Vascular endothelial growth factor (VEGF) expression is regulated by sequence elements in the 3′ UTR of VEGF mRNA. AUF1/hnRNP D suppresses VEGF 3′ UTR–dependent expression. Peptides with arginine–glycine–glycine motifs derived from AUF1 also suppress VEGF expression.
Abstract
Vascular endothelial growth factor (VEGF) is a regulator of vascularization in development and is a key growth factor in tissue repair. In disease, VEGF contributes to vascularization of solid tumors and arthritic joints. This study examines the role of the mRNA-binding protein AUF1/heterogeneous nuclear ribonucleoprotein D (AUF1) in VEGF gene expression. We show that overexpression of AUF1 in mouse macrophage-like RAW-264.7 cells suppresses endogenous VEGF protein levels. To study 3′ untranslated region (UTR)–mediated regulation, we introduced the 3′ UTR of VEGF mRNA into a luciferase reporter gene. Coexpression of AUF1 represses VEGF-3′ UTR reporter expression in RAW-264.7 cells and in mouse bone marrow–derived macrophages. The C-terminus of AUF1 contains arginine–glycine–glycine (RGG) repeat motifs that are dimethylated. Deletion of the RGG domain of AUF1 eliminated the repressive effects of AUF1. Surprisingly, expression of an AUF1-RGG peptide reduced endogenous VEGF protein levels and repressed VEGF-3′ UTR reporter activity in RAW-264.7 cells. These findings demonstrate that AUF1 regulates VEGF expression, and this study identifies an RGG peptide that suppresses VEGF gene expression.
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a growth factor critical for blood vessel growth (Ferrara, 2004). In addition, VEGF acts as a cytokine that stimulates immune cells (Ferrara, 2004) and increases vascular permeability (Fava et al., 1994). In development, VEGF is so critical to blood vessel formation that deletion of one VEGF allele in mice results in embryonic lethality (Ferrara, 2004). In adults, VEGF acts in a limited and temporal way in processes that require the formation of new tissue, such as wound healing (Bao et al., 2009), the proliferative stage of the menstrual cycle (Critchley et al., 2001), and bone repair (Holstein et al., 2011). In disease, neovascularization is key to the growth of solid tumors (Ferrara, 2004), loss of sight in intraocular vascularization (Ferrara, 2004), and nourishing of inflamed tissue, including the arthritic joint (Paleolog, 2009) and atherosclerotic plaques (Salomonsson et al., 2002). Therapeutic treatments with VEGF to induce angiogenesis are being studied (Ferrara, 2004), as are therapies that block VEGF protein or VEGF receptors, primarily in solid cancers (Ferrara, 2004).
In addition to modulating blood vessel growth, therapies targeting VEGF also affect activation of immune cells that bear VEGF receptors. The macrophage is a central mediator of the innate immune response and plays a critical role in inflammatory processes (Gordon, 2007; Paleolog, 2009). Recent studies show that the macrophage combines the innate immune response (activation of Toll receptors by bacteria/lipopolysaccharide [LPS]) with the adaptive immune response (Fc-receptor recognition of immune complexes; Rittirsch et al., 2009). VEGF activates a family of cellular receptors (Hiratsuka et al., 2001). Acting as a cytokine, VEGF facilitates macrophage infiltration into inflamed sites (Fava et al., 1994). In blood vessel growth, VEGFR-2 is the key receptor and is found on endothelial cells (Hiratsuka et al., 2001). Another receptor, VEGFR-1, is also found on endothelial cells but is considered a decoy receptor that down-regulates VEGF activity (Hiratsuka et al., 2001). The relationship between VEGF and macrophage activation has not been explored in depth, in part because the macrophage expresses the VEGFR-1 receptor (Flt-1) but not VEGFR-2 (Flk-1; Hiratsuka et al., 2001). For these reasons, the role of VEGF activation of macrophages must be seen not simply with respect to blood vessel growth, but also in terms of a cytokine promoting macrophage entry into tissue (vascular permeability) and activating macrophages through VEGFR-1, perhaps by an autocrine mechanism.
Posttranscriptional regulation of gene expression is controlled by sequence elements in mRNA and by mRNA-binding proteins that recognize mRNA sequence elements. The mRNA regulatory protein HuR controls VEGF translation by stabilizing VEGF mRNA (Goldberg-Cohen et al., 2002). In contrast, AUF1/heterogeneous nuclear ribonucleoprotein (hnRNP) D (AUF1) regulates many mRNAs by destabilizing mRNA, although stabilization of mRNA has also been reported (Kiledjian et al., 1997; Sela-Brown et al., 2000). Genetic deletion of AUF1 in the mouse results in multiple disease phenotypes, including endotoxic shock (Lu et al., 2006), pruritic inflammatory skin disease (Sadri and Schneider, 2009), and B cell dysfunction (Sadri et al., 2010). However, the effect of AUF1 on VEGF gene expression has not been studied. Translational regulation of VEGF gene expression by other mRNA-regulatory proteins has been the subject of recent review (Yoo et al., 2006), and adenosine-uridine–rich elements (AUREs) have been characterized (Chen and Shyu, 1995; Xu et al., 1997; Ray and Fox, 2007). Genetic separation of the VEGF 3′ untranslated region (UTR) from the coding region by insertion of a reporter gene resulted in embryonic lethality in mice, demonstrating the importance of the 3′ UTR in regulating VEGF gene expression (Miquerol et al., 2000). We previously identified a region of VEGF 3′ UTR that contains an AURE, and we hypothesized that the AU-rich element affected gene expression (Du et al., 2006). We identified a similar AU-rich region in the 3′ UTR of glucose transporter-1 (GLUT1) mRNA (Griffin et al., 2004). A recent report shows that the AU-rich element in human VEGF mRNA regulates gene expression by alternating secondary structures of RNA (Ray and Fox, 2007). These facts led us to investigate the role of regulatory mRNA-binding proteins that control translation of VEGF mRNA in macrophages.
This study evaluates the effects of AUF1 on expression of endogenous VEGF protein and VEGF 3′ UTR–dependent reporters. We are the first to show that AUF1 affects endogenous VEGF protein levels and VEGF-3′ UTR–dependent gene expression. To investigate the mechanism of AUF1 action and identify novel regulators of VEGF gene expression, we focus on a regulatory region of the AUF1 protein. The C-terminal region contains multiple arginine–glycine–glycine (RGG) motifs in an RGG domain. The arginine residue in the third RGG motif is enzymatically methylated on the guanidinium side chain (Ong et al., 2004). The effects of blockade of arginine methylation are examined. Finally, we design peptides modeled on the AUF1 RGG domain and measure the effects on endogenous VEGF protein levels and VEGF-3′ UTR–dependent reporter activity. The identification of rationally designed peptide regulators of AUF1 may yield novel methods to repress VEGF gene expression.
