Abstract
The bed bug, Cimex lectularius, has a unique mode of copulation termed “traumatic” insemination [Carayon, J. (1966) in Monograph of the Cimicidae, ed. Usinger, R. (Entomol. Soc. Am., Philadelphia), pp. 81–167] during which the male pierces the female's abdominal wall with his external genitalia and inseminates into her body cavity [Carayon, J. (1966) in Monograph of the Cimicidae, ed. Usinger, R. (Entomol. Soc. Am., Philadelphia), pp. 81–167]. Under controlled natural conditions, traumatic insemination was frequent and temporally restricted. We show for the first time, to our knowledge, that traumatic insemination results in (i) last-male sperm precedence, (ii) suboptimal remating frequencies for the maintenance of female fertility, and (iii) reduced longevity and reproductive success in females. Experimental females did not receive indirect benefits from multiple mating. We conclude that traumatic insemination is probably a coercive male copulatory strategy that results in a sexual conflict of interests.
Recently, there has been a shift away from the view that the sexes share a common goal during reproduction and a move toward the concept that males and females are often in conflict over reproductive outcomes (1–6). Sexual conflict occurs because of the potentially different fitness optima for each sex resulting from copulation, such as conflicts over isogamous and anisogamous reproduction (7), copulation duration and mating frequency (3, 7, 8), and relative parental investment (1, 2). Conflicts can also arise when copulation is costly to one partner (usually the female, e.g., ref. 9), because of adaptations in the male that are associated with sperm competition (10). A well studied example of this phenomenon is sperm displacement in Drosophila melanogaster. Female D. melanogaster are polyandrous and their mates transfer proteins within the ejaculate that act to disable and kill the sperm of rival males. Male success in sperm competition is, in part, determined by these proteins (11). However, the proteins are also toxic to the female and reduce female longevity and fitness (refs. 12 and 13, but see ref. 14). The resulting conflict has produced a rapid coevolutionary arms race between the sexes (15).
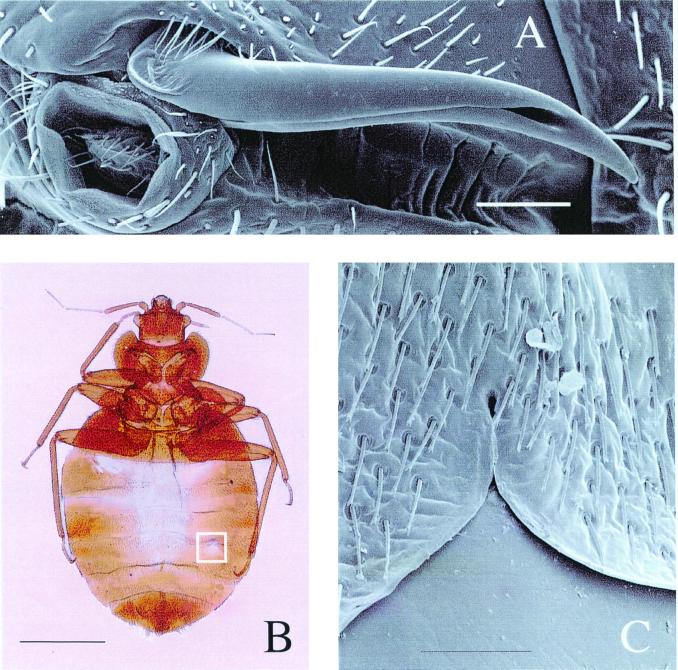
Extragenital insemination (internal insemination without the involvement of the female's genitalia) is rare but taxonomically widespread in invertebrates (e.g., refs. 16–21). It has been described in two orders of insects (Hemiptera and Strepsiptera) of which the hemipteran family, the Cimicidae, are the most widely cited example (e.g., refs. 22–24). The cimicids are the so-called “bed bugs.” In this taxon, copulation consists of abdominal wounding of the female by the introduction of a specialized male intromittent organ (Fig. 1A) through an external groove overlying the pleural membrane in the female's abdominal wall (ref. 25; Fig. 1 B and C). This groove is the external manifestation of the female bed bug's paragenital system, which is morphologically and embryologically different (26) from the genital tract. The genital tract is not used for copulation in any members of the bed bug family (26) but functions solely in egg laying. Once the intromittent organ pierces the body wall it enters the mesospermalege, a female paragenital organ containing hemocytes (26). Sperm then migrate to the ovaries (where fertilization takes place) via the hemocele and various specialized paragenital structures (27).
