Abstract
Since the secretory pathway is essential for Candida albicans to transition from a commensal organism to a pathogen, an understanding of how this pathway functions may be beneficial for identifying novel drug targets to prevent candidiasis. We have cloned the C. albicans KAR2 gene, which performs many roles during the translocation of proteins into the endoplasmic reticulum (ER) during the first committed step of the secretory pathway in many eukaryotes. Our results show that C. albicans KAR2 is essential, and that the encoded protein rescues a temperature-sensitive growth defect found in a Saccharomyces cerevisiae strain harboring a mutant form of the Kar2 protein. Additionally, S. cerevisiae containing CaKAR2 as the sole copy of this essential gene are viable, and ER microsomes prepared from this strain exhibit wild-type levels of post-translational translocation during in vitro translocation assays. Finally, ER microsomes isolated from a C. albicans strain expressing reduced amounts of KAR2 mRNA are defective for in vitro translocation of a secreted substrate protein, establishing a new method to study ER translocation in this organism. Together, these results suggest that C. albicans Kar2p functions during the translocation of proteins into the ER during the first step of the secretory pathway.
Keywords: KAR2, Secretory pathway, Candida albicans, ER translocation, Translocation assay
Introduction
The pathogenic yeast Candida albicans causes many forms of human disease, especially in immunocompromised individuals (Ruhnke 2002; Kullberg and Filler 2002; Filler and Kullberg 2002). In order for this organism to switch from its avirulent commensal state to a virulent one, a number of physiological changes must occur. These changes include the morphogenesis of at least a subset of cells from a budding yeast form to pseudohyphal and true hyphal morphologies (Sudbery et al. 2004; Romani et al. 2003). This transition requires the secretion of new cell wall proteins that are important for cell wall expansion (Chaffin 2008; Chaffin et al. 1998; Rico et al. 1991), as well as for aiding in adhesion to host tissues (Tronchin et al. 1991) and implanted medical devices (Meda et al. 2007; Verstrepen and Klis 2006). In addition to secreted cell wall proteins, infectious forms of C. albicans also require the secretion of aspartyl proteases (reviewed in Gagnon-Arsenault et al. 2006; Sohn et al. 2006; Naglik et al. 2003; Hube 1996) and various lipases and phospho-lipases (reviewed in Schaller et al. 2005) that presumably aid in host tissue invasion. Thus, protein secretion is a key component of the virulence arsenal of C. albicans.
In support of the virulence role for protein secretion, many specific C. albicans proteins that function in the secretory pathway have been shown to be important for the transport of virulence factors and the establishment of virulence characteristics in this fungus. For example, Vps1p (Bernardo et al. 2008) and Vps4p (Lee et al. 2009; Thomas et al. 2009) are C. albicans vacuolar protein sorting proteins that have been observed to be involved in protease secretion, filamentation, biofilm formation and in vivo virulence. Additionally, the deletion of the C. albicans PMR1 gene, which is involved in Golgi-level glycosylation of secretory proteins, leads to a decrease in virulence in a murine system (Bates et al. 2005). Finally, secreted C. albicans proteins have been found to affect the morphology and motility of infected cultured epithelial cells (Sandovsky-Losica and Segal 2006). Because protein secretion is essential for the establishment of C. albicans infections, an understanding of the mechanisms of protein secretion in this organism could result in the identification of new chemotherapeutic targets.
Despite the fact that the transition from the a virulent form of C. albicans to a pathogenic one requires the secretion of proteins to the cell surface and surrounding matrix, few studies have investigated the basic biology of the early secretory pathway in C. albicans, namely the translocation of secretory proteins into the endoplasmic reticulum (ER; reviewed in Fonzi 2009). A few C. albicans proteins presumed to play roles in the early secretory pathway have been shown to function similarly to their Saccharomyces cerevisiae orthologs, such as SEC65 (Regnacq et al. 1998) and SPC3, an ER resident protein involved in N-terminal signal sequence cleavage (De la Rosa et al. 2004a). Other C. albicans proteins that are involved later in the secretory pathway have also been shown to function similarly to their S. cerevisiae homologues, including SEC4 (Mao et al. 1999; Clément et al. 1998), SEC14 (Monteoliva et al. 1996), SEC18 (Nieto et al. 1993) and VPS1 (Bernardo et al. 2008). Interestingly, a few C. albicans proteins involved in the secretory pathway do not possess the ability to complement the function of their S. cerevisiae homologues. Genes encoding such proteins include SEC61 (De la Rosa et al. 2004b) and SEC20 (Weber et al. 2001). While there may be multiple explanations as to why these C. albicans proteins do not complement their S. cerevisiae homologues, these results suggest that one cannot know beforehand whether a given gene will or will not function similarly to its S. cerevisiae homologue. Given the importance of C. albicans as a human pathogen, it seems pertinent to study the mechanisms of protein secretion in C. albicans.
The first committed step in the secretory pathway is the translocation of presecretory proteins into the ER, and in S. cerevisiae a key player in this process is the Kar2 protein. This ER lumenal Hsp70 molecular chaperone protein (Normington et al. 1989; Rose et al. 1989) is involved in many aspects of both post- and co-translational translocation (Matlack et al. 1999; Gething 1999; Brodsky et al. 1995), as well as during the retrotranslocation of aberrant soluble proteins out of the ER and back to the cytosol for degradation (Nishikawa et al. 2005; 2001; Kabani et al. 2003; Plemper et al. 1997), a process which appears to involve many of the same proteins in C. albicans (Wimalasena et al. 2008). During import processes in S. cerevisiae, the Kar2 protein has been shown to aid in the translocation of presecretory proteins into the ER lumen through the Sec61p complex translocation pore (Matlack et al. 1999; Brodsky et al. 1995; Panzner et al. 1995; Brodsky and Schekman 1993; Sanders et al. 1992), to serve as a gate that “plugs” the translocon from the lumenal side of the ER membrane (Alder et al. 2005; Haigh and Johnson 2002; Hamman et al. 1998), and to act as a chaperone that aids in the folding of presecretory proteins once they are fully translocated into the ER lumen (Gething 1999; Simons et al. 1995).
To date, the KAR2 gene in C. albicans has not been directly characterized. Because of the many roles that Kar2p plays in the early secretory pathway in other eukaryotes, and because the secretory pathway is critical for C. albicans virulence, we have cloned the KAR2 gene from C. albicans and begun to characterize the function of the encoded protein. In this study, we have identified and initially characterized orf19.2013 in the C. albicans genome that encodes a predicted protein that is 71% identical and 81% similar to S. cerevisiae Kar2p at the amino acid level. Our results indicate that C. albicans Kar2p is essential, much like its S. cerevisiae ortholog. In addition, C. albicans Kar2p functions similarly to its S. cerevisiae ortholog, and C. albicans microsomes containing reduced amounts of Kar2p are defective for the translocation of a secretory protein substrate. Together, these results indicate that C. albicans Kar2p functions during the translocation of secretory proteins into the ER of this human pathogen and is likely critical for this fungus to cause disease in humans.
Materials and methods
Strains and general methods
Strains used in these studies are listed in Table 1. Routine DNA and protein methods used in this study were performed following standard protocols generally as outlined in Sambrook and Russell (2001).
Table 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| C. albicans strains | ||
| BPY104 | As BWP17, but KAR2/MET3p-KAR2 | This study |
| BPY116a and BPY116b | As BWP17, but kar2Δ::UAU1/MET3p-KAR2 | This study |
| BWP17 | Δura3::imm434/Δura3::imm434 Δhis1::hisG/Δhis1::hisG Δarg4::hisG/Δarg4::hisG | Wilson et al. (1999) |
| NIH3172 | Clinical isolate | ATCC #14053 |
| SC5314 | Clinical isolate | Gillum et al. (1984) |
| S. cerevisiae strains | ||
| MMY101 | As RSY586, but containing pYEX-BX | This study |
| MMY102 | As RSY586, but containing pCaKAR2-BX | This study |
| MMY105 | As YJ034W BY4743, but containing pCaKAR2-BX | This study |
| MMY108 | MATα his3Δ1 leu2Δ0 ura3 Δ0 lys2Δ Δkar2::kanMX and containing pCaKAR2-BX | This study |
| RSY586 (MS137) | MATα ura3-52 leu2-3, 112 ade2-101 kar2-159 | Brodsky et al. (1995) |
| RSY607 | MATα ura3-52 leu2-3, 112 pep4::URA3 | Brodsky et al. (1993) |
| YJ034W BY4743 | MATα/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 lys2Δ0/+ met15Δ0/+ ura3Δ0/uraΔ0 Δkar2::kanMX/+ | ATCC #4021388 |
Cloning of the C. albicans KAR2 gene
Genomic DNA was isolated from C. albicans strain NIH3172 (ATCC, Manassas, VA; ATCC #14053). This strain was grown in 7.0 mL of YPD (1% yeast extract, 2% bacto-peptone, 2% dextrose) overnight at 30°C. The cells were then harvested, washed with water, sedimented as before and then resuspended in 200 µL of lysis buffer (2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris pH 8.0, 1 mM EDTA), 200 µL of phenol/chloroform and 300 µL of 425–600 µm glass beads (Sigma–Aldrich, St. Louis, MO). The tubes were then agitated in a vortex mixer for 4 min followed by the addition of 200 µL of TE (10 mM Tris pH 7.6, 1 mM EDTA) and centrifugation in a microcentrifuge for 5 min. The aqueous layer was then removed and the DNA was ethanol precipitated. The pellet was suspended in TE, treated with RNase A and ethanol precipitated again. The resulting DNA pellet was then suspended in 50 µL of TE and stored at −20°C.