RESULTS
AUF1 decreases VEGF protein levels
To determine whether the mRNA-binding protein AUF1 affects endogenous VEGF protein production, we cloned the p37 isoform of human AUF1 into the pcDNA-3.1-V5-His expression construct. Cultured RAW-264.7 macrophage-like cells were transfected with AUF1 and, after 4 h, treated with LPS (50 ng/ml) for 18 h. As shown in Figure 1, immunoblotting of VEGF protein levels shows that AUF1 decreased VEGF modestly (22%) but significantly. This result is especially interesting since not all cells are transfected successfully by transient transfection.
FIGURE 1:
Effects of overexpressing AUF1 on endogenous VEGF protein levels. Cultured RAW-264.7 cells were grown to 50% confluence and transfected with full-length AUF1 (AUF1) or the empty parental vector pcDNA-3.1 (PC). After 4 h, cells were treated with LPS for 18 h. Lysates were probed for VEGF and α-tubulin. Two of six experiments are shown. For six experiments, VEGF protein (normalized to α-tubulin) was decreased by 22% (p < 0.04).
The 3′ UTR region of VEGF represses reporter gene activity
To evaluate the role of the 3′ UTR of VEGF mRNA in gene expression, we created a luciferase reporter construct (Du et al., 2006). The 3′ UTR of VEGF mRNA destabilizes mRNA, and when the 3′ UTR of VEGF mRNA is introduced into the 3′ UTR of a heterologous reporter gene, the reporter activity decreases in resting macrophages (Du et al., 2006; Ray and Fox, 2007). When cells are activated, reporters with the VEGF-3′ UTR have increased activity (Du et al., 2006; Ray and Fox, 2007). As shown in Figure 2, introducing the 3′ UTR of VEGF into the 3′ UTR of the parental firefly luciferase reporter (3.1-Luc) to create the VEGF-3′ UTR-Luc reporter decreased gene activity in many cell types (Figure 2). The VEGF-3′ UTR decreased reporter activity in RAW-264.7 macrophage cells (60% decrease; Figure 2A), as previously reported (Du et al., 2006). The VEGF-3′ UTR also decreased reporter activity in mouse primary bone marrow–derived macrophages (BMDMs; 50% decrease; Figure 2B). The VEGF mRNA is regulated posttranscriptionally in the rat kidney cell (Feliers et al., 2005), and the VEGF-3′ UTR reduced reporter activity in the rat kidney cell line NRK-52E by 80% (Figure 2C). The reporter also showed reduced activity in the human embryonic kidney (HEK-293) cell line (70% decrease; Figure 2D).
FIGURE 2:
Effects of the 3′ UTR of VEGF mRNA on reporter activity in multiple cell types. (A) VEGF-3′ UTR-luciferase (VEGF-3′ UTR-Luc) or the empty parental vector (3.1-Luc) were transfected into RAW-264.7 cells using Lipofectamine 2000. Luciferase activity was measured in cell lysates. (B) Bone marrow–derived macrophages were obtained from C57/Bl6 mouse femurs and cultured in L-400 media. After 6 d, cells were trypsinized and transfected with VEGF-3′ UTR-Luc or 3.1-Luc by electroporation (Amaxa). (C) Normal rat kidney cells (NRK-52E) were cultured for <30 passages and transfected with VEGF-3′ UTR-Luc or 3.1-Luc using Lipofectamine 2000. (D) HEK-293 cells were transfected with VEGF-3′ UTR-Luc or 3.1-Luc using Lipofectamine 2000. All transfections (A–D) were incubated for 18–24 h before cell lysis. Results are normalized to 3.1-Luc transfection, and are representative of two or more experiments. Shown are mean and SD. p < 0.001 for A and C. p < 0.01 for B and D.
AUF1 decreases the activity of the VEGF-3′ UTR reporter
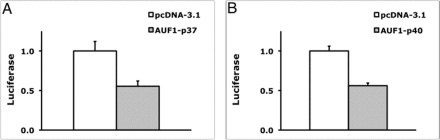
To determine whether AUF1 affects VEGF-3′ UTR–dependent expression, we cotransfected AUF1 in RAW-264.7 cells with the VEGF-3′ UTR-Luc reporter. As shown in Figure 3A, overexpression of AUF1 decreased luciferase reporter activity by 45%. The mRNA of AUF1 is alternatively spliced to produce four isoforms. The smaller isoforms (p37 and p40) are more predominant in the cytoplasm and are likely more important in regulation of mRNA stability (He and Schneider, 2006). Overexpression of the p40 isoform also reduced VEGF-3′ UTR-Luc activity (45% decrease; Figure 3B). Overexpression of AUF1 did not affect the activity of the parental reporter, 3.1-Luc (Supplemental Table S1).
FIGURE 3:
Effects of overexpressing AUF1 on VEGF-3′ UTR-luciferase. Cultured RAW-264.7 cells were cotransfected with VEGF-3′ UTR-Luc and either AUF1-p37 (A) or AUF1-p40 (B). After 24 h, cells were lysed and luciferase activity measured. Results are normalized to transfection with pcDNA-3.1 and are representative of two or more experiments. Shown are mean and SD. p < 0.001 for A and p < 0.0001 for B.
AUF1 associates with VEGF mRNA
To determine whether AUF1 associates with VEGF mRNA in RAW-264.7 cells, we immunoprecipitated AUF1 from RAW-264.7 cell lysates and measured VEGF mRNA in the pulldown. The presence of AUF1 isoforms in the pulldown was confirmed by immunoblotting (Figure 4A). AUF1 has been reported to act on multiple genes (Mazan-Mamczarz et al., 2009), and PCR was performed to identify other AURE-containing mRNAs pulled down with AUF1. As shown in Figure 4B, PCR products for VEGF, GLUT1, and hexokinase-2 (HK2) could be amplified from cDNA prepared from anti-AUF1 pulldowns. Pulldowns without and with anti-AUF1 antibody contained the very abundant 18S RNA (Figure 4B). Beads alone (no anti-AUF1 antibody added) and pulldowns with sera (mouse, rat, goat, bovine) brought down no VEGF mRNA. For comparison of VEGF mRNA associated with AUF1, we performed parallel pulldowns with a control antibody (anti-Tie2). By real-time PCR analysis, there was 3.6-fold-greater VEGF mRNA associated with AUF1 than with the control. This result demonstrates that VEGF mRNA associates with AUF1 protein in macrophages, and future studies will determine whether AUF1 interacts directly with the VEGF mRNA.
FIGURE 4:
Immunoprecipitation of mRNA associated with AUF1. (A) AUF1 protein was isolated with anti-AUF1 antibody and protein A beads and the pulldown analyzed for AUF1 protein. Control pulldowns were performed with no anti-AUF1 antibody (No Ab). (B) To identify mRNA associated with AUF1, RNA was purified from pulldown beads and the cDNA amplified for VEGF, GLUT1, HK2, or 18S RNA. PCR products are shown.