Figure 1.
(A) The intromittent organ of the male bed bug (a modified paramere). The groove in which the paramere sits when not in use is visible underneath the paramere. (Bar = 0.1 mm.) (B) The site of copulation on the ventrum of the female's abdomen. The male always copulates at this site (the ectospermalege). This structure directly overlies the mesospermalege into which the sperm are ejaculated. (Bar = 1.5 mm.) (C) Detail of the ectospermalege showing the incurving of the female's sternite, which acts as a guide for the male's intromittent organ. (Bar = 0.1 mm.) A and C are used by permission of Andrew Syred (Microscopix, U.K.).
Male Cimex lectularius produce an abdominal wound in females during mating, a phenomenon that is likely to be costly to females. In this article, we ask the question, “Do female bed bugs pay a cost for mating?” We first make observations of the bed bug mating system that provide a foundation for the design—and interpreting the results—of subsequent experiments. We measured (i) natural sex ratios (to define experimental parameters and to determine whether females avoided males in the wild), (ii) mating rates (to determine the extent and nature of polygamy), (iii) sperm precedence (to determine male fertilization success in a polygamous system), and (iv) the female remating optimum (to determine whether, and by how much, natural remating rates exceed the optimum that maintains maximum fecundity). We then examined the possibility that there is a sexual conflict of interests over mating frequencies in bed bugs based on the assumption that there is a cost to females of traumatic insemination. We first measured the longevity and fecundity consequences for females of being exposed to (i) the remating rate that maintains maximum fecundity and (ii) the natural remating rate. A difference in the longevity/fitness outcomes of these treatments indicates the potential for sexual conflict. However, any difference may be offset by indirect benefits (i.e., differences in offspring fitness). We therefore also examine whether the different mating regimes produce different outcomes in terms of offspring fitness traits.
Materials and Methods
Stock Maintenance.
C. lectularius cultures were maintained in an incubator at 26 ± 1°C and about 70% relative humidity. Bugs were fed weekly on rabbit blood (28). Bugs in these conditions went through a juvenile instar about every 6 days and eggs hatched within 10 days of laying. To produce virgins of similar age, final instar bugs were separated from the stock cultures every week and isolated in individual containers after feeding. The isolated adult virgin bugs resulting from this procedure were sexed under a stereo microscope by examining the ventral surface of the abdomen for the presence of the ectospermalege (a female-specific structure, Fig. 1). All bugs were measured by using image analysis software (OPTIMAS 6.1, VSG, U.K.) and the total length and maximum thoracic width were recorded. Size measurements always were taken on adult bugs starved for 5 days to control for the expanded size of the abdomen after feeding. The size of animals is presented as a size index that is derived from total body length × maximum thoracic width.
Natural Sex Ratios of C. lectularius Populations.
Samples of bugs from seven different natural populations (chicken farms in Dubai and the United Arab Emirates) were collected and sexed to determine whether sex-ratio biases occurred in the field.
Mating-System Observations.
Observations of copulations were carried out under dim red light conditions (60-W bulb) in a constant-temperature room (26 ± 2°C). Five satiated bugs of each sex (10 in total) were marked individually by using enamel paint and then were placed in a 5-cm diameter Petri dish lined with clean filter paper [this situation reflected natural density and sex ratio (29)]. The dish was placed under a Sony (Tokyo) video camera (TR-2000E), and the behavior of the bugs was recorded for 3 days. The frequency of copulation, duration of copulation, and remating interval were noted for each bug.
Sperm Precedence.
Sperm precedence was estimated by using reciprocal matings between irradiated sterile males [sterilized with 30 krad (30) from a closed-beam 137Cs source at 384 rad⋅min−1] and normal males. Paternity was assigned by counting fertile and sterile eggs (see ref. 31).
Female Fertility.
To investigate the fertilizing capacity of a single copulation, 20 virgin females each received a single copulation from a virgin male. The number and proportion of fertile eggs produced were counted over eight clutches (one clutch per week).