In order to clone the C. albicans KAR2 gene, which lacks CUG codons in the encoded mRNA, a standard PCR approach was employed. Genomic DNA from C. albicans strain NIH3172 was used in PCR reactions containing primers fcaKAR2bam (5′-GCATGGATCCCAACTTACCTTCCACGGTTTT-3′) and rcaKAR2sal (5′-GCATGTCGACGGTCCGTTCATTTCATGGCT-3′) (restriction sites are underlined; primers designed using the Candida Genome Database (CGD), http://www.candidagenome.org/), which amplified a 2,414 bp fragment containing the CaKAR2 gene flanked by BamHI and SalI restriction sites. The PCR product was purified using a PCR purification kit (Qiagen, Valencia, CA). Next, the PCR product and the yeast expression vector pYEX-BX (Clontech, Mountain View, CA) were cut with BamHI and SalI (Fermentas, Hanover, MD) in double digests followed by a purification reaction using the PCR purification kit. Ligation reactions were then performed overnight at 23°C. Ligation products were transformed into competent DH5α cells and plated on LB + 50 mg/mL ampicillin. Plasmid minipreps were performed and the correct ligation product was confirmed using a diagnostic PstI digestion. The resulting plasmid was named pCaKar2-BX.
Construction of a conditional kar2 null mutant in C. albicans
The C. albicans BWP17 strain was routinely cultured on YPD. The selective medium was YNB (1.2% Difco yeast nitrogen base, 5% (NH4)2SO4, 10% succinic acid, 6% NaOH, 2% glucose) supplemented with the appropriate amino acids and/or uridine at a concentration of 80 µg/ml. All strains were grown at 30°C.
Plasmid pCaDIS-KAR2 was constructed as follows. A 1,000 bp fragment of the 5′ end of the C. albicans KAR2 gene was PCR amplified from BWP17 genomic DNA, using Deep Vent Polymerase (New England Biolabs, Ipswich, MA) and primers F-CA-KAR2-BamHI (5′-GCATGGATCCATGAGATCATTACAATCTTCTT-3′) and R1 kb-CA-KAR2-BamHI (5′-GATAGGATCCCATCAACAAAGGAGTCGA-3′) (restriction sites are underlined, primers designed using CGD). The PCR product was digested with BamHI, purified with a Qiagen PCR cleanup kit, and ligated into pCaDIS (Care et al. 1999). pCaDIS-KAR2 was then linearized with KpnI and transformed into strain BWP17 (Wilson et al. 1999) using the lithium acetate/PEG protocol outlined by Gietz and Woods (2002).
Clone CAGA643 was obtained as part of the TIGR library of transposon insertions into the C. albicans genome that is available to the research community (http://www.candidagenome.org/CommunityNews.shtml). This clone has the Tn7-UAU1 transposon inserted within the open reading frame of C. albicans KAR2 at approximately position 541 bp (Davis et al. 2002). Prior to transformation into yeast containing one KAR2 allele under control of the MET3 promoter, the plasmid was digested with NotI to generate a linear ~10 kb fragment, which contained the UAU1 cassette and KAR2 flanking homology.
Southern blot analysis was performed as previously described by Sambrook and Russell (2001). Genomic DNA was isolated from the parental strain, BWP17, as well as from each transformant. A total of 1 µg of genomic DNA from each transformant was digested with AlwNI. The template DNA for the probe was a PCR amplified 1,529 bp fragment, starting 38 bp after the stop codon of the KAR2 gene, which used primers F-CaKar2-Arg4-KpnI (5′-CGTAGGTACCGCATTCCAATGTTTAGTTCTC-3′) and R-CaKar2-Arg4-KpnI (5′-CGTAGGTACCATGCGAATGGTCATGGTTGT-3′) designed using the CGD. The fragment was digested with SnaBI, resolved on an agarose gel, and the 1,234 bp piece was gel purified, followed by radiolabeling with the Prime-a-Gene Labeling System (Promega, Madison, WI) and random oligonucleotide primers.
Complementation of S. cerevisiae KAR2 in vivo
Strains MMY101 and MMY102 were grown for 7 days at 23°C on SC–URA plates (0.67% yeast nitrogen base + (NH4)2SO4, 2% glucose, all supplements except uracil), while strains RSY607 and RSY586 were grown for 3 days at 23°C on YPD plates. Two colonies from each plate were suspended in 200 µL of sterile water. The OD600 for each of the four cultures was determined and normalized by adding the appropriate amounts of sterile water. Tenfold serial dilutions of each culture were prepared using sterile water, and 5 µL of each dilution were spotted onto each of two YPD plates. One plate was incubated at 23°C and the other was incubated at 37°C. Digital photographs were taken of the plates after 2 days of incubation at their respective temperatures followed by one additional day at 23°C.
In order to generate a S. cerevisiae strain containing only the C. albicans KAR2 gene, tetrad dissection was utilized. The hemizygous diploid S. cerevisiae strain YJ034W BY4743 (ATCC #4021388) was used, which contains one wild-type allele of the KAR2 gene and one KAR2 allele that has been replaced with the kanMX gene, conferring resistance to G418 (Wach et al. 1994). This strain was transformed with plasmid pCaKAR2-BX and plated on SC-URA. After screening positive transformants for the presence of pCaKAR2-BX, these cells, named strain MMY105, were sporulated and subjected to standard tetrad dissection on YPD plates (Rose et al. 1990). The plates were then incubated at 30°C until the spores grew into visible colonies.
Cells from the four colonies resulting from the growth of the four spores of one tetrad were streaked onto SC-URA and YPD + 100 µg/mL G418 (USB, Cleveland, OH). These plates were incubated at 30°C for 2 days. Cells from the same four colonies were also grown overnight in liquid YPD at 30°C. The cells were then harvested from 750 µL of the culture and extracts were prepared by suspending the cells in 20 µL of SDS-PAGE sample buffer (65 mM Tris, pH 6.8, 2 mM β-mercaptoethanol, 2% SDS, 0.25 mg/mL bromophenol blue, 10% glycerol). The samples were then heated at 70°C for 10 min followed by the addition of 0.12 g of 425–600 µm micron glass beads (Sigma–Aldrich). Finally, the samples were agitated with a vortex mixer for 1 min and extracts were frozen at −20°C.
Extracts were then diluted 1:10 and run on a 15% SDS-PAGE gel. The proteins were transferred to Protran BA85 nitrocellulose (Schleicher and Schuell, Keene, NH), blocked in 2% milk in TBST (50 mM Tris, pH 7.4, 150 mM NaCl, 0.2% Tween-20) and cut just below the 60 kD Benchmark molecular weight marker (Invitrogen, Carlsbad, CA). The top portion of the blot was incubated overnight at 4°C with a 1:3,000 dilution of a polyclonal antibody that was generated against the S. cerevisiae Kar2 protein (Brodsky and Schekman 1993). The bottom portion of the blot was incubated in the same manner, but with an antibody generated against the S. cerevisiae Sec61 protein (Stirling et al. 1992). The blots were then washed with TBST and incubated for 1 h at 23°C in a 1:3,000 dilution of HRP-conjugated α rabbit IgG secondary antibody (Invitrogen). After adding chemiluminescence reagents (Pierce Biotechnology, Rockford, IL), the blots were exposed to film and developed.