Sequence homology of AUF1
To identify functional domains of AUF1, several studies have used sequence analysis (DeMaria et al., 1997; He and Schneider, 2006). To extend these studies, we identified regions of AUF1-p37 that are homologous to other species. Homology analysis was performed using BLAST (National Center for Biotechnology Information) and is shown in Figure 5A. Different homologies were found for three regions, encoded by exons 1, 3–5, and 6 and 8. The AUF1-p37 isoform lacks exons 2 and 7 (Figure 5B). As shown in Figure 5B, the central region of AUF1 contains two RNA recognition motifs (RRMs) that are highly conserved (Figure 5A). The C-terminal region, encoded by exons 6 and 8, is also conserved, except for opossum and panda (Figure 5A). The N-terminus shows many nonidentical residues between species (Figure 5A), with the most variation occurring in serine and threonine residues. Studies have shown that the N- and C-terminal regions contribute to, but are not primarily involved in, RNA binding (DeMaria et al., 1997). The N- and C-terminal regions are both candidate regulatory regions (DeMaria et al., 1997; He and Schenider, 2006), and the role of the C-terminus in AUF1 function is the subject of this study. The amino acid sequences of AUF1-p37 and other AUF1 regions are shown in Figure 5C.
FIGURE 5:
(A) Sequence analysis of AUF1. Shown are comparisons of the nine most closely related species with human AUF1-p37. Exons 2 and 7 are alternatively spliced out of the p37 isoform. Vertical lines show single–amino acid variations or single–amino acid gaps as compared with human. (B) Diagram showing the structure of AUF1, including two polyalanine motifs (poly-Ala; 11-AAAAA-15 and 37-AAAAA-41), two RRMs, a polyglutamine stretch (poly-Q; 245-QQQQQ-249), and three RGG repeats (253-RGG-255, 263-RGG-265, and 277-RGG-279). Alternative splicing that retains exon 2, exon 7, or both produces the other AUF1 isoforms (AUF1-p40, -p42, -p45; not shown). Below are shown AUF1 deletion constructs and AUF1-RGG peptides: AUF1-Δ21-Ala (aa 13–33 deleted), AUF1-ΔQRGG (aa 206–280 deleted), AUF1-Ex6+8 (aa 234–287), and AUF1-QRGG (aa 245–287). (C). (i) Amino acid sequence of human AUF1-p37. Shown in boldface italic font are the polyalanine motifs in exon 1 and the polyglutamine and RGG repeat motifs in the C-terminal region. G–K denotes the end of exon 1. K–C denotes the beginning of exon 6. (ii) The polyalanine region of AUF1 exon 1. The amino acids removed in the AUF1-Δ21-Ala deletant are underlined. (iii) The region deleted in AUF1-ΔQRGG. (iv) The amino acid sequence of the AUF1-Ex6+8 (top) and AUF1-QRGG (bottom) peptides.
C-Terminal RGG motifs
Many regulatory mRNA-binding proteins shuttle between the cytoplasm and nucleus, and localization to the cytoplasm increases effects on regulated mRNA (He and Schenider, 2006). The RGG repeat protein motif affects cytoplasmic localization of another mRNA-binding protein, hnRNP A2 (Nichols et al., 2000). Arginine residues in RGG motifs of hnRNP A2 are methylated, and blockade of methylation with adenosine dialdehyde (AdOx) eliminated preferential nuclear localization of hnRNP A2 (Nichols et al., 2000). The RGG domain in AUF1-p37 includes three RGG motifs within a 27–amino acid (aa) sequence (Figure 5B), and the arginine in the third RGG motif of AUF1 is dimethylated (Ong et al., 2004). Treatment of several cell types with AdOx did not affect cytoplasmic/nuclear localization of AUF1 (He and Schenider, 2006). However, AdOx treatment of RAW-264.7 cells affects AUF1 protein levels (see later discussion).
Deletion of N- or C-terminal region affects AUF1 activity
Previous studies showed that N- and C-terminal regions are required for maximal RNA binding (DeMaria et al., 1997). To determine whether the N- and C-terminal regions are necessary for regulating VEGF gene expression in RAW-264.7 cells, we deleted portions of the N- and C-termini from AUF1. The RGG domain and a nearby polyglutamine region (poly-Q) contribute to RNA binding (DeMaria et al., 1997) and were removed (Figure 5B). The AUF1-ΔQRGG mutant was expressed in RAW-264.7 cells and showed a loss of the inhibitory effect on VEGF-3′ UTR-Luc reporter activity (Figure 6A). Deletion of 21 aa in the N-terminal region within an alanine-rich region (AUF1-Δ21-Ala; Figure 5B) also eliminated the inhibitory effects on VEGF reporter (Figure 6B), as did a double deletant (AUF1-Δ21-Ala+ΔQRGG; Figure 6B). Deletions may reduce affects on reporter activity by reducing deletant protein levels, and protein levels of AUF1 deletants were measured next.
FIGURE 6:
Deletion of C- and N-terminal regions reduces activity. (A) The AUF1 or AUF1-ΔQRGG (dQRGG) expression plasmids were cotransfected into RAW-264.7 cells with VEGF-3′ UTR-Luc (see Figure 5B). Data are normalized to cells transfected with the empty expression plasmid pcDNA-3.1 (pcDNA). Deletion of the QRGG region inactivated the inhibitory effect of AUF1 on VEGF-3′ UTR-Luc. p < 0.0001, pcDNA vs. AUF1; pcDNA vs. ΔQRGG was not significantly different. p < 0.0001 AUF1 vs. ΔQRGG. (B) Deletion of 21 aa (AUF1-Δ21-Ala, d21) from the polyalanine region of the full-length AUF1 inactivated AUF1. Effects of the double deletant (both the polyalanine and the QRGG region deleted) are also shown (d21+dQRGG). p < 0.0001, pcDNA vs. AUF1; pcDNA vs. Δ21 was not significantly different; p < 0.03, pcDNA vs. Δ21+ΔQRGG; p < 0.0001, AUF1 vs. Δ21; p < 0.0001, AUF1 vs. Δ21+ΔQRGG; p < 0.02, Δ21 vs. Δ21+ΔQRGG. Results are normalized to transfection with pcDNA and are representative of two or more experiments. Shown are mean and SD.
Effects of deletions on AUF1 protein levels
To determine whether N- or C-terminal deletions affect protein levels, full-length AUF1 or AUF1 deletants were expressed in RAW-264.7 cells and immunoblotted with anti-V5 antibody. Deletion of 21 amino acids from the polyalanine region decreased AUF1 protein levels (Figure 7). However, protein levels of the AUF1-ΔQRGG mutant were similar to those of full-length AUF1 (Figure 7). The double deletant (AUF1-Δ21-Ala+ΔQRGG) was expressed at lower levels, similar to the AUF1-Δ21-Ala deletant (Figure 7). Larger deletions of the polyalanine region (up to 34 aa) also reduced protein levels (Supplemental Figure S1).