Cost of Remating Experiment.
Virgin female bugs were collected on the day after adult eclosion and measured by using image analysis software (OPTIMAS 6.1). During the experiment, all bugs were kept under a 12-h light/dark cycle at 26 ± 1°C with a relative humidity of 70%. Females were then allocated at random to one of two experimental treatments. In the first treatment, 45 females were fed to satiation and allowed to copulate once with a virgin male before being isolated in a 7-cm3 plastic vial containing clean filter paper. A male whose paramere was glued (Super Attak, Loctite, London, U.K.) into the genital groove (and so was unable to mate) then was introduced into each vial (to act as a control for the presence of a noncopulating male). These females were fed every 7 days and the number of eggs produced was counted each week. After 4 weeks, the females were allowed to copulate again with a virgin male to maintain the maximum fecundity; this treatment is hereafter called the “low-mating” treatment. Glued males showed similar mounting frequencies (1.32 ± 0.215 mounts per min) to unmanipulated males (1.18 ± 0.162 mounts per min; 18 df, t = −0.52, P = 0.61; measured in a 5-min block during the first hour after introduction). (Mounting is a stereotyped behavior in which the male “jumps” onto the female's dorsum for <5 sec and then dismounts. Apart from copulation, this is the only overt physical interaction between individuals). The second group was treated identically to the low-mating group except each female was allocated a virgin male at the beginning of the experiment and the pair was allowed to copulate for the remainder of their lives [isolated pairs of males and females copulated an average of 5.0 ± 1.41 (n = 20) times during a 7-day period]. This treatment group was termed the “reference” mating group and represents the natural pattern of mating behavior observed in our cultures (see Results).
Measurement of Indirect Benefits to Females from Multiple Mating.
Virgin females where allocated at random to one of two experimental treatments. In the first treatment group, females were allowed to copulate once with a virgin male (allocated at random), whereas in the second treatment group, females were allowed to copulate once with each of five virgin males (allocated at random). Females then were isolated and fed at weekly intervals, and the eggs were collected for five clutches.
The fertile and sterile eggs were counted and all eggs were measured, (OPTIMAS 6.1) as was the hatching date for each egg. The offspring were fed at weekly intervals and the date of adult eclosion as well as adult size were recorded also.
Statistical Analysis.
All analyses were performed by using STATVIEW 5.0 (SAS Institute, Cary, NC) statistical software for Macintosh. Data were checked for normality and homogeneity of variances. Where parametric test assumptions were violated, the appropriate nonparametric test was used.
Results
Natural Sex Ratios.
None of the seven sampled populations had a sex ratio that deviated from unity (χ2 = 0, n = 16; χ2 = 4.0, n = 14; χ2 = 0.38, n = 23; χ2 = 0.38, n = 11; χ2 = 0.53, n = 47; χ2 = 0.06, n = 17; χ2 = 0.38, n = 19; all P > 0.05).
Copulatory Behavior.
Under our culture conditions, copulation occurred only during the 36 h after a blood meal. During this period, females copulated 5.0 ± 3.16 times (n = 20) and males moved through the culture copulating with any engorged adult females they encountered. Eighty-nine videotaped reproductive interactions (240 h worth) failed to reveal any overt female resistance behavior during the relatively rapid (88.63 ± 5.36 sec, mean ± SE, n = 89) process of traumatic insemination
Sperm Precedence.
There was significant last-male sperm precedence in C. lectularius after two matings, separated by the median remating interval found in our cultures (17 min, lowest interquartile = 5.0, highest interquartile = 134.1, n = 41). (P2 = 0.68 ± 0.03, n = 40; one-sample t test: t = 4.76 and df = 39; P < 0.001.)
Female Remating Optimum Based on the Maintenance of Fertility.
Maximum fertility was maintained for a minimum of 4 weeks (n = 20) after only a single insemination (28). Because blood meals are taken every 6–7 days ad libitum (29), females are subjected to a 20-fold greater exposure to traumatic insemination (i.e., they receive about 20 copulations in 4 weeks under natural conditions) than is necessary to maintain maximum fertility.
Consequence of Natural Remating Frequencies for Females.