Microsome preparation and in vitro translocation reactions
ER microsomes were prepared from S. cerevisiae strains MMY101, MMY102, MMY108, RSY607 and RSY586 after the cells were grown in their respective media (SC-URA for the MMY strains and YPD for the RSY strains) to an OD600 of ~2.0. C. albicans strain BPY116 cells were grown in YNB + His medium and then used to inoculate fresh YNB + His and YNB + His + 2.5 mM Met, Cys media to an OD600 of 0.2. Microsomes were prepared from these cells after 6 and 8 h of growth at 30°C. S. cerevisiae microsomes were purified as previously described (Brodsky et al. 1993). Microsomes isolated from C. albicans cells were prepared in the same manner except that commercial zymolyase (100T; US Biologicals, Swampscott, MA) was used at a concentration of 0.3 mg/mL of suspended cells. Microsomes were then used in in vitro post-translational translocation assays, which were performed as described (Morrow and Brodsky 2005; Brodsky et al. 1993, Rothblatt et al. 1989), except that translocation reactions were incubated at 20°C for 40 min, cytosol was added to 5 mg of protein/mL and the protein pellets were washed twice with acetone after TCA precipitation. The substrate used in these reactions was in vitro transcribed/translated S. cerevisiae 3Δg-ppαf, which is lacking the three N-linked glycosylation sites (McCracken and Brodsky 1996) and was prepared using a rabbit reticulocyte lysate system (Promega), and l-[35S] methionine (Perkin Elmer, Waltham, MA). Translocation assays using microsomes prepared from C. albicans cells contained cytosol isolated from wild-type C. albicans cells (SC5314), prepared as described for S. cerevisiae (Sorger and Pelham 1987). The amounts of all microsomes used in each translocation reaction were normalized by adjusting the absorbance at 280 nm of a 1:100 dilution in 2% SDS with buffer 88 (20 mM HEPES, pH 6.8, 150 mM potassium acetate, 5 mM magnesium acetate, 250 mM sorbitol). Translocation efficiencies were calculated by dividing the amount of protease-protected pαf in the trypsin treated reaction by the total of the 3Δg-ppαf and pαf in the untreated reaction. Standard error of the mean (SE) was then calculated for translocation reactions using each type of microsome.
Quantitative real-time PCR
BPY116 C. albcians cells were grown over night in YNB + His and then diluted to an OD600 of 0.2 in either YNB + His or YNB + His + 2.5 mM Met and Cys. The cells were grown for 8 h at 30°C and 3 ODs of cells were then harvested. Total RNA was purified from these cells using the Masterpure Yeast RNA Purification Kit (Epicentre Biotechnologies, Madison, WI) followed by DNase I treatment using a Turbo DNA-free kit (Applied Biosystems, Austin, TX). Complementary DNA was made using 500 ng of total RNA, Omniscript reverse transcriptase (Qiagen) and oligo dT primers (Applied Biosystems). Quantitative PCR was then performed using the SYBR green IQ mastermix (Bio-Rad, Hercules, CA), an iCycler thermocycler (Bio-Rad) and KAR2-specific primers fCa-Kar2realtime1 (5′-CCAATCACAGCAAAATTATACGGTGG-3′) and rCaKar2realtime1 (5′-CATCGTGATCGAATTCATCATCTGAA-3′), designed using the CGD. Real-time PCR primers were designed to amplify ~100 bp products within 300 bp of the translational stop codon for each respective gene. Primers amplifying actin (ACT1) and alpha-tubulin (TUB1) cDNA were used for normalization controls. Data were analyzed as normalized fold expression as described (Livak and Schmittgen 2001). Results presented are the mean and standard deviations of two biological replicates consisting of six total technical replicates for each gene and condition, except where indicated otherwise.
Results
The C. albicans KAR2 gene is essential
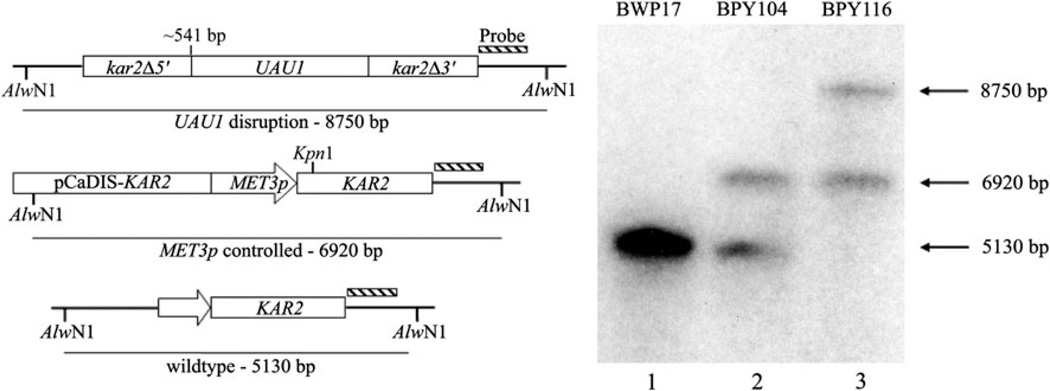
The requirement of the C. albicans KAR2 gene for yeast survival has not been investigated, although the S. cerevisiae KAR2 gene is essential (Rose et al. 1989; Normington et al. 1989). Therefore, a conditional null mutant for the C. albicans KAR2 gene was generated by placing one allele under control of the tightly controlled C. albicans MET3 promoter (Care et al. 1999) before disrupting the other allele with a UAU1 cassette (Enloe et al. 2000). Southern blot analysis confirmed that strain BPY116 contains one intact KAR2 allele, which is controlled by the MET3 promoter (Fig. 1).
Fig. 1.
Confirmation of KAR2 conditional null mutant in Candida albicans through Southern blot analysis. The diagram on the left depicts the three expected restriction fragments after the digestion of genomic DNAs with AlwNI. When probed, BWP17 (lane 1) results in a single band, representing two wild-type KAR2 alleles. Placing one KAR2 allele under control of the MET3 promoter (BPY104; lane 2) increases the size of the restriction fragment of one allele so that two bands are evident. The UAU1 disruption of the remaining wild-type KAR2 allele (BPY116; lane 3) corresponds to the emergence of the largest restriction fragment, the continued presence of the MET3 controlled allele, and the disappearance of the band corresponding to the wild-type size
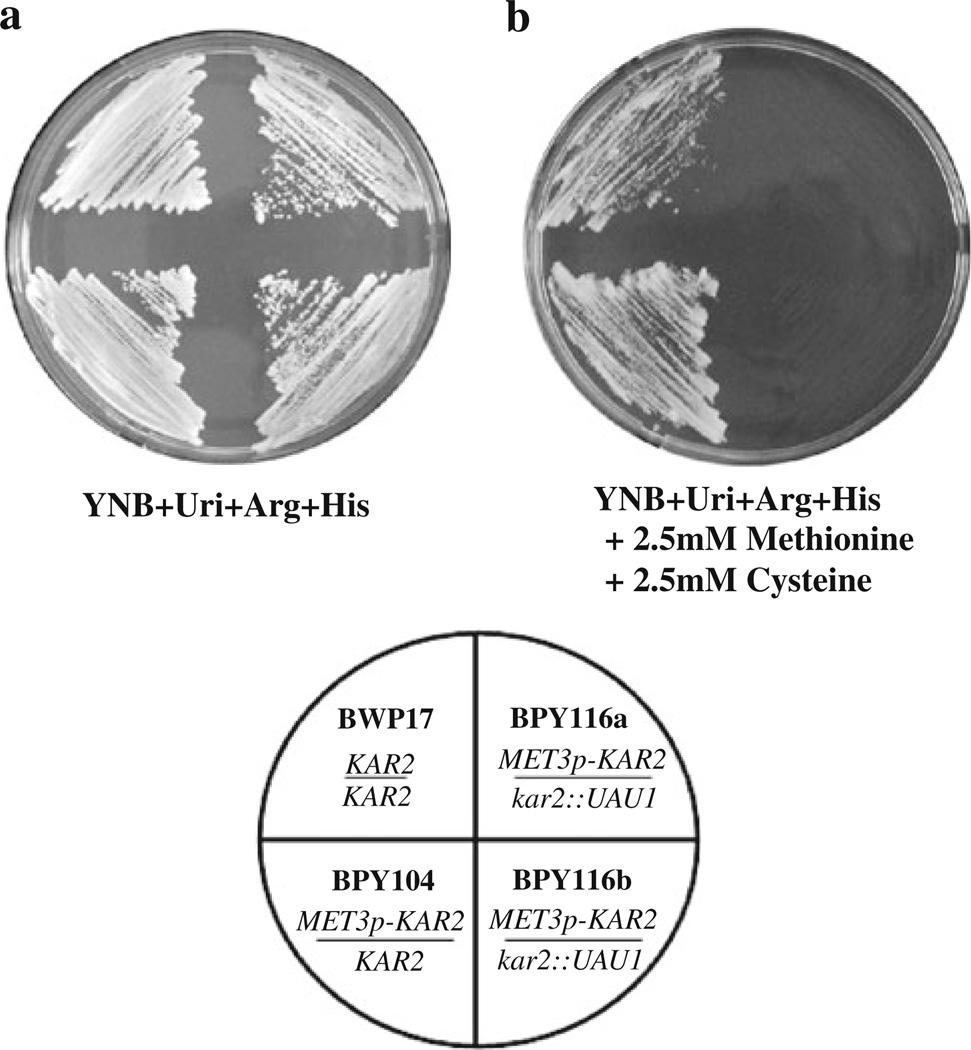
To determine if C. albicans KAR2 is essential, the MET3 promoter was used to repress the transcription of the lone functional KAR2 allele in strain BPY116 through the addition of 2.5 mM methionine and cysteine to the growth media (Care et al. 1999). Two independent isolates (BPY116a and BPY116b) were plated on the appropriate selective media either lacking (Fig. 2a) or containing 2.5 mM methionine and cysteine (Fig. 2b). After incubation at 30°C, neither of the conditional null mutants were viable in the presence of 2.5 mM methionine and cysteine (Fig. 2b). These results suggest that a functional copy of the KAR2 gene is essential in C. albicans.