FIGURE 7:
Effects of N- and C-terminal deletions on protein levels. Lysates of RAW-264.7 cells expressing V5-tagged full-length AUF1-p37 (FL) or the polyalanine deletant (AUF1-Δ21; d21) or the QRGG deletant (dQ/RGG) were immunoblotted with anti-V5 antibody. The d21+dQ/RGG deletant lacks both the polyalanine and the QRGG regions. Results are representative of three experiments.
Proximal to the polyalanine region are candidate ubiquitination sites, and deletion of the polyalanine motifs may affect protein degradation via ubiquitin-mediated proteasomal degradation. To evaluate whether proteasomal inhibition affected AUF1 or AUF1 deletants, cells were transfected and then treated with MG132. Proteasomal inhibition increased endogenous AUF1-V5 levels (Supplemental Figure S2A). Protein levels of both the AUF1-∆21-Ala deletant and the AUF1-∆QRGG also increased with MG132, but the increases were less (Supplemental Figure S2A). The double deletant (AUF1-∆21+∆QRGG) was not affected by MG132 (Supplemental Figure S2B), suggesting that both N- and C-terminal regions affect proteasomal degradation of AUF1.
To determine whether decreased protein levels resulted from reduced transfection efficiency, in separate experiments, we cotransfected AUF1 or AUF1 deletants with a parental luciferase vector (Renilla luciferase). The transfection efficiency of all constructs was the same (Supplemental Figure S3). Finally, to evaluate whether the protein levels result from changes in protein turnover, we performed cycloheximide (CYX) inhibition studies (50 μg/ml for 0, 1, or 4 h). Treatment with CYX inhibits protein translation, and the rate of protein degradation can be measured. As shown in Figure 8, although with CYX treatment the double deletant appeared to have more rapid decay than the wild-type AUF1, the difference was not significantly different (Figure 8). Hence the two deletants with reduced protein levels (AUF1-Δ21-Ala and AUF1-Δ21-Ala+ΔQRGG; Figure 7) displayed decay similar as wild type. More studies are needed to explain why the AUF1-Δ21-Ala and AUF1-Δ21-Ala+ΔQRGG deletant proteins are expressed at lower levels. To summarize these protein studies: 1) the loss of activity on VEGF reporters by deletants lacking the N-terminal polyalanine region (Figure 6B) likely results from reduced protein levels (by an unknown mechanism); and 2) loss of activity on VEGF reporters by the AUF1-∆QRGG C-terminal deletant (Figure 6A) does not result from reduced levels of the deletant protein.
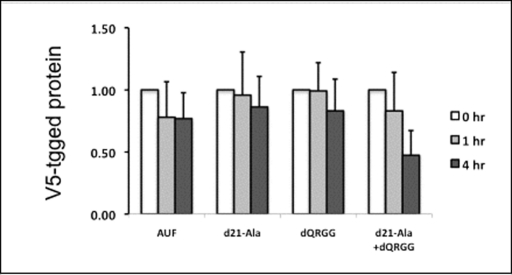
FIGURE 8:
Effects of cycloheximide inhibition on V5-tagged protein synthesis. Cultured RAW-264.7 cells were transfected with the full-length AUF1-V5 protein (AUF), the N-terminal deletant AUF1-d21-Ala (d21-Ala), the C-terminal deletant (dQRGG), or the double deletant (d21-Ala+dQRGG). After 24 h, cells were treated with cycloheximide for 0, 1, or 4 h. Cell lysates were immunoblotted with anti-V5 antibody, and protein levels were normalized to α-tubulin levels. Levels of each protein type are expressed relative to untreated controls. The change in deletant protein levels was not significantly different from AUF1 at any time point. Compared to AUF1, the double-deletant protein levels were decreased by 40% after 4 h, but the decrease was not significant (p < 0.10). Results are the mean and SD for three separate experiments.
Blockade of arginine methylation reduces AUF1 protein
To extend our analysis of the C-terminal regulatory region, we next determined whether methylation of arginine residues affects protein levels. Cultured RAW-264.7 cells were treated with an arginine methylation inhibitor, AdOx, for 48 h. Both endogenous AUF1 (Figure 9A) and AUF1-V5 (Figure 9B) protein levels were decreased with AdOx treatment. However, the AUF1-ΔQRGG mutant was less affected by AdOx treatment (Figure 9B). Hence deletion of the QRGG region did not affect protein levels (Figure 7), but blockade of arginine methylation decreased AUF1 protein levels by a mechanism that is not clear. Together these results suggest that deletion of the QRGG motif is a functional deletion and that arginine methylation is important to AUF1 protein levels.
FIGURE 9:
Effects of adenosine dialdehyde on AUF1 protein. (A) Cells (RAW-264.7) were treated with AdOx (5 µM) for 48 h, and lysates were immunoblotted for AUF1 isoforms. Loading control was α-tubulin. (B) The effects of AdOx (5 μM for 48 h) were measured on RAW-264.7 cells transfected with AUF1 (WT) or AUF1-ΔQRGG-V5 (dQRGG). Triplicate samples were immunoblotted with anti-V5 antibody. WT, dimethyl sulfoxide (DMSO) vs. AdOx, p < 0.0001; AUF1-ΔQRGG, DMSO vs. AdOx, p < 0.001. Results are normalized to DMSO treatment and are representative of two or more experiments. Shown are mean and SD.
Effects of AUF1-RGG peptides on VEGF gene expression
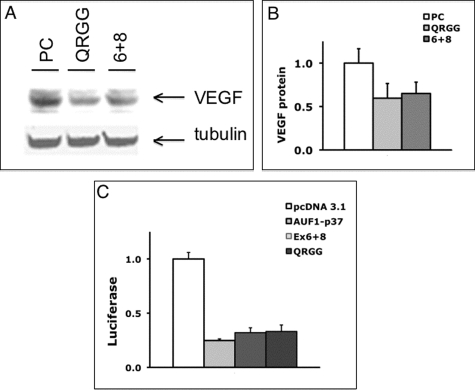
The reported functions of the RGG domain in the C-terminal region include homodimer formation (Kajita et al., 1995), RNA binding (Raman et al., 2001), and protein–protein interaction with regulatory proteins (Raman et al., 2001; He and Schneider, 2006). We hypothesized that a peptide modeled on the C-terminus might interfere with binding of AUF1 with its binding partners and act as a dominant-negative peptide. Two AUF1-RGG peptides were created in expression vectors. One peptide contains the region encoded by exons 6 and 8 (AUF1-Ex6+8; Figure 5B). The second is a shorter peptide whose N-terminus begins at the polyglutamine sequence (AUF1-QRGG; Figure 5B). As shown earlier (Figure 1), treatment of RAW-264.7 cells with full-length AUF1 decreased endogenous VEGF protein levels. To determine whether expression of the RGG peptides affects endogenous VEGF expression, RAW-264.7 cells were transfected with the empty vector (PC), the AUF1-QRGG peptide (QRGG), or the AUF1-Ex6+8 peptide (6+8). As shown in Figure 10, A and B, endogenous VEGF levels were decreased by 35% (AUF1-Ex6+8) or 40% (AUF1-QRGG). To determine whether the AUF1-RGG peptides affect expression of the VEGF-3′ UTR reporter, we cotransfected RAW-264.7 cells with VEGF-3′ UTR-Luc together with AUF1-6+8 or AUF1-QRGG. The amount of repression of reporter activity was the same with AUF1-RGG peptides and full-length AUF1 (Figure 10C). As a negative control, a small scrambled peptide did not affect reporter activity when compared with overexpressed AUF1 (Supplemental Figure S4). Hence peptides modeled on the RGG domain of AUF1 are effective inhibitors of endogenous VEGF gene expression and likely act by a 3′ UTR–mediated mechanism. Future studies will identify the active motif in AUF1-RGG peptides and further characterize the mechanism by which AUF1-RGG peptides repress VEGF gene expression.