There was no difference in the size of animals allocated to the experimental groups (females: reference group, 13.72 ± 0.24 mm2; low-mating group, 14.03 ± 0.21 mm2; t = 0.977, 88 df, P = 0.33; males: reference group, 12.69 ± 0.24 mm2; low-mating group = 12.73 ± 0.22 mm2; t = 0.107, 88 df, P = 0.91).
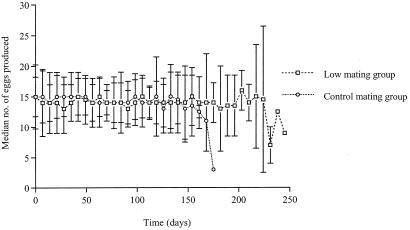
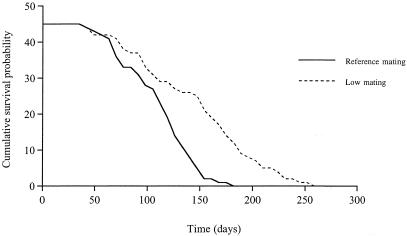
Females produced fertile eggs at the same rate regardless of which group they were from (Mann–Whitney U tests, P > 0.05; Fig. 2). Females from the reference group, however, died at a significantly higher rate than females from the low-mating group (log rank test: χ2 = 20.48, 1 df, P < 0.0001; Fig. 3) and consequently had significantly lower survival probabilities (reference group: 110.91 ± 4.98 days; low-mating group: 147.15 ± 8.37 days; t = 3.72, 88 df, P = 0.0004).
Figure 2.
Median egg production of females from the low-mating and reference groups. Bars show the interquartile range.
Figure 3.
Survival curves for females in the reference and the low-mating groups. The cumulative survival probability is the number of females alive at the end of a given sampling period (7 days).
Females in the reference group produced significantly (t = 3.69, 88 df, P < 0.001) fewer fertile eggs over their lifetimes (224.71 ± 16.06 eggs, n = 45) compared with females in the low-mating group (294.35 ± 9.89 eggs, n = 45). As a result of this reduced lifespan, females in the reference group suffered a 24% reduction in lifetime reproductive output.
Does Multiple Mating Produce Indirect Benefits for Females?
There was no difference between the sizes of males (unpaired t test: t37 = 1.07, P = 0.29) or females (unpaired t test: t37 = 0.24, P = 0.81) used in the treatment groups.
Likewise, there were no differences across five successive clutches of eggs from low-mating and reference-group females in terms of egg size (ANOVA, F1, 20 = 0.981, P = 0.32), egg development rate (ANOVA, F1, 20 = 0.38, P = 0.5), or the imaginal size of offspring (ANOVA, F1, 20 = 0.02, P = 0.88), suggesting that females in the reference group did not receive compensatory indirect fitness gains by means of the measured offspring traits.
Discussion
Our results have shown that natural patterns of traumatic insemination carry a measurable fitness cost for female C. lectularius, thereby providing evidence that the conditions for a sexual conflict over the mating frequency are present.
Frequent remating by male bed bugs probably occurs, at least in part, because the pattern of sperm precedence (P2 = 0.68) favors the last male to copulate. The observations of copulation, and the lack of any overt female resistance behavior during copulation, suggest that females have no behavioral control over mating frequencies. Indeed, the evolution of a piercing intromittent organ that bypasses the normal reproductive system may have provided males with the opportunity to avoid female adaptations that control copulation frequency. Females are unlikely to avoid traumatic insemination by dispersing away from areas where they encounter males given the sex ratios we observed in natural populations. Alternatively, multiple mating may provide fitness gains for females that offset, or exceed, the costs. Females that mate with several different males to get “good genes” or allelic diversity in their offspring may produce more viable offspring (24, 32). However, no viability differences were found in a suite of putative fitness traits in the offspring of singly or multiply mated female C. lectularius. Another possible benefit of multiple mating is that females may be avoiding genetically incompatible sperm that would lead to infertility of eggs or reduced offspring fitness (33, 34). Because no difference in the number of infertile eggs laid by the high- and low-mating group was found, this is also an unlikely explanation.