Fig. 2.
The Candida albicans KAR2 gene is essential. Strains BWP17 (KAR2/KAR2), BPY104 (MET3p-KAR2/KAR2), BPY116a, and BPY116b (both MET3p-KAR2/kar2::UAU1) were streaked onto plates under conditions in which the MET3p-controlled KAR2 gene was either induced in medium lacking methionine and cysteine (a) or repressed in medium containing 2.5 mM methionine and 2.5 mM cysteine (b). Both plates were grown at 30°C for ~48 h. The conditional null mutants (BPY116a and BPY116b) are inviable when KAR2 expression is repressed by the MET3 promoter (b)
CaKAR2 rescues the growth of a kar2 Saccharomyces cerevisiae mutant strain
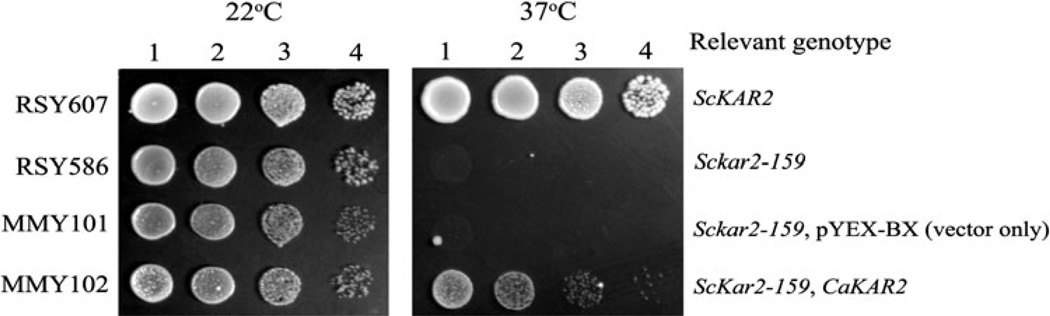
To better understand the function of the C. albicans Kar2 protein, we first determined if the CaKAR2 gene can rescue the temperature-sensitive growth phenotype observed for a S. cerevisiae strain containing the kar2-159 mutation, which is inviable at 37°C (Fig. 3; Vogel et al. 1990). This strain is defective for both the translocation of secretory proteins into the ER lumen (Vogel et al. 1990) and during the export of soluble secretory proteins back into the cytosol for degradation (Nishikawa et al. 2001).
Fig. 3.
The C. albicans KAR2 gene can rescue a temperature-sensitive allele of the S. cerevisiae kar2 gene. A S. cerevisiae strain containing the kar2-159 mutation (RSY586) was transformed with a plasmid expressing the C. albicans KAR2 gene, generating strain MMY102. Liquid cultures of these cells were grown in appropriate media overnight along with a wild-type S. cerevisiae strain (RSY607) and strain MMY101, which is RSY586 containing the empty vector. The cultures were normalized to a common OD600, and 5 µl of each culture (1) and tenfold serial dilutions (2–4) were then spotted onto two YPD plates. One plate was incubated at 22°C and the other at 37°C for ~48 h
A S. cerevisiae kar2-159 strain (RSY586) was transformed with plasmid pCaKAR2-BX, containing the C. albicans KAR2 gene under control of the CUP1 promoter, and with the control plasmid (pYEX-BX), generating strains MMY102 and MMY101, respectively. An isogenic wild-type stain, RSY607, was also examined. As seen in Fig. 3, all four strains grow well at the permissive temperature (22°C). However, the presence of the CaKAR2 gene, which encodes no CUG codons, rescues growth at the nonpermissive temperature (Fig. 3; MMY102), while the vector-only control continues to display a temperature-sensitive phenotype (Fig. 3; MMY101). These results indicate that the CaKAR2 gene partially rescues the kar2-159 thermosensitive growth defect in S. cerevisiae.
CaKAR2 can serve as the sole copy of the KAR2 gene in S. cerevisiae
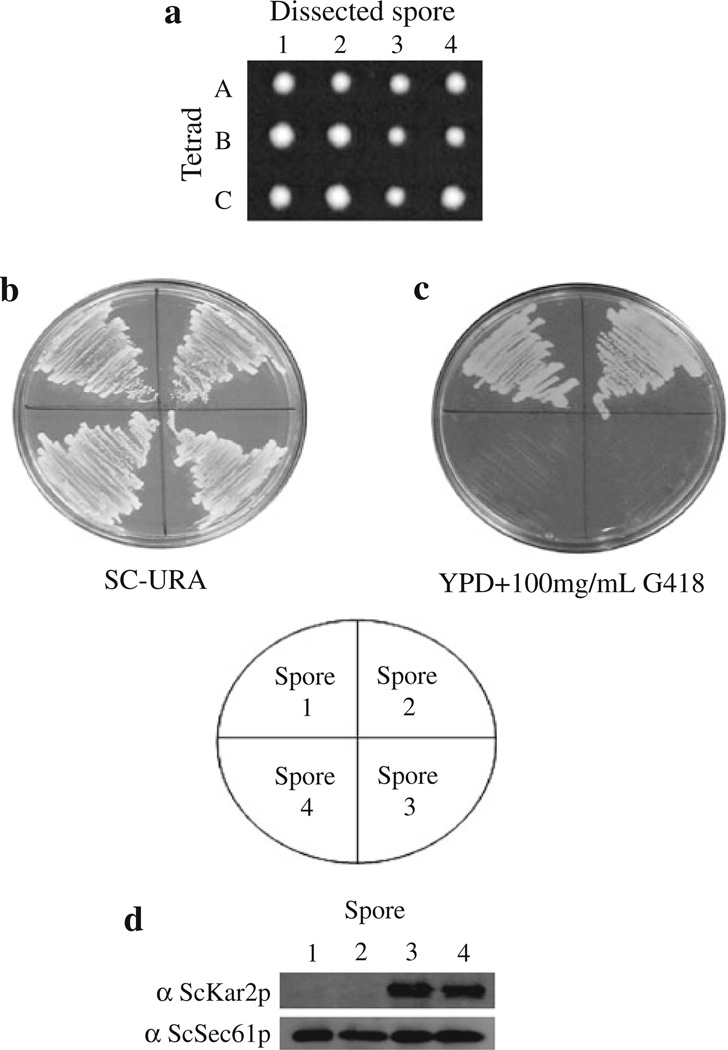
Since it is possible that the mutant form of S. cerevisiae Kar2p in strain MMY102 could work synergistically with C. albicans Kar2p to allow the cells to live at the non-permissive temperature, a S. cerevisiae strain containing the C. albicans KAR2 gene as the sole copy of the gene was produced. A classical tetrad dissection approach was employed to generate such a strain from a KAR2 hemizygous diploid S. cerevisiae strain (YJ034W BY4743) containing one wild-type KAR2 allele and a KAR2 allele that is deleted with the kanMX gene, conveying resistance to G418 (this strain does not contain the kar2-159 mutation). This strain was transformed with plasmid pCaKAR2-BX, containing the wild-type C. albicans KAR2 gene, generating strain MMY105, which was then sporulated. Spores obtained after dissection resulted in four equally sized colonies (Fig. 4a), while the diploid parent strain lacking the CaKAR2 plasmid resulted in only two colonies (data not shown). Since ScKAR2 is an essential gene in S. cerevisiae (Rose et al. 1989; Normington et al. 1989), these results indicate that the C. albicans KAR2 gene can replace the essential functions of KAR2 in S. cerevisiae.
Fig. 4.