FIGURE 10:
Effects of AUF1-RGG peptides on endogenous VEGF protein levels. Cultured RAW-264.7 cells were transfected with the empty parental vector, pcDNA-3.1 (PC), the AUF1-QRGG peptide vector (QRGG), or the AUF1-6+8 peptide vector (6+8). After 4 h, cells were treated with LPS for 18 h. Lysates were probed for VEGF and α-tubulin. (A) Immunoblot for VEGF or α-tubulin. (B) Levels of VEGF protein normalized to α-tubulin for six experiments. As compared with pcDNA-3.1, both AUF1-QRGG (p < 0.002) and AUF1-6+8 (p < 0.003) significantly decreased VEGF protein. (C) Effects of AUF1 peptides on VEGF-3′ UTR reporter activity. Cells (RAW-264.7) were transfected with VEGF-3′ UTR-Luc and were cotransfected with pcDNA-3.1, AUF1 (AUF1-p37), or AUF1-RGG peptides (Ex6+8 or QRGG). The luciferase activity in cell lysates from triplicate wells was read after 24 h. p < 0.0001 for pcDNA vs. AUF, AUF1-Ex6+8, or AUF1-QRGG. No other comparators were significantly different. Results are normalized to pcDNA treatment and are representative of two or more experiments.
DISCUSSION
Vascular endothelial growth factor is critical as a growth factor in blood vessel growth and as a cytokine in immune cell activation (Ferrara, 2004). Our studies (Du et al., 2006) and the studies of others (De Bandt et al., 2003) demonstrate that VEGF acts as a cytokine in macrophages, activating the cell through VEGFR-1 (De Bandt et al., 2003). Hence macrophages both respond to and produce VEGF. In human disease, macrophage cells may exacerbate disease when overactivated or may play a role in fighting disease when killing bacteria or as tumor-infiltrating macrophages (Gordon, 2007; Stockmann et al., 2008). Growth factor stimulation of macrophages leads to increased VEGF gene transcription (Jiang and Liu, 2009) and stabilization of VEGF mRNA (Du et al., 2006). This study demonstrates that the mRNA-binding protein AUF1/hnRNP D regulates expression of VEGF.
Expression of genes is primarily regulated by transcription acting on gene promoter and enhancer sequences. However, genes that encode proteins involved in cell activation, such as cytokines and growth factors, are often regulated posttranscriptionally (Xu et al., 1997). Posttranscriptional regulatory steps include 1) splicing and processing, 2) nuclear export, 3) cytoplasmic localization, 4) translational efficiency, and 5) degradation (Ferrara, 2004). Our previous studies show that a region of the 3′ UTR of VEGF mRNA that contains an adenosine-uridine–rich element regulates VEGF gene expression (Du et al., 2006). Our earlier reports also identified a similar AU-rich element in the 3′ UTR of GLUT1 mRNA (Griffin et al., 2004). Another report showed that the AU-rich element in human VEGF mRNA regulates gene expression in macrophages by alternating secondary structures of RNA (Ray and Fox, 2007). In a renal cell carcinoma model, Xin et al. (2012) show that AUF1 binds VEGF AU-rich elements under both normoxia and hypoxia and that regulation of larger isoforms of AUF1 (p42 and p45) is mediated by a VHL-RNP complex. The AU-rich elements in 3′ UTR regions regulate gene expression in concert with mRNA-binding proteins (Xu et al., 1997). We report here that overexpression of the AUF1 mRNA-binding protein decreases endogenous VEGF protein levels (Figure 1), establishing the biological significance of AUF1 action on VEGF gene expression. This study investigates posttranscriptional regulation of VEGF mRNA by the AUF1 mRNA-binding protein.
Regulation by mRNA-binding proteins on 3′ UTR elements is analyzed by introducing the 3′ UTR from regulated mRNA (VEGF, GLUT1, tumor necrosis factor-α [TNF]) into the 3′ UTR of reporter genes (firefly luciferase or green fluorescent protein; Griffin et al., 2004; Du et al., 2006; Nichols et al., 2010). In this study, the 3′ UTR of VEGF was placed in the 3′ UTR of a luciferase reporter, as described previously (Du et al., 2006). In resting macrophages and other cell types, the 3′ UTR decreases reporter expression (Figure 2; Du et al., 2006). When cells are stimulated, reporter expression increases (Du et al., 2006). This study establishes that AUF1 regulates 3′ UTR–dependent gene expression by inhibiting VEGF-3′ UTR–dependent luciferase reporter expression in RAW-264.7 cells (Figure 3). Attempts to knock down AUF1 using small inhibitory RNA (siRNA) in RAW-264.7 and BMDM cells by transient transfection was only modestly successful (∼10–30% decrease), and although VEGF mRNA and VEGF-3′ UTR-Luc activity increased modestly, the increase was not significant (unpublished data). Future studies will use knockdown-stable cell lines in which all cells are affected by siRNA.
