furthermore, self-reports of PA onset, upon which the timing of our analysis partially depended, are subject to uncertainty (1,32). However, the correspondence between reported PA onset and physiological change points was strikingly close. Finally, this study is based on only 13 attacks. Our patients sometimes told us they were eager to experience an attack, “so they could finally get evidence that it was truly real.” While this intention may have paradoxically reduced the number of attacks reported, expectations for having a PA was unrelated to PA occurrence.
ACKNOWLEDGMENTS
This study was supported by Grant R01MH56094 from the National Institutes of Mental Health(WTR, FHW), the Department of Veterans Affairs, and the generous research support of the Beth and Russell Siegelman Foundation (AEM).
Footnotes
A total of 17 (14 daytime and 3 nocturnal) were reported. Two daytime attacks were excluded from analysis because self-reported PA symptoms did not meet the required number of DSM-IV PA symptoms. Two nocturnal PAs were excluded due to missing control variables (i.e., motion, speech).
The 10 min criterion was used because after that time point, more than half of the patients reported that the PA had subsided.
Because we imposed a minimum time length of 5 min for a CP, only 60 of 70 time points were tested for a CP. Thus, for 8 physiological measures, the p level for significance was set at .05/(60*8)=.0001.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors reported no biomedical financial interests or potential conflicts of interest. We are indebted to the helpful comments by Dr. Israel Liberzon on an earlier draft of this manuscript.
REFERENCES
- 1.Craske MG, Kircanski K, Epstein A, Wittchen HU, Pine DS, Lewis-Fernandez R, et al. Panic disorder: a review of DSM-IV panic disorder and proposals for DSM-V. Depress Anxiety. 2010;27:93–112. doi: 10.1002/da.20654. [DOI] [PubMed] [Google Scholar]
- 2.Baillie AJ, Rapee RM. Panic attacks as risk markers for mental disorders. Soc Psychiatry PsychiatrEpidemiol. 2005;40:240–244. doi: 10.1007/s00127-005-0892-3. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin RD, Hamilton SP. Panic attack as a marker of core psychopathological processes. Psychopathology. 2001;34:278–288. doi: 10.1159/000049326. [DOI] [PubMed] [Google Scholar]
- 4.Reed V, Wittchen HU. DSM-IV panic attacks and panic disorder in a community sample of adolescents and young adults: how specific are panic attacks? J Psychiatr Res. 1998;32:335–345. doi: 10.1016/s0022-3956(98)00014-4. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 6.Klein DF, Klein HM. The substantive effect of variations in panic measurement and agoraphobia definition. J Anxiety Disord. 1989;3:45–56. [Google Scholar]
- 7.Freud S. Joan Riviere (Trans.), Collected Papers. Vol.1. Hogarth Press; London: 1924. Obsessions and phobias; their psychical mechanisms and their aetiology. (Original work published in 1895) [Google Scholar]
- 8.Wilkinson DJC, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, et al. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry. 1998;55:511–520. doi: 10.1001/archpsyc.55.6.511. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RR, Ianni P, Ettedgui E, Puthezhath N. Ambulatory monitoring of panic disorder. Arch Gen Psychiatry. 1985;42:244–248. doi: 10.1001/archpsyc.1985.01790260038004. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AS, Barlow DH, Blanchard EB. Psychophysiology of relaxation-associated panic attacks. J AbnormPsychol. 1985;94:96–101. doi: 10.1037//0021-843x.94.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Lader M, Mathews A. Physiological changes during spontaneous panic attacks. J Psychosom Res. 1970;14:377–382. doi: 10.1016/0022-3999(70)90004-8. [DOI] [PubMed] [Google Scholar]
- 12.Goetz RR, Klein DF, Gully R, Kahn J, Liebowitz MR, Fyer AJ, et al. Panic attacks during placebo procedures in the laboratory: physiology and symptomatology. Arch Gen Psychiatry. 1993;50:280–285. doi: 10.1001/archpsyc.1993.01820160050006. [DOI] [PubMed] [Google Scholar]
- 13.Taylor CB, Telch MJ, Havvik D. Ambulatory heart rate changes during panic attacks. J Psychiatr Res. 1983;17:261–266. doi: 10.1016/0022-3956(82)90004-8. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CB, Sheikh MD, Agras WS, Roth WT, Margraf J, Ehlers A, et al. Ambulatory heart rate changes in patients with panic attacks. Am J Psychiatry. 1986;143:478–482. doi: 10.1176/ajp.143.4.478. [DOI] [PubMed] [Google Scholar]