The C. albicans KAR2 gene can serve as the sole copy of this essential gene in S. cerevisiae. Hemizygous diploid S. cerevisiae strain YJ034W BY4743, which has one copy of the ScKAR2 gene disrupted and one wild-type ScKAR2 gene, was transformed with plasmid pCaKAR2-BX containing the C. albicans KAR2 gene. The cells were then sporulated, and the four spores were dissected from each tetrad on YPD plates and incubated for ~72 h at 30°C (a). Three typical dissected tetrads are shown (A–C). The four spores from each tetrad resulted in normal sized colonies (1–4). To show that two of the spores from the tetrads lack the S. cerevisiae KAR2 gene, colonies from all four spores of one tetrad were streaked onto a SC-URA plate (b) as well as a YPD plate supplemented with 100 µg/mL G418 (c) and grown at 30°C for ~48 h. Lysates were made from cells derived from each spore, and the proteins were analyzed by western blot using antibodies generated against the S. cerevisiae Kar2 and Sec61 proteins (d)
To establish that two of the four colonies obtained during tetrad dissection were comprised of cells containing only the CaKAR2 gene, cells from each of the four spores from a single tetrad were streaked onto both SC-URA and YPD + 100 µg/mL G418. As can be seen in Fig. 4b, all four of the colonies contained cells that could grow in the absence of uracil in the growth media, suggesting that all four contained the plasmid harboring the C. albicans KAR2 gene. In addition, only two of the four colonies could grow in the presence of 100 µg/mL G418 (Fig. 4c; spores 1 and 2), which indicates that the cells from these two spores lack the S. cerevisiae copy of KAR2 (Fig. 4c).
ScKar2 protein expression was also assessed in cells derived from the four spores to verify that only two spores were expressing ScKar2p. Cell extracts were prepared from cells derived from all four spores. These extracts were then analyzed by western blot using an antibody generated against S. cerevisiae Kar2p (Brodsky and Schekman 1993). As can be seen in Fig. 4d, while all four lanes had comparable amounts of protein, as indicated by similar amounts of αScSec61p immunoreactivity, only the cells derived from the spores that were susceptible to G418 possessed ScKar2p (spores 3 and 4). These results suggest that the S. cerevisiae Kar2 protein is absent from cells derived from two of the four spores dissected from strain MMY105, and that the anti-ScKar2p antiserum does not cross react with the C. albicans Kar2 protein.
CaKar2p supports the translocation of a presecretory protein into ER-derived microsomes from S. cerevisiae
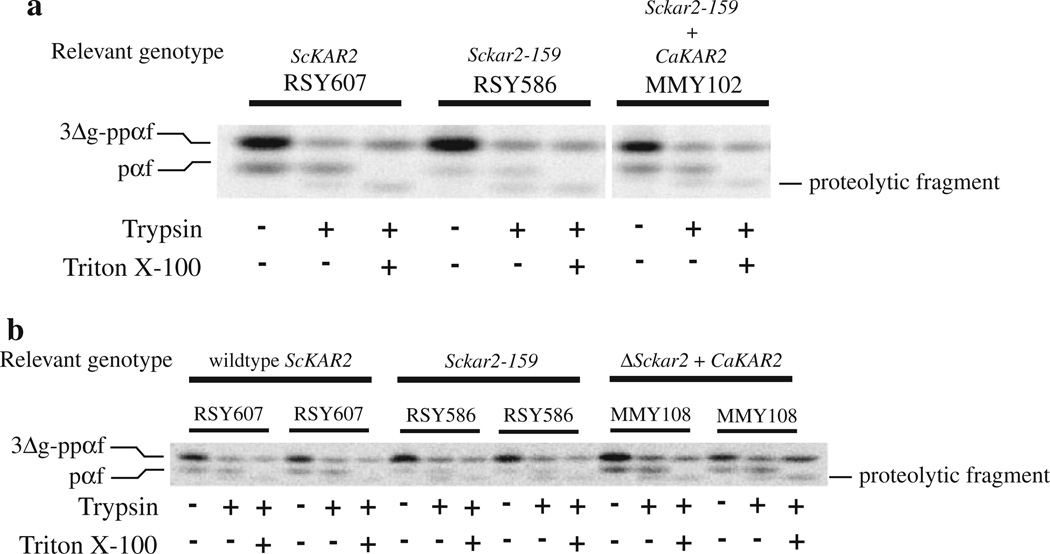
The data presented above support the idea that CaKar2p can functionally replace Kar2p from S. cerevisiae. To establish this fact at the level of CaKar2p’s ability to translocate presecretory proteins into the ER, a well-established in vitro translocation assay was employed (Morrow and Brodsky 2005; Morrow and Brodsky 2001; Brodsky et al. 1993). Briefly, yeast-derived ER microsomes were combined with in vitro translated 35S-labeled prepro alpha factor lacking the three N-linked glycosylation sites (3Δg–ppαf; McCracken and Brodsky 1996), purified yeast cytosol, and an ATP regenerating system. After incubation, the reactions were separated into three tubes. One tube remained untreated while one was treated with trypsin, and the other with trypsin and Triton X-100. Upon successful translocation, the 3Δg–ppαf signal sequence is cleaved from this ~18.6 kD secreted yeast mating pheromone, resulting in the 18 kD pro-alpha factor (pαf) species, which is contained within the microsomes and protected from the exogenously added protease. The Triton X-100 treated reaction serves as a control to show that the protease-protected pαf is dependant on an intact membrane and is not simply protease resistant due to aggregation. It should be noted that S. cerevisiae 3Δg–ppαf is prone to aggregation, which generates a subpopulation of protease resistant 3Δg–ppαf (Brodsky and Schekman 1993).
ER microsomes were purified from S. cerevisiae strain MMY102, which contains the Sckar2-159 allele in addition to the plasmid expressing the C. albicans KAR2 gene (described above). Microsomes were also purified from an isogenic wild-type S. cerevisiae strain (RSY607) and RSY586, which contains the kar2-159 allele but lacks the CaKAR2-expressing plasmid. ER microsomes prepared from RSY586 have been shown to be defective in in vitro translocation reactions performed at 20°C (Brodsky et al. 1995, 1993; Sanders et al. 1992). All three microsome preparations were then used in in vitro translocation assays as described above. Figure 5a shows that microsomes isolated from wild-type yeast cells (RSY607) translocate 3Δg–ppαf, forming trypsin-protected pαf (lane 2), while the kar2-159 mutant microsomes lacking CaKar2p (RSY586) are defective for this process. Microsomes prepared from wild-type yeast converted 22.5% (SE = ±2.7%, n = 4) of the 3Δg–ppαf to protease-protected pαf, while microsomes prepared from the kar2-159 mutant strain only converted 7.1% (SE = ±1.0%, n = 4) of the 3Δg–ppαf to protease-protected pαf. In contrast, microsomes prepared from strain MMY102, containing both the mutant S. cerevisiae and wild-type C. albicans Kar2 proteins, translocate 3Δg–ppαf to levels approaching that of wild type (16.5%, SE = ±1.6%, n = 4; Fig. 5a). These results indicate that CaKar2p replaces the function of the S. cerevisiae Kar2 protein during post-translational translocation.
Fig. 5.
The C. albicans Kar2 protein can replace the function of S. cerevisiae Kar2p during translocation in vitro. ER microsomes were prepared from a wild-type S. cerevisiae strain (RSY607), a kar2 mutant strain (RSY586) and strain MMY102 (a), which is RSY586 expressing wild-type C. albicans Kar2p, or MMY108 (b), which is a S. cerevisiae strain containing C. albicans Kar2p as the only form of Kar2p in the cells. These microsomes were used in in vitro translocation reactions using 35S-labeled 3Δg–ppαf as the translocation substrate (see text). After translocation, the reactions were split equally into three tubes and either left untreated, or treated with trypsin or trypsin and Triton X-100. The proteins were then TCA precipitated and resolved by 18% SDS-PAGE in gels containing urea and subjected to phosphorimage analysis. The presence of trypsin-protected pαf indicates substrate translocation. Duplicate translocation reactions are shown in b
If the C. albicans Kar2 protein functions similarly to the S. cerevisiae Kar2 protein during translocation, then microsomes prepared from a S. cerevisiae strain containing only the C. albicans KAR2 gene should also be able to translocate 3Δg–ppαf. To this end, S. cerevisiae strain MMY108, containing only CaKar2p (from the tetrad dissection), was grown to log phase and microsomes were prepared. These microsomes, as well as microsomes prepared from wild type and the kar2-159 mutant, which serve as positive and negative controls, respectively, were then used in translocation assays as described above. As shown in Fig. 5b, microsomes containing C. albicans Kar2p as the only version of the protein (MMY108) translocate 3Δg–ppαf as effectively as those containing wild-type S. cerevisiae Kar2p. Quantitation of the protease-protected pαf suggests that 23.3% (SE = ±2.3%, n = 16) and 23.5% (SE = ±3.1%, n = 16) of the 3Δg–ppαf was converted to protease-protected pαf in reactions containing microsomes from wild type and CaKar2p-containing cells, respectively. These results suggest that the C. albicans Kar2 protein functions much like the S. cerevisiae version of the protein, and can replace its function during the translocation of presecretory proteins into the ER.