To understand the mechanism by which AUF1 inhibits VEGF expression, this study investigates the regulatory regions lying N- and C-terminal to the highly conserved RNA-binding domain (Figure 5). Deletion of a polyalanine-rich region in the N-terminus reduced activity on the VEGF-3′ UTR-Luc reporter (Figure 6B), a result most likely due to reduced protein levels (Figure 7). The N-terminus is important for protein folding, and loss of N-terminal sequence may reduce the level of successfully folded protein (Peroutka et al., 2011). The C-terminus of AUF1 contains a region with three repeat RGG motifs. Arginine residues in RGG motifs are enzymatically dimethylated by protein arginine methyltransferase. This posttranslational modification produces many effects on biological activity (Bedford and Clarke, 2009; Yu, 2011). Dimethylation of RGG motifs alters the structure of RNA to which RGG motifs bind but does not affect the affinity of binding (Raman et al. 2001). Blockade of arginine methylation with adenosine dialdehyde decreased AUF1 protein levels, suggesting a role for methylated arginines in protein stability (Figure 9). However, deletion of the RGG domain did not affect protein levels (Figure 7). Together these results suggest that AdOx produces AUF1 proteins with unmethylated RGG motifs, and the unmethylated arginines in RGG motifs mark the protein for decay. Loss of the RGG domain inactivated the repressive effects of AUF1 on the VEGF-3′ UTR reporter (Figure 6A), which suggested that this region of AUF1 is a candidate for regulatory peptides.
To understand the mechanism of AUF1 action and identify possible regulatory AUF1-RGG peptides, two C-terminal sequences (AUF1-Ex6+8 and AUF1-QRGG) were studied. Expression of these AUF1-RGG peptides was anticipated to disrupt protein–protein interactions (DeMaria et al., 1997; He and Schneider, 2006) or interfere with binding of AUF1 to RNA (Raman et al., 2001) and reverse the inhibitory effect of AUF1 on VEGF gene expression. Surprisingly, AUF1-RGG peptides reduced VEGF-3′ UTR-Luc activity to the same degree as full-length AUF1 (Figure 10C). Perhaps more interesting, expression of AUF1-RGG peptides also reduced endogenous VEGF protein levels (Figure 10, A and B), reductions that were greater than found with the full-length AUF1 (Figure 1). A likely mechanism of action by AUF1-RGG peptides is inactivation of an inhibitory regulator of AUF1. The 14-3-3σ protein binds to a portion of the RGG domain of AUF1-p37 and -p40 but not to the larger isoforms (He and Schenider, 2006). The interaction site overlaps with a putative nuclear localization signal, and 14-3-3σ appears to block localization of AUF1 to the nucleus and increases the effects of AUF1 on mRNA (He and Schenider, 2006). The 14-3-3σ protein increases cytoplasmic levels of AUF1 and would be predicted to decrease VEGF gene expression. Interruption of 14-3-3σ by AUF1-RGG peptides would reverse the action of 14-3-3σ and increase VEGF gene expression. On the basis of our results, it is more likely that the AUF1-RGG peptides promote or mimic the action of 14-3-3σ. There is no apparent homology between 14-3-3σ and the AUF1-RGG peptides.
Our results demonstrate that AUF1-RGG peptides derived from AUF1 can regulate VEGF gene expression. The most likely mechanism for AUF1-RGG peptide action is binding to AUF1 and increasing AUF1 activity. Alternatively, AUF1-RGG peptides may interact with other regulatory proteins that are binding partners of the RGG domain of AUF1 (He and Schenider, 2006). Determining how AUF1-RGG peptides act to change VEGF gene expression in macrophages is the focus of ongoing studies. Design of novel biologically active AUF1 peptides will identify the binding partner and optimize the pharmacokinetics of this reagent, which may be valuable in reducing VEGF gene expression.
MATERIALS AND METHODS
Reagents
AdOx, LPS, CYX, and MG132 were from Sigma-Aldrich (St. Louis, MO).
Cell culture
The RAW-264.7 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained as previously described (Griffin et al., 2004; Du et al., 2006). Medium was low-glucose DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS). HEK-293 cells were obtained from ATCC and maintained in DMEM media supplemented with 10% FBS. Cells were grown without antibiotics. Cell treatments with LPS, AdOx, or CYX are described in the text.
BMDMs were obtained from male C57/Bl6 mice. Cells were collected from femoral shafts by flushing with RPMI-1640 containing 10% FBS. The cell suspensions were passed through a 21-gauge needle in RPMI-1640 to disperse cell clumps and then filtered using a 70-μm nylon cell strainer to remove bone fragments. Adherent bone marrow cells (stroma and mature macrophages) were removed by incubation in tissue culture plates at 37°C overnight. The nonadherent bone marrow cells were then cultured in RPMI-1640, 10% FBS, 20% L-cell conditioned medium, and gentamicin (50 μg/ml). The media was supplemented every 2–3 d, and the macrophages were harvested after 7–10 d.
Cell transfection and luciferase assay
For transfection studies, cultured cells were plated ∼24 h prior to transfection in 48-well plates with sufficient cells to give ∼50% confluence at the time of transfection. Plasmid DNA was complexed with Lipofectamine 2000, as described by the manufacturer (Invitrogen, Carlsbad, CA). Cells were transfected with luciferase constructs at ∼0.2 μg of plasmid DNA per 100,000 cells. Transfection efficiency under experimental conditions was monitored in separate experiments by cotransfection with 1) a plasmid expressing firefly luciferase plus 2) sufficient plasmid expressing Renilla sea pansy luciferase (pRL-SV40; Promega, Madison, WI) to give 2–10% of the firefly reporter activity. Correction for Renilla activity did not change the variation in the firefly data (Supplemental Table S2). Cells were lysed after 18–24 h with Cell Culture Lysis Reagent or Passive Lysis Buffer (for Renilla) (Promega) and the lysate frozen at −80°C. The luciferase activity in the lysate of ∼20,000 cells (20 μl of a 100-μl lysate of a well from a 48-well plate) was determined following addition of luciferin substrate (Promega) in LMax (Molecular Dynamics, Sunnyvale, CA) or Centro LB 960 (Berthold, Oak Ridge, TN) luminometers. Transfections were performed in triplicate or quadruplicate. The effect of AUF1-binding proteins on VEGF-3′ UTR–dependent luciferase production was measured by cotransfecting the AUF1 expression plasmids (pcDNA-3.1 is the parent vector) together with luciferase reporter plasmids. Reporter activity of cotransfected cells is expressed relative to the activity of cells transfected with the empty expression plasmid, pcDNA-3.1. To determine whether experimental conditions affected the luciferase reporter, parallel wells were transfected with the parent luciferase (3.1-luciferase) instead of the VEGF-3′ UTR-luciferase. Experimental conditions did not affect the parent luciferase (Supplemental Table S1). All experiments were performed two or more times.
For transfection studies of BMDMs, cells were suspended with trypsin and counted. Cells were incubated with plasmid DNA and electroporated using the Amaxa BMDM buffer, as described by the manufacturer (Lonza, Basel, Switzerland). Cells were lysed after 24 h and luciferase activity measured in 20 μl of cell lysate.