Our results indicate that females need to copulate only approximately once every four blood meals to maximize their lifetime reproductive success; the normal copulation rate (20 times the optimum) results in females suffering reduced longevity without a compensatory increase in egg-laying rates. This result mirrors the pattern of the mating cost in D. melanogaster (12). However, in D. melanogaster, the remating rate of females is influenced by female nutritional status [females thus have some control over their remating rate and therefore the expression of the mating cost (14)].
The mechanism that reduces longevity in female C. lectularius exposed to natural remating frequencies is not clear. The mode of copulation and insemination in C. lectularius produces potentially novel mechanisms for a cost of mating. Repeated copulatory wounding will result in the expression of cuticle-repair systems that are potentially costly in energetic terms (35) and so may divert resources away from somatic maintenance resulting in reduced longevity. Another cost of mating in C. lectularius may be associated with the need to respond to infection by pathogens introduced into the female as a consequence of traumatic insemination. Exposure to sexually transmitted diseases is a cost of mating that has been well documented in several taxa (36, 37), and it is likely to be an important cost in traumatically inseminating organisms because the intromittent organ is introduced directly into the female's hemocele.
Female counteradaptations to the costs of multiple insemination, regardless of the mechanism, are predicted by theory (38–41), yet female bed bugs show no overt behavioral counteradaptation that reduces the potential costs of copulation (unlike some other insect species, e.g., ref. 42). One way female cimicids may have responded to the costs imposed by traumatic insemination is by physiological and anatomical adaptation (i.e., the spermalege). The mesospermalege prevents sperm from directly entering the female's hemocele and is replete with phagocytic hemocytes (26), an observation that has led to the suggestion that this structure may function to kill sperm (24). The idea that the paragenital system evolved to enable female mate choice (24) is partly based on this suggestion; females may derive direct benefits by selecting sperm that result in better adapted offspring. However, some of our results are not consistent with this explanation (multiple-mated females do not show increased offspring performance under the conditions of our experiments). A plausible and, in light of our results, more parsimonious explanation for the evolution of the cimicid paragenital system in general, and the mesospermalege in particular, is that it functions to diminish the potential effects of wounding and infection caused by traumatic insemination. In light of our results, we propose that the mesospermalege evolved by means of natural, rather than sexual, selection and functions to reduce the fitness cost imposed on females by multiple traumatic inseminations that they cannot avoid. This idea remains to be tested, as does the question of why the proposed spermalege-associated amelioration of the cost of traumatic insemination is incomplete in C. lectularius.
Acknowledgments
We thank Allison Davids and Heather Jenkins for technical support, Steve Christmas (Leeds University, Leeds, U.K.) for irradiating the animals, Andrew Brooks (EHA, Liverpool, U.K.) for supplying bugs, and Sophie Armitage, T. R. Birkhead, Helen Crudgington, Ben Hatchwell, Francis Ratnieks, Jon Ryder, and John Thompson for their constructive comments. Andrew Syred and Microscopix provided the SEMs. A.D.S. was supported by a Biotechnology and Biological Sciences Research Council Studentship.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Trivers R L. In: Sexual Selection and the Descent of Man. Campbell B, editor. Chicago: Aldine-Atherton; 1972. pp. 1871–1971. [Google Scholar]
- 2.Trivers R L. Am Zool. 1974;14:249–264. [Google Scholar]
- 3.Parker G A. In: Sexual Selection and Reproductive Competition in Insects. Blum M, Blum N, editors. London: Academic; 1979. pp. 123–166. [Google Scholar]
