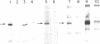Abstract
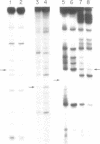
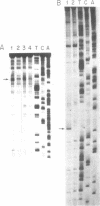
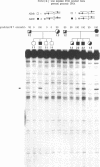
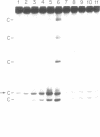
In the presence of tetramethylammonium chloride, potassium permanganate specifically modifies mismatched thymines. Similarly, the modification of mismatched cytosines by hydroxylamine was enhanced by tetraethylammonium chloride. Modification followed by piperidine cleavage permits specific identification of the T and C mismatches and by extension, when the opposite DNA strand is analyzed, of A and G mismatches as well. These reactions can be performed conveniently with DNA immobilized on Hybond M-G paper. We describe conditions that exploit these reactions to detect mismatches, e.g. point mutations or genetic polymorphisms, using either synthetic oligonucleotide probes or PCR amplification of specific genomic DNA sequences.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Lilley D. M. Single base mismatches in DNA. Long- and short-range structure probed by analysis of axis trajectory and local chemical reactivity. J Mol Biol. 1989 Oct 20;209(4):583–597. doi: 10.1016/0022-2836(89)90596-2. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Campbell R. D. Chemical reactivity of matched cytosine and thymine bases near mismatched and unmatched bases in a heteroduplex between DNA strands with multiple differences. Nucleic Acids Res. 1989 Jun 12;17(11):4223–4233. doi: 10.1093/nar/17.11.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Green P., Helms C., Cartinhour S., Weiffenbach B., Stephens K., Keith T. P., Bowden D. W., Smith D. R., Lander E. S. A genetic linkage map of the human genome. Cell. 1987 Oct 23;51(2):319–337. doi: 10.1016/0092-8674(87)90158-9. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K. A., Rudersdorf R., Neill S. D., Dougherty J. P., Brown E. L., Fritsch E. F. The thermal stability of oligonucleotide duplexes is sequence independent in tetraalkylammonium salt solutions: application to identifying recombinant DNA clones. Nucleic Acids Res. 1988 May 25;16(10):4637–4650. doi: 10.1093/nar/16.10.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena S. D., Behe M. J. Influence of tetraalkyl ammonium ions on the structure of poly (rG-dC).poly (rG-dC): unexpected transitions among the Z, A and B conformations. Nucleic Acids Res. 1987 May 11;15(9):3907–3916. doi: 10.1093/nar/15.9.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchakdjian M., Li B. F., Swann P. F., Patel D. J. Pyrimidine.pyrimidine base-pair mismatches in DNA. A nuclear magnetic resonance study of T.T pairing at neutral pH and C.C pairing at acidic pH in dodecanucleotide duplexes. J Mol Biol. 1988 Jul 5;202(1):139–155. doi: 10.1016/0022-2836(88)90526-8. [DOI] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Von Hippel P. H. Alteration of the relative stability of dA-dT and dG-dC base pairs in DNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):298–302. doi: 10.1073/pnas.70.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Montandon A. J., Bobrow M., Bentley D. R. Detection of novel genetic markers by mismatch analysis. Nucleic Acids Res. 1989 Aug 11;17(15):5961–5971. doi: 10.1093/nar/17.15.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Jung R., Hunger H. D. Solid-phase methods for sequencing of oligodeoxyribonucleotides and DNA. Methods Enzymol. 1987;155:301–331. doi: 10.1016/0076-6879(87)55022-4. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Theophilus B. D., Latham T., Grabowski G. A., Smith F. I. Comparison of RNase A, a chemical cleavage and GC-clamped denaturing gradient gel electrophoresis for the detection of mutations in exon 9 of the human acid beta-glucosidase gene. Nucleic Acids Res. 1989 Oct 11;17(19):7707–7722. doi: 10.1093/nar/17.19.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter E., Yamamoto F., Almoguera C., Perucho M. A method to detect and characterize point mutations in transcribed genes: amplification and overexpression of the mutant c-Ki-ras allele in human tumor cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7575–7579. doi: 10.1073/pnas.82.22.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Antonarakis S. E., Goff S. C., Orkin S. H., Boehm C. D., Kazazian H. H., Jr On the origin and spread of beta-thalassemia: recurrent observation of four mutations in different ethnic groups. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6529–6532. doi: 10.1073/pnas.83.17.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Pustell J., Spoerel N., Kafatos F. C. Coding and potential regulatory sequences of a cluster of chorion genes in Drosophila melanogaster. Chromosoma. 1985;92(2):124–135. doi: 10.1007/BF00328464. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]