Immunoblotting
Total protein was measured using either Bradford assay (Bio-Rad, Hercules, CA) or bicinchoninic acid (Pierce, Thermo Fisher Scientific, Rockford, IL). Equal amounts of protein were separated on Invitrogen 4–12% Tris-glycine gels and transferred to polyvinylidene fluoride membranes using the I-blot system (Invitrogen) or by wet transfer (Bio-Rad). In some experiments, all four AUF1 isoforms could be resolved using gradient gels and the I-Blot transfer system. Precision Plus dual-color protein standard (Bio-Rad) was used to determine molecular weight. Blots were probed with antibodies to AUF1 (Millipore, Billerica, MA), V5 (Invitrogen), or Tie2 (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed with horseradish peroxidase (HRP) secondary antibodies (mouse and rabbit secondary HRP antibodies were from Sigma-Aldrich) using X-Omat film (Kodak, Rochester, NY) or imaged using the VersaDoc system (Bio-Rad). Protein levels were quantitated by scanning immunoblots or digital images. The image intensities were measured using ImageJ (National Institutes of Health, Bethesda, MD). Protein bands were normalized to α-tubulin (Sigma-Aldrich).
RNA isolation and real-time PCR
Total cellular RNA was isolated using the QiaShredder columns and the RNeasy kit (Qiagen, Valencia, CA). The RNA was quantified using a NanoDrop (Thermo Fisher Scientific, Waltham, MA). Next, 1–5 μg of RNA was treated with Turbo-DNA-free (Ambion, Austin, TX), and cDNA was created using the Maxima First Strand cDNA kit (Fermentas, Glen Burnie, MD). Real-time PCR was performed with RT2 Real Time SYBR Master Mix (SABiosciences, Frederick, MD), using standard conditions. Levels of mRNA (Ct) were normalized to the level of 18S RNA. All samples were run in triplicate. Melting curves were performed to confirm single PCR products. Primer pairs are as follows. Mouse VEGF: sense, aagtgatcaagttcatggatgtcta; antisense, aagctcatctctcctatgtgctg. Mouse hexokinase 2: sense, aactgagtttgacagagagatcgac; antisense, gtcttcaatatccgagacatctttg. Mouse GLUT1: sense, caatgctgtgttctactactcaacg; antisense, gatgctcagataggacatccaag. 18S rRNA: sense, gcatatgcttgtctcaaagattaag; antisense, tattagctctagaattaccacagttatcca. Primer pairs cross introns, except the 18S RNA gene, which lacks introns. To verify the size of PCR products, amplicons were separated on a 2% NuSieve DNA gels, stained with Syber Gold (Molecular Probes, Invitrogen), and imaged by VersaDoc.
AUF1/mRNA immunoprecipitation
RAW-264.7 cells were plated with 1 × 107 cells in 10-cm plates. The next day, the cells were lysed in 1 ml of lysis buffer (LB; 10 mM Tris-HCl, pH 8.0, 1.5 mM MgCl2, 100 mM NaCl, 0.5% Tween-20, complete protease cocktail [Roche, Indianapolis, IN]) plus RNAse Protect (Sigma-Aldrich). Lysate was precleared with 50 μl of equilibrated protein A magnetic beads (Millipore). A 20-μg amount of antibody to AUF1 or Tie2 (control) was bound to beads. Antibody-bound beads were incubated with 300 μg of total cellular protein overnight rotating at 4°C. Beads were washed repeatedly with LB, and RNA was purified (RNeasy). Levels of mRNA were measured by PCR, followed by DNA gel electrophoresis or by real-time PCR.
Plasmid construction
AUF1 expression constructs.
Human AUF1-p37 and AUF1-p40 expression plasmids were created using AUF1 plasmids as template (generous gift of Robert Schneider, New York University, New York, NY). Coding regions were amplified by PCR and cloned into pcDNA-3.1-V5-His (Invitrogen). The parental vector control for AUF1 overexpression experiments was pcDNA-3.1. The polyalanine AUF1-p37 deletant (∆21-Ala; aa 13–33 deleted) was created by low-stringency (low annealing temperature) whole-plasmid PCR. A deletion construct of the C-terminal region of AUF1-p37 (∆QRGG, aa 206–278 deleted) was generated using the PCR-based QuikChange Mutagenesis Kit (Stratagene, Santa Clara, CA). The ∆QRGG construct retains a hybrid RGG motif at the site of the deletion. A double deletant (∆21-Ala+∆QRGG) was created by using PCR cloning using ∆21-Ala as template. Fragments of AUF1 were created by PCR and cloned into pcDNA-3.1-V5-His. The AUF1-Ex6+8 construct includes the region encoded by exons 6 and 8 (aa 234–287), and AUF1-QRGG includes the region from the polyglutamine motif to the C-terminus (aa 244–287). The sense primer introduced an initiating methionine codon. Amino acid numbering is based on human AUF1-p37, GenBank reference file NM_001003810. All AUF1 C-terminal fragments were cloned using the pcDNA-3.1-V5-His cloning construct (Invitrogen). DNA sequencing on both strands verified the integrity of all constructs.
Luciferase constructs.
The parental (3.1-luciferase) and full-length VEGF-3′ UTR-luciferase (mouse VEGF-3′ UTR, nt-209-1747, GenBank AF317892) expression plasmids were constructed as previously described (Du et al., 2006). Briefly, the luciferase gene from pGL3 (Promega) was cloned into pcDNA-3.1. Then the VEGF 3′ UTR was cloned into the 3′ UTR of the luciferase gene.
Statistical analysis
Experimental conditions were analyzed in triplicate or quadruplicate. Results are shown as mean and SD. Significant differences in results were determined by t test using GraphPad Prism (GraphPad Software, La Jolla, CA), and p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by Veterans Administration Merit Review Awards (R.C.N., R.F., P.M., R.B.R.), a Veterans Administration Career Development Award (B.L.P), and P20RR16437 (R.C.N.) from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources. We thank Jason Pfeiffer and Dale Mierke for helpful discussions.
Abbreviations used:
- aa
amino acid
- AdOx
adenosine dialdehyde
- AUF1
AUF1/heterogeneous nuclear ribonucleoprotein D mRNA-binding protein
- AURE
adenosine-uridine–rich element
- BMDM
bone marrow–derived macrophage
- GLUT1
glucose transporter-1
- HK2
hexokinase-2
- hnRNP
heterogeneous nuclear ribonucleoprotein
- LPS
lipopolysaccharide
- Luc
luciferase
- RGG
arginine–glycine–glycine
- RRM
RNA recognition motifs
- siRNA
small inhibitory RNA
- TNF
tumor necrosis factor-α
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-06-0545) on February 29, 2012.