- 15.Gaffney FA, Fenton BJ, Lane LD, Lake CR. Hemodynamic, ventilatory, and biochemical responses of panic patients and normal controls with sodium lactate infusion and spontaneous panic attacks. Arch Gen Psychiatry. 1988;45:53–60. doi: 10.1001/archpsyc.1988.01800250063008. [DOI] [PubMed] [Google Scholar]
- 16.Cameron OG, Lee MA, Curtis GC, McCann DS. Endocrine and physiological changes during ‘spontaneous’ panic attacks. Psychoneuroendocrinology. 1987;12:321–331. doi: 10.1016/0306-4530(87)90061-8. [DOI] [PubMed] [Google Scholar]
- 17.Bystritsky A, Craske M, Maidenberg E, Vapnik T, Shapiro D. Ambulatory monitoring of panic patients during regular activity: a preliminary report. Biol Psychiatry. 1995;15:684–9. doi: 10.1016/0006-3223(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 18.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 19.Ley RA. Blood, breath and fears: A hyperventilation theory of panic attacks and agoraphobia. ClinPsychol Rev. 1985;5:271–285. [Google Scholar]
- 20.Hibbert G, Pilsbury D. Hyperventilation in panic attacks: ambulant monitoring of transcutaneous carbon dioxide. Br J Psychiatry. 1988;153:76–80. doi: 10.1192/bjp.153.1.76. [DOI] [PubMed] [Google Scholar]
- 21.Garssen B, Buikhuise M, van Dyck R. Hyperventilation and panic attacks. Am J Psychiatry. 1996;153:513–518. doi: 10.1176/ajp.153.4.513. [DOI] [PubMed] [Google Scholar]
- 22.Martinez JM, Papp LA, Coplan JD, Anderson DE, Mueller CM, Klein DF, Gorman JM. Ambulatory monitoring of respiration in anxiety. Anxiety. 1996;2:296–302. [PubMed] [Google Scholar]
- 23.Salkovskis PM, Warwick HM, Clark DM, Wessels DJ. A demonstration of acute hyperventilation during naturally occurring panic attacks. Behav Res Ther. 1986;24:91–94. doi: 10.1016/0005-7967(86)90156-7. [DOI] [PubMed] [Google Scholar]
- 24.Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders-Patient. Edition (SCID-IP, Version 2.0) Biometrics Research Dept, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- 26.Shear MK, Brown TA, Barlow DH, Money R, Sholomsskas DE, Woods SW, et al. Multicenter collaborative Panic Disorder Severity Scale. Am J Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 27.Chadha TS, Watson H, Birch S, Jenouri GA, Schneider AW, Cohn MA, et al. Validation of respiratory inductive plethysmography using different calibration procedures. Am Rev Respir Dis. 1982;125:644–649. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm FH, Grossman P. Emotions beyond the laboratory: Theoretical fundaments, study design, and analytic strategies for advanced ambulatory assessment. BiolPsychol. 2010;84:552–569. doi: 10.1016/j.biopsycho.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Conrad A, Isaac L, Roth WT. The psychophysiology of generalized anxiety disorder: 1. Pretreatment characteristics. Psychophysiology. 2008;45:366–376. doi: 10.1111/j.1469-8986.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindquist MA, Waugh C, Wager TD. Modeling state-related fMRI activity using change-point theory. Neuroimage. 2007;35:1125–1141. doi: 10.1016/j.neuroimage.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfield D, Zhou E, Wilhelm FH, Conrad A, Roth WT, Meuret AE. Change point analysis for longitudinal physiological data: detection of cardio-respiratory changes preceding panic attacks. Biol Psychology. 2010;84:112–120. doi: 10.1016/j.biopsycho.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow DH, Brown TA, Craske MG. Definitions of panic attacks and panic disorder in the DSM-IV: implications for research. J AbnormPsychol. 1994;103:553–564. doi: 10.1037//0021-843x.103.3.553. [DOI] [PubMed] [Google Scholar]
- 33.Sinha S, Papp LA, Gorman JM. How study of respiratory physiology aided our understanding of abnormal brain function in panic disorder. J Affect Disord. 2000;61:191–200. doi: 10.1016/s0165-0327(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 34.Street LL, Craske MG, Barlow DH. Sensations, cognitions and the perception of cues associated with expected and unexpected panic attacks. Behav Res Ther. 1989;27:189–198. doi: 10.1016/0005-7967(89)90078-8. [DOI] [PubMed] [Google Scholar]
- 35.Kenardy J, Taylor CB. Expected versus unexpected panic attacks: a naturalistic prospective study. J Anxiety Disord. 1999;13:435–445. doi: 10.1016/s0887-6185(99)00013-4. [DOI] [PubMed] [Google Scholar]