CaKar2p is involved in the process of ER translocation in C. albicans
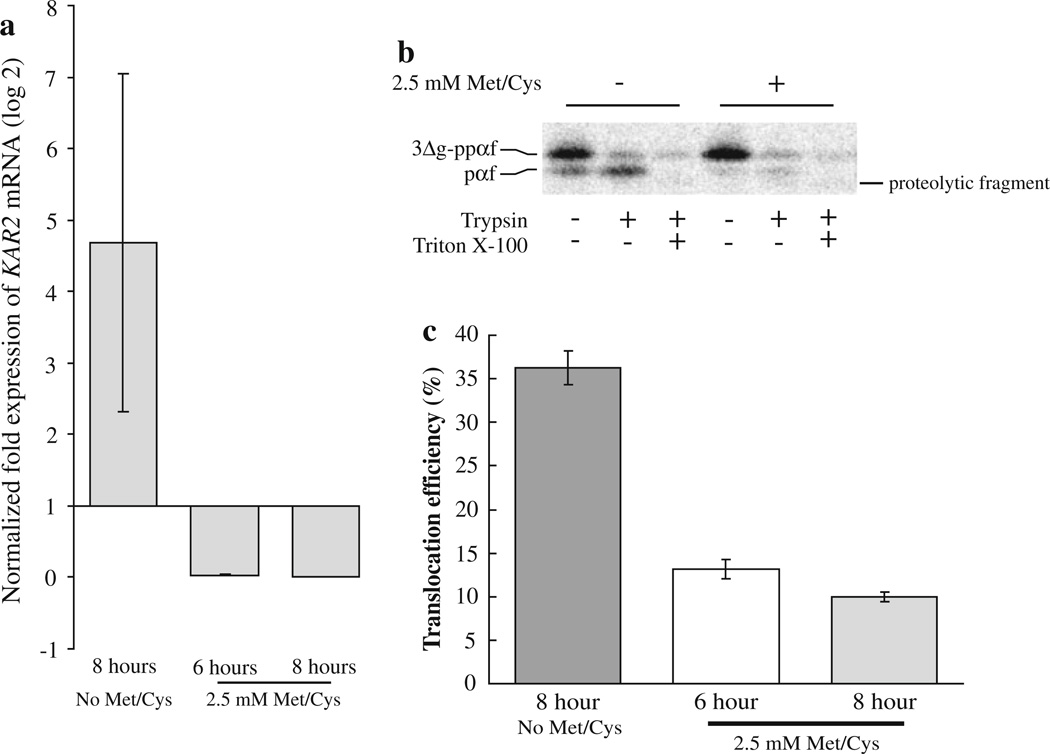
In order to determine if Kar2p plays a role during the translocation of presecretory proteins into the ER in C. albicans, we performed in vitro translocation assays using ER microsomes prepared from C. albicans strain BPY116 either expressing or not expressing CaKar2p. To verify that strain BPY116 expresses KAR2 mRNA when grown in the absence of methionine, but not when grown in the presence of methionine, a qRT-PCR experiment was performed. A concentration of 2.5 mM methionine and cysteine was used in these experiments since this concentration did not have an affect on the growth of wild-type C. albicans cells (data not shown). BPY116 cells grown in the presence of 2.5 mM methionine and cysteine for 6 and 8 h express ~14-fold (SD = ±5.4) and over 1,000-fold (2,422-fold, SD = ±1,227) less KAR2 mRNA, respectively, as compared to BPY116 cells grown for 8 h in the absence of methionine and cysteine (Fig. 6a). These results strongly suggest that the inducible promoter in strain BPY116 functions as expected with regard to KAR2 mRNA accumulation.
Fig. 6.
Kar2p functions during the translocation of a substrate protein into the C. albicans ER in vitro. BPY116 C. albicans cells were grown in YNB + His (No Met/Cys; inducing conditions) or YNB + His + 2.5 mM methionine and cysteine (repressing conditions) for 6 and 8 h. Total RNA was purified from three ODs of cells, and qRT-PCR was performed to determine the amount of KAR2 mRNA present in the cells (a). The data represent the means of two biological replicates with a total of six technical replicates for the repressed cultures (6 h = 0.028, SD = ±0.010, 8 h = 0.0019, SD = ± 9.9 × 10−4), and the mean of three technical replicates for the induced culture (4.68, SD = ±2.37). KAR2 mRNA levels were normalized to actin and alpha-tubulin genes in each condition, and expression levels are relative to KAR2 expression at the time of inoculation (zero time point, expression = 1). ER microsomes were then purified from strain BPY116 after growth for 8 h in media either containing (+) or lacking (−) 2.5 mM methionine and cysteine. The microsomes were combined with C. albicans cytosol, 35S-labeled S. cerevisiae 3Δg–ppαf and an ATP regenerating system. Translocation assays were performed as described, and typical reactions are shown (b). The presence of trypsin-protected pαf indicates successful translocation. Translocation reactions were also performed using microsomes prepared from strain BPY116 after growth under repressing conditions for 6 and 8 h (n = 15 and 18, respectively). Average translocation efficiencies were compared to translocation efficiencies into microsomes prepared from BPY116 cells grown under inducing conditions for 8 h (n = 18). Standard error of the means are shown as error bars (c)
Candida albicans strain BPY116 was grown in medium lacking methionine and cysteine and then used to inoculate two cultures, one lacking methionine and cysteine and the other containing 2.5 mM concentrations of the two amino acids. After 6 and 8 h of growth, the cells were harvested and ER microsomes were isolated. These microsomes were then used in in vitro translocation assays as described above, but using cytosol isolated from wild-type C. albicans cells. As can be seen in Fig. 6b, after 8 h of growth, microsomes prepared from C. albicans cells expressing Kar2p (no Met/Cys) displayed a significantly higher translocation efficiency (36.3%, SE = ±1.96%, n = 18) than those prepared from cells expressing reduced amounts of KAR2 mRNA (2.5 mM Met/Cys; 10.0%, SE = ±0.54%, n = 18). In addition, the efficiency of translocation into microsomes prepared from cells expressing reduced amounts of KAR2 mRNA decreased over the amount of time grown in medium containing methionine and cysteine (Fig. 6c), suggesting that Kar2p turnover results in decreased translocation activity. Together, these data support the conclusion that Kar2p is required for the translocation of presecretory proteins into the C. albicans ER.
Discussion
We have determined that the C. albicans KAR2 gene is essential and functions during the translocation of proteins into the endoplasmic reticulum, indicating the importance of the encoded Hsp70 molecular chaperone protein in C. albcians. It is probable that the absence of Kar2p function during the process of secretory protein transport into the ER is the reason for inviability under the conditions used in this study, a suggestion that is supported by the fact that reduced Kar2p expression results in a translocation defect in C. albicans (Fig. 6b, c). This is likely because translocation into the ER is the first committed step of the secretory pathway, which is an essential process for protein targeting in the cell. Since ScKar2p has also been shown to play an essential role during protein folding in S. cerevisiae (Simons et al. 1995), it is also possible that the deletion of CaKAR2 results in inviability due to a defect in protein folding.
As well as its essential roles during translocation and protein folding, it is possible that the C. albicans Kar2 protein may also display functional overlap with other non-essential roles that Kar2p plays in S. cerevisiae, such as during the process of ER-associated degradation (ERAD, Nishikawa et al. 2005; 2001; Kabani et al. 2003; Plemper et al. 1997) and karyogamy (Ng and Walter 1996; Latterich and Schekman 1994; Rose et al. 1989; Normington et al. 1989). Interestingly, a recent study has identified that C. albicans Jem1p, which is a co-chaperone protein that interacts with Kar2p and functions during ERAD and karyogamy, can complement karyogamy and ERAD defects in S. cerevisiae (Makio et al. 2008). Given the facts that our study shows that CaKar2p can replace the essential functions of ScKar2p, and that Makio et al. (2008) showed that CaJem1p can effectively interact with ScKar2p during ERAD and karyogamy, together these results suggest that Kar2p and Jem1p may interact in C. albicans as they do in S. cerevisiae during ERAD. In support of this hypothesis, Wimalasena et al. (2008) have shown that some of the machinery involved in C. albicans ERAD is regulated similar to that of S. cerevisiae.