REFERENCES
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;3:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Shyu AB. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation—a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- De Bandt M, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol. 2003;171:4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- DeMaria CT, Sun Y, Long L, Wagner BJ, Brewer G. Structural determinants in AUF1 required for high affinity binding to A + U-rich elements. J Biol Chem. 1997;272:27635–27643. doi: 10.1074/jbc.272.44.27635. [DOI] [PubMed] [Google Scholar]
- Du M, Roy KM, Zhong L, Shen Z, Meyers HE, Nichols RC. VEGF gene expression is regulated post-transcriptionally in macrophages. FEBS J. 2006;273:732–745. doi: 10.1111/j.1742-4658.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Spencer-Green G, Yeo KT, Yeo TK, Berse B, Jackman RW, Senger DR, Dvorak HF, Brown LF. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliers D, Duraisamy S, Barnes JL, Ghosh-Choudhury G, Kasinath BS. Translational regulation of vascular endothelial growth factor expression in renal epithelial cells by angiotensin II. Am J Physiol Renal Physiol. 2005;288:F521–F529. doi: 10.1152/ajprenal.00271.2004. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Goldberg-Cohen I, Furneauxb H, Levy AP. A 40-bp RNA element that mediates stabilization of vascular endothelial growth factor mRNA by HuR. J Biol Chem. 2002;277:13635–13640. doi: 10.1074/jbc.M108703200. [DOI] [PubMed] [Google Scholar]
- Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Hamilton BJ, Roy KM, Du M, Willson AM, Keenan BJ, Wang XW, Nichols RC. Post-transcriptional regulation of glucose transporter-1 by an AU-rich element in the 3′ UTR and by hnRNP A2. Biochem Biophys Res Commun. 2004;318:977–982. doi: 10.1016/j.bbrc.2004.04.128. [DOI] [PubMed] [Google Scholar]
- He C, Schneider R. 14-3-3sigma is a p37 AUF1-binding protein that facilitates AUF1 transport and AU-rich mRNA decay. EMBO J. 2006;25:3823–3831. doi: 10.1038/sj.emboj.7601264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Maru Y, Okada A, Seiki M, Noda T, Shibuya M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;6:1207–1213. [PubMed] [Google Scholar]
- Holstein JH, Becker SC, Fiedler M, Garcia P, Histing T, Klein M, Laschke MW, Corsten M, Pohlemann T, Menger MD. Intravital microscopic studies of angiogenesis during bone defect healing in mice calvaria. Injury. 2011;42:765–771. doi: 10.1016/j.injury.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita Y, Nakayama J, Aizawa M, Ishikawa F. The UUAG-specific RNA binding protein, heterogeneous nuclear ribonucleoprotein D0. Common modular structure and binding properties of the 2xRBD-Gly family. J Biol Chem. 1995;270:22167–22175. doi: 10.1074/jbc.270.38.22167. [DOI] [PubMed] [Google Scholar]
- Kiledjian M, DeMaria CT, Brewer G, Novick K. Identification of AUF1 (heterogeneous nuclear ribonucleoprotein D) as a component of the alpha-globin mRNA stability complex. Mol Cell Biol. 1997;17:4870–4876. doi: 10.1128/mcb.17.8.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Sadri N, Schneider RJ. Endotoxic shock in AUF1 knockout mice mediated by failure to degrade proinflammatory cytokine mRNAs. Genes Dev. 2006;20:3174–3184. doi: 10.1101/gad.1467606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazan-Mamczarz K, Kuwano Y, Zhan M, White EJ, Martindale JL, Lal A, Gorospe M. Identification of a signature motif in target mRNAs of RNA-binding protein AUF1. Nucleic Acids Res. 2009;37:204–214. doi: 10.1093/nar/gkn929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- Nichols RC, Botson J, Wang XW, Hamilton BJ, Collins JE, Uribe V, Brooks SA, Zan M, Rigby WF. A flexible approach to studying post-transcriptional gene regulation in stably transfected mammalian cells. Mol Biotechnol. 2010;48:210–217. doi: 10.1007/s12033-010-9360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RC, Wang XW, Tang J, Hamilton BJ, High FA, Herschman HR, Rigby WF. The RGG domain in hnRNP A2 affects subcellular localization. Exp Cell Res. 2000;256:522–532. doi: 10.1006/excr.2000.4827. [DOI] [PubMed] [Google Scholar]
- Ong SE, Mittler G, Mann M. Identifying and quantifying in vivo methylation sites by heavy methyl SILAC. Nat Methods. 2004;1:119–126. doi: 10.1038/nmeth715. [DOI] [PubMed] [Google Scholar]
- Paleolog EM. The vasculature in rheumatoid arthritis: cause or consequence? Int J Exp Pathol. 2009;90:249–261. doi: 10.1111/j.1365-2613.2009.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka RJ, Orcutt SJ, Strickler JE, Butt TR. SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol Biol. 2011;705:15–30. doi: 10.1007/978-1-61737-967-3_2. [DOI] [PubMed] [Google Scholar]
- Raman B, et al. N(omega)-arginine dimethylation modulates the interaction between a Gly/Arg-rich peptide from human nucleolin and nucleic acids. Nucleic Acids Res. 2001;29:3377–3384. doi: 10.1093/nar/29.16.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:33603372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, et al. Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 2009;5:e1000464. doi: 10.1371/journal.ppat.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri N, Lu JY, Badura ML, Schneider RJ. AUF1 is involved in splenic follicular B cell maintenance. BMC Immunol. 2010;11:1. doi: 10.1186/1471-2172-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri N, Schneider RJ. Auf1/hNRPD-deficient mice develop pruritic inflammatory skin disease. J Invest Dermatol. 2009;129:657–670. doi: 10.1038/jid.2008.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson L, Pettersson S, Englund MC, Wiklund O, Ohlsson BG. Post-transcriptional regulation of VEGF expression by oxidised LDL in human macrophages. Eur J Clin. 2002;32:767–774. doi: 10.1046/j.1365-2362.2002.01072.x. [DOI] [PubMed] [Google Scholar]
- Sela-Brown A, Silver J, Brewer G, Naveh-Many T. Identification of AUF1 as a parathyroid hormone mRNA 3′-untranslated region-binding protein that determines parathyroid hormone mRNA stability. J Biol Chem. 2000;275:7424–7429. doi: 10.1074/jbc.275.10.7424. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;4:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Brown JA, Gong C, Fan H, Brewer G, Gnarra JR. Association of the von Hippel-Lindau protein with AUF1 and posttranscriptional regulation of VEGFA mRNA. Mol Cancer Res. 2012;10:108–120. doi: 10.1158/1541-7786.MCR-11-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Chen CY, Shyu AB. Modulation of the fate of cytoplasmic mRNA by AU-rich elements: key sequence features controlling mRNA deadenylation and decay. Mol Cell Biol. 1997;17:4611–4621. doi: 10.1128/mcb.17.8.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo PS, Mulkeen AL, Cha CH. Post-transcriptional regulation of vascular endothelial growth factor: implications for tumor angiogenesis. World J Gastroenterol. 2006;12:4937–4942. doi: 10.3748/wjg.v12.i31.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC. The role of protein arginine methylation in mRNP dynamics. Mol Biol Int. 2011;2011:163827. doi: 10.4061/2011/163827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.