- 4.Parker G A. In: Sperm Competition and the Evolution of Animal Mating Systems. Smith R L, editor. London: Academic; 1984. pp. 2–61. [Google Scholar]
- 5.Hammerstein P, Parker G A. In: Sexual Selection: Testing the Alternatives. Bradbury J W, Andersson M B, editors. New York: Wiley; 1987. pp. 119–141. [Google Scholar]
- 6.Alexander R D, Marshall D C, Cooley J R. In: The Evolution of Mating Systems in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 4–31. [Google Scholar]
- 7.Arnqvist G. Anim Behav. 1989;43:559–567. [Google Scholar]
- 8.Arnqvist G. In: The Evolution of Mating Systems in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 146–163. [Google Scholar]
- 9.Jackson R R, Pollard S D. In: The Evolution of Mating Systems in Insects and Arachnids. Choe J C, Crespi B J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 340–351. [Google Scholar]
- 10.Stockley P. Trends Ecol Evol. 1997;12:154–159. doi: 10.1016/s0169-5347(97)01000-8. [DOI] [PubMed] [Google Scholar]
- 11.Clark A G, Aguadé M, Prout T, Harshman L G, Langley C H. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler K, Partridge L. Nature (London) 1989;338:760–761. [Google Scholar]
- 13.Chapman T, Liddle L F, Kalb J M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman T, Partridge L. Proc R Soc London B. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 15.Rice W. Nature (London) 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 16.Mann K H. Leeches (Hirundinae). Their Structure, Physiology, Ecology and Embryology. Oxford: Pergamon; 1962. [Google Scholar]
- 17.Schroeder P C, Hermans C O. In: Reproduction of Marine Invertebrates. Geise A, Pierce J, editors. Vol. 3. New York: Academic; 1975. [Google Scholar]
- 18.Norman M D, Lu C C. Nature (London) 1997;389:683–684. [Google Scholar]
- 19.Schaller R. Annu Rev Entomol. 1971;16:407–446. [Google Scholar]
- 20.Hyman L H. The Invertebrates: Acanthocephala, Aschelminthes and Entoprocta. New York: McGraw–Hill; 1951. [Google Scholar]
- 21.Henley C. In: Reproduction of Marine Invertebrates. Geise A, Pierce J, editors. Vol. 1. New York: Academic; 1974. [Google Scholar]
- 22.Thornhill R, Alcock J. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard Univ. Press; 1983. [Google Scholar]
- 23.Eberhard W G. Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard Univ. Press; 1985. [Google Scholar]
- 24.Eberhard W G. Female Control: Sexual Selection Through Cryptic Female Choice. Princeton: Princeton Univ. Press; 1996. [Google Scholar]
- 25.Cragg F. Indian J Med Res. 1915;2:698–705. [Google Scholar]
- 26.Carayon J. In: Monograph of the Cimicidae. Usinger R, editor. Philadelphia: Entomol. Soc. Am.; 1966. pp. 81–167. [Google Scholar]
- 27.Abraham R. Z Parasitenkd. 1934;6:560–591. [Google Scholar]
- 28.Davis N. Ann Entomol Soc Am. 1956;49:466–493. [Google Scholar]
- 29.Mellanby K. Parasitology. 1939;31:200–211. [Google Scholar]
- 30.Younes M W F, Emara T E A. J Egypt Germ Soc Zool. 1995;17:217–225. [Google Scholar]
- 31.Boorman E, Parker G A. Ecol Entomol. 1976;1:145–155. [Google Scholar]
- 32.Kirkpatrick M, Ryan M J. Nature (London) 1991;350:33–38. [Google Scholar]
- 33.Zeh J A, Zeh D W. Proc R Soc London Ser B. 1996;263:1711–1717. [Google Scholar]
- 34.Zeh J A, Zeh D W. Proc R Soc London Ser B. 1997;264:69–75. [Google Scholar]
- 35.Chapman R F. The Insects: Structure and Function. Cambridge, U.K.: Cambridge Univ. Press; 1998. [Google Scholar]
- 36.Hurst G D D, Sharpe R G, Broomfield A H, Walker L E, Majerus T M O, Zakharov I A, Majerus M E N. Ecol Entomol. 1995;20:230–236. [Google Scholar]
- 37.Lockhart A B, Thrall P H, Antonovics J. Biol Rev. 1996;71:415–471. doi: 10.1111/j.1469-185x.1996.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 38.Rice W R, Holland B. Behav Ecol Sociobiol. 1997;41:1–10. [Google Scholar]
- 39.Holland B, Rice W R. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker G A, Partridge L. Philos Trans R Soc London. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Partridge L, Hurst L D. Science. 1998;281:2003–2008. doi: 10.1126/science.281.5385.2003. [DOI] [PubMed] [Google Scholar]
- 42.Crudgington H, Siva-Jothy M T. Nature (London) 2000;407:855–856. doi: 10.1038/35038154. [DOI] [PubMed] [Google Scholar]