In addition to our in vivo data, we also determined that the C. albicans Kar2 protein functions in place of S. cerevisiae Kar2p during the translocation of a presecretory protein into the ER lumen. In fact, translocation efficiencies using S. cerevisiae microsomes containing only CaKar2p were nearly identical to the efficiencies obtained when using microsomes containing only wild-type ScKar2p. These results suggest that C. albicans Kar2 protein can completely replace the function of the S. cerevisiae Kar2 protein during translocation. However, in translocation reactions using microsomes containing both a mutant form of ScKar2p in addition to wild-type C. albicans Kar2p, translocation efficiencies were approximately 73% of microsomes containing only wild-type ScKar2 protein (16.5% for CaKar2p + mutant ScKar2p and 22.5% for wild-type ScKar2p). In these translocation reactions, it is possible that the presence of a mutant form of S. cerevisiae Kar2p inhibits the complete rescue of translocation activity by C. albicans Kar2p. This could be due to the fact that the mutant form of the S. cerevisiae protein may engage in normal complex formation with ScSec61p, ScSec63p and other proteins, thus sequestering these other factors from forming functional complexes with the C. albicans Kar2 protein. Since Kar2p must associate with Sec63p and Sec61p to perform its many roles during the translocation of proteins into the ER (Alder et al. 2005; Young et al. 2001; McClellan et al. 1998; Matlack et al. 1997; Lyman and Schekman 1995; Sanders et al. 1992), it is possible that this sequestration could inhibit the translocation process in these microsomes.
Importantly, we were able to successfully use an in vitro translocation assay to show that C. albicans microsomes isolated from a strain expressing reduced amounts of Kar2p display a translocation defect when compared to microsomes containing normal levels of Kar2p (Fig. 6). This is the first example of the use of an in vitro assay to study ER translocation using components derived from C. albicans, which provides a very useful system for studying the roles of other proteins involved in ER translocation. Our translocation assays using C. albicans cytosol and ER microsomes isolated from a C. albicans strain expressing Kar2p from the MET3 promoter displayed translocation efficiencies (~36%; Fig 6c, no met/cys) that surpass translocation efficiencies using components derived from wild-type S. cerevisiae (~23%; see Figs. 5a, b, RSY607), indicating that this assay functions similarly with C. albicans components. It is possible that this increased efficiency is due to the enhanced KAR2 expression from the MET3 promoter used in strain BPY116. In vitro translocation assays using ER-derived microsomes and reconstituted proteoliposomes (Sanders et al. 1992) have been used to extensively investigate ER translocation processes in S. cerevisiae including the establishment of the minimal components necessary for post-translational translocation (Panzner et al. 1995), the components that interact with the signal sequence of secretory proteins (Plath et al. 1998), and specific chaperone interactions (Brodsky et al. 1993). Additionally, in vitro translocation reactions are the first step in cell-free ERAD assays that have been used to dissect the mechanisms of this process in S. cerevisiae (Lee et al. 2004, 2005; McCracken and Brodsky 1996). The development and use of this translocation assay using C. albicans components may assist in establishing a deeper understanding of how protein import into the ER and ERAD occur in this human pathogen.
Finally, while the classic secretory pathway appears to be relatively conserved in eukaryotes, alternative secretory pathways are used in C. albicans (reviewed in Fonzi 2009 and Nombela et al. 2006). In addition, the C. albicans Sec61 protein appears to be unable to functionally replace its orthologue in S. cerevisiae, despite being 76% similar and codon-corrected for expression in S. cerevisiae (de la Rosa et al. 2004b). The Sec20 protein in C. albicans has also been suggested to be unable to complement its orthologue in S. cerevisiae (Weber et al. 2001). While there are multiple possible explanations as to why these C. albicans proteins do not complement the functions of their S. cerevisiae homologues, these studies suggest that while the secretory pathway is highly conserved in eukaryotic systems, specific differences may exist in C. albicans. Since the classic secretory pathway is essential for viability and virulence of this organism, differences in the function(s) of proteins involved in this pathway may serve as potential future chemotherapeutic targets for preventing and treating candidiasis. It will be interesting to examine the direct role of the secretory pathway on C. albicans virulence in future studies.
Acknowledgments
This research was funded by grant number P20 RR-16455-10 from the National Center for Research Resources (NCRR), a component of the National Institute of Health (NIH). We would like to thank Drs David Butler and Kurt Toenjes (Montana State University-Billings), Dr. Robert Cramer (Montana State University-Bozeman) and Dr. Jeffrey Brodsky (University of Pittsburgh) for providing strains, αScKar2p and αScSec61p antisera, qRT-PCR facility use, technical assistance and critically reviewing this manuscript. In addition, we also thank Drs Karen Arndt and Margaret Shirra (University of Pittsburgh) for assistance with tetrad dissections and Dr. Peter Sudbery (University of Sheffield) for providing plasmid pCaDis. Finally, we would like to thank Drs Qi Zhao and William C. Nierman (TIGR), Frank J. Smith and Aaron P. Mitchell (Carnegie Mellon University), and NIH grant 1R01AI057804 for the UAU1-containing clone, CAGA643.
References
- Alder NN, Shen Y, Brodsky JL, Hendershot LM, Johnson AE. The molecular mechanism underlying BiP-mediated gating of the Sec61 translocon of the endoplasmic reticulum. J Cell Biol. 2005;168:389–399. doi: 10.1083/jcb.200409174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, Brown AJ, Odds FC, Gow NA. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem. 2005;280:23408–23415. doi: 10.1074/jbc.M502162200. [DOI] [PubMed] [Google Scholar]
- Bernardo SM, Khalique Z, Kot J, Jones JK, Lee SA. Candida albicans VPS1 contributes to protease secretion, filamentation and biofilm formation. Fungal Genet Biol. 2008;45:861–877. doi: 10.1016/j.fgb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteo-liposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Hamamoto S, Feldheim D, Schekman R. Reconstitution of protein translocation from solubilized yeast membranes reveals topologically distinct roles for BiP and cytosolic Hsc70. J Cell Biol. 1993;120:95–102. doi: 10.1083/jcb.120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Goeckeler J, Schekman R. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care RS, Trevethick J, Binley KM, Sudbery PE. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol. 1999;34:792–798. doi: 10.1046/j.1365-2958.1999.01641.x. [DOI] [PubMed] [Google Scholar]
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008;72:495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin WL, López-Ribot JL, Casanova M, Gozalbo D, Martínez JP. Cell wall and secreted proteins of Candida albicans: identification, function and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément M, Fournier H, De Repentigny L, Belhumeur P. Isolation and characterization of the Candida albicans SEC4 gene. Yeast. 1998;14:675–680. doi: 10.1002/(SICI)1097-0061(199805)14:7<675::AID-YEA252>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Davis DA, Bruno VM, Loza L, Filler SG, Mitchell AP. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics. 2002;162:1573–1581. doi: 10.1093/genetics/162.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rosa JM, González JM, Gutiérrez F, Ruíz T, Rodríguez L. Characterization of Candida albicans orthologue of the Saccharomyces cerevisiae signal-peptidase-subunit encoding gene SPC3. Yeast. 2004a;21:883–894. doi: 10.1002/yea.1137. [DOI] [PubMed] [Google Scholar]
- De la Rosa JM, Ruíz T, Fonzi WA, Rodríguez L. Analysis of heterologous expression of Candida albicans SEC61 gene reveals differences in Sec61p homologues related to species-specific functionality. Fungal Genet Biol. 2004b;41:941–953. doi: 10.1016/j.fgb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Enloe B, Diamond A, Mitchell AP. A single-transformation gene function test in diploid Candida albicans. J Bacteriol. 2000;182:5730–5736. doi: 10.1128/jb.182.20.5730-5736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler SG, Kullberg BJ. Deep-seated candidal infections. In: Calderone RA, editor. Candida and candidiasis. Washington, DC: ASM Press; 2002. pp. 341–348. [Google Scholar]
- Fonzi WA. The protein secretory pathway of Candida albicans. Mycoses. 2009;52:291–303. doi: 10.1111/j.1439-0507.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- Gagnon-Arsenault I, Tremblay J, Bourbonnais Y. Fungal yapsins and cell wall: a unique family of aspartic peptidases for a distinctive cellular function. FEMS Yeast Res. 2006;6:966–978. doi: 10.1111/j.1567-1364.2006.00129.x. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol. 1999;10:465–472. doi: 10.1006/scdb.1999.0318. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Haigh NG, Johnson AE. A new role for BiP: closing the aqueous translocon pore during protein integration into the ER membrane. J Cell Biol. 2002;156:261–270. doi: 10.1083/jcb.200110074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman BD, Hendershot LM, Johnson AE. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 1998;92:747–758. doi: 10.1016/s0092-8674(00)81403-8. [DOI] [PubMed] [Google Scholar]
- Hube B. Candida albicans secreted aspartyl proteinases. Curr Top Med Mycol. 1996;7:55–69. [PubMed] [Google Scholar]
- Kabani M, Kelley SS, Morrow MW, Montgomery DL, Sivendran R, Rose MD, Gierasch LM, Brodsky JL. Dependence of endoplasmic reticulum-associated degradation on the peptide binding domain and concentration of BiP. Mol Biol Cell. 2003;14:3437–3448. doi: 10.1091/mbc.E02-12-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg BJ, Filler SG. Candidemia. In: Calderone RA, editor. Candida and candidiasis. Washington, DC: ASM Press; 2002. pp. 327–340. [Google Scholar]
- Latterich M, Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994;78:87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Liu C, Harty C, McCracken AA, Latterich M, Römisch K, DeMartino GN, Thomas PJ, Brodsky JL. Uncoupling retro-translocation and degradation in the ER-associated degradation of a soluble protein. EMBO J. 2004;23:2206–2215. doi: 10.1038/sj.emboj.7600232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, McCracken AA, Brodsky JL. Reconstitution of endoplasmic reticulum-associated degradation using yeast membranes and cytosol. Methods Mol Biol. 2005;301:175–184. doi: 10.1385/1-59259-895-1:175. [DOI] [PubMed] [Google Scholar]
- Lee SA, Jones J, Hardison S, Kot J, Khalique Z, Bernardo SM, Lazzell A, Monteagudo C, Lopez-Ribot J. Candida albicans VPS4 is required for secretion of aspartyl proteases and in vivo virulence. Mycopathologia. 2009;167:55–63. doi: 10.1007/s11046-008-9155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2T−ΔΔC method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J Cell Biol. 1995;131:1163–1171. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makio T, Nishikawa S, Nakayama T, Nagai H, Endo T. Identification and characterization of a Jem1p ortholog of Candida albicans: dissection of Jem1p functions in karyogamy and protein quality control in Saccharomyces cerevisiae. Genes Cells. 2008;13:1015–1026. doi: 10.1111/j.1365-2443.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- Mao Y, Kalb VF, Wong B. Overexpression of a dominant-negative allele of SEC4 inhibits growth and protein secretion in Candida albicans. J Bacteriol. 1999;181:7235–7242. doi: 10.1128/jb.181.23.7235-7242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack KE, Plath K, Misselwitz B, Rapoport TA. Protein transport by purified yeast Sec somplex and Kar2p without membranes. Science. 1997;277:938–941. doi: 10.1126/science.277.5328.938. [DOI] [PubMed] [Google Scholar]
- Matlack KES, Misselwitz B, Plath K, Rapoport TA. BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell. 1999;97:553–564. doi: 10.1016/s0092-8674(00)80767-9. [DOI] [PubMed] [Google Scholar]
- McClellan AJ, Endres JB, Vogel JP, Palazzi D, Rose MD, Brodsky JL. Specific molecular chaperone interactions and an ATP-dependent conformational change are required during posttranslational protein translocation into the yeast ER. Mol Biol Cell. 1998;9:3533–3545. doi: 10.1091/mbc.9.12.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken AA, Brodsky JL. Assembly of ER-associated protein degradation in vitro: dependence on cytosol, calnexin and ATP. J Cell Biol. 1996;132:291–298. doi: 10.1083/jcb.132.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda MS, Lopez AJ, Guyot A. Candida inferior vena cava filter infection and septic thrombophlebitis. Br J Radiol. 2007;80:e48–e49. doi: 10.1259/bjr/13944004. [DOI] [PubMed] [Google Scholar]
- Monteoliva L, Sánchez M, Pla J, Gil C, Nombela C. Cloning of Candida albcians SEC14 gene homologue coding for a putative essential function. Yeast. 1996;12:1097–1105. doi: 10.1002/(SICI)1097-0061(19960915)12:11%3C1097::AID-YEA990%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Morrow MW, Brodsky JL. Yeast ribosomes bind to highly purified reconstituted Sec61p complex and to mammalian p180. Traffic. 2001;2:705–716. doi: 10.1034/j.1600-0854.2001.21005.x. [DOI] [PubMed] [Google Scholar]
- Morrow MW, Brodsky JL. The encyclopedia of life sciences. Vol. 15. New York: Nature Publishing Group; 2005. Protein import into endoplasmic reticulum: methods; pp. 346–348. [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Micro Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT, Walter P. ER membrane protein complex required for nuclear fusion. J Cell Biol. 1996;132:499–509. doi: 10.1083/jcb.132.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto A, Sanz P, Sentandreu R, Agudo LDC. Cloning and characterization of the SEC18 gene from Candida albicans. Yeast. 1993;9:875–887. doi: 10.1002/yea.320090808. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Fewell SW, Kato Y, Brodsky JL, Endo T. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J Cell Biol. 2001;153:1061–1069. doi: 10.1083/jcb.153.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Brodsky JL, Nakatsukasa K. Roles of molecular chaperones in endoplasmic reticulum (ER) quality control and ER-associated degradation (ERAD) J Biochem. 2005;137:551–555. doi: 10.1093/jb/mvi068. [DOI] [PubMed] [Google Scholar]
- Nombela C, Gil C, Chaffin L. Non-conventional protein secretion in yeast. Trends Micro. 2006;14:15–21. doi: 10.1016/j.tim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Normington K, Kohno K, Kozutsumi Y, Gething MJ, Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989;57:1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in pottranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Regnacq M, Hewitt E, Allen J, Rosamond J, Stirling CJ. Deletion analysis of yeast Sec65p reveals a central domain that is sufficient for function in vivo. Mol Microbiol. 1998;29:753–762. doi: 10.1046/j.1365-2958.1998.00969.x. [DOI] [PubMed] [Google Scholar]
- Rico H, Herrero E, Miragall F, Sentandreu R. An electron microscopy study of wall expansion during Candida albicans yeast and mycelial growth using concanavalin A-ferritin labeling of mannoproteins. Arch Microbiol. 1991;156:111–114. doi: 10.1007/BF00290982. [DOI] [PubMed] [Google Scholar]
- Romani L, Bistoni F, Puccetti P. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr Opin Microbiol. 2003;6:338–343. doi: 10.1016/s1369-5274(03)00081-x. [DOI] [PubMed] [Google Scholar]
- Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Springs Harbor, NY: Cold Springs Harbor Laboratory Press; 1990. [Google Scholar]
- Rothblatt JA, Deshaies RJ, Sanders SL, Daum G, Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum of yeast. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnke M. Skin and mucous membrane infections. In: Calderone RA, editor. Candida and candidiasis. Washington, DC: ASM Press; 2002. pp. 307–325. [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman RW. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Sandovsky-Losica H, Segal E. Infection of HEp2 epithelial cells with Candida albicans: adherence and postadherence events. FEMS Immunol Med Microbiol. 2006;46:470–475. doi: 10.1111/j.1574-695X.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- Simons JF, Ferro-Novick S, Rose MD, Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Schwenk J, Urban C, Lechner J, Schweikert M, Rupp S. Getting in touch with Candida albicans: the cell wall of a fungal pathogen. Curr Drug Targets. 2006;7:505–512. doi: 10.2174/138945006776359395. [DOI] [PubMed] [Google Scholar]
- Sorger PK, Pelham HRB. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987;6:3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Lopez-Ribot JL, Lee SA. A proteomic analysis of secretory proteins of a pre-vacuolar mutant of Candida albicans. J Proteomics. 2009;73:342–351. doi: 10.1016/j.jprot.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G, Bouchara JP, Annaix V, Robert R, Senet JM. Fungal cell adhesion molecules in Candida albicans. Eur J Epidemiol. 1991;7:23–33. doi: 10.1007/BF00221338. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Misra LM, Rose MD. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pöhlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Weber Y, Santore UJ, Ernst JF, Swoboda RK. Divergence of eukaryotic secretory components: the Candida albicans homolog of the Saccharomyces cerevisiae Sec20 protein is N terminally truncated, and its levels determine antifungal drug resistance and growth. J Bacteriol. 2001;183:46–54. doi: 10.1128/JB.183.1.46-54.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, Brown AJP, Archer DB. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth of Candida albicans. Fungal Genet Biol. 2008;45:1235–1247. doi: 10.1016/j.fgb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Young BP, Craven RA, Reid PJ, Willer M, Stirling C. Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J. 2001;20:262–271. doi: 10.1093/emboj/20.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]