Abstract
CRF receptor 1 (CRF1), a key neuroendocrine mediator of the stress response, has two known agonists corticotropin-releasing factor (CRF) and urocortin 1 (Ucn1). Here we report that endothelin-converting enzyme-1 (ECE-1) differentially degrades CRF and Ucn1; ECE-1 cleaves Ucn1, but not CRF, at critical residue Arginine-34/35′, which is essential for ligand-receptor binding. At near KD agonist concentration (30 nm), both Ucn1- and CRF-mediated Ca2+ mobilization are ECE-1 dependent. Interestingly, at high agonist concentration (100 nm), Ucn1-mediated Ca2+ mobilization remains ECE-1 dependent, whereas CRF-mediated mobilization becomes independent of ECE-1 activity. At high agonist concentration, ECE-1 inhibition disrupted Ucn1-, but not CRF-induced CRF1 recycling and resensitization, but did not prolong the association of CRF1 with β-arrestins. RNA interference-mediated knockdown of Rab suggests that both Ucn1- and CRF-induced CRF1 resensitization is dependent on activity of Rab11, but not of Rab4. CRF1 behaves like a class A G protein-coupled receptor with respect to transient β-arrestins interaction. We propose that differential degradation by ECE-1 is a novel mechanism by which CRF1 receptor is protected from overactivation by physiologically relevant high concentrations of higher affinity ligand to mediate distinct resensitization and downstream signaling.
The corticotropin-releasing factor (CRF) and urocortin 1 (Ucn 1–3) family of neuropeptides and their receptors, CRF1 and CRF2, mediate neuroendocrine stress and immune responses (1–4) in part by activating the hypothalamic-pituitary-adrenal axis. Stress can augment and activate the brain-gut axis, resulting in peripheral release of CRF, Ucn1, and Substance P among other neuropeptides to degranulate mast cells and initiate immune responses. Ucn1 binds to CRF1 with 6- to 10-fold higher affinity than CRF (5, 6), but whether this higher binding affinity results in a more efficient trafficking and signaling by Ucn1-bound CRF1 over CRF is unclear. The mechanisms by which CRF1 binds its ligands, traffics, and signals differentially in the presence of its multiple ligands remains to be determined.
CRF1 belongs to the family of G protein-coupled receptors (GPCR), and like a number of other GPCR present on the cell surface, is internalized upon agonist stimulation. The movement of internalized receptors between various intracellular vesicular compartments is regulated by specific Rab GTPase (7). The pH in perinuclear recycling endosomes is more alkaline than that of early endosomes (8, 9), and endosomal pH is a key determinant for the activity of metalloendopeptidases that cleave agonists from bound receptors to promote recycling. Endothelin-converting enzyme 1 (ECE-1) is a metalloendopeptidase that shuttles between plasma and endosomal membranes. There are four known ECE-1 isoforms (a–d) that share a common catalytic domain but have different subcellular distributions (10). ECE-1 has recently been shown to regulate recycling and resensitization of some GPCR by degrading their agonists in endosomes (10, 11). This degradation disrupts the agonist-GPCR-β-arrestins (βARR) complex and frees the receptor to recycle to the cell surface, which mediates resensitization and also controls the duration of signaling of the receptor at the endosomal membranes (12). Whether ECE-1 cleaves the CRF family of neuropeptides and controls trafficking and signaling of CRF1 is unknown.
GPCR can be classified according to their interaction with βARR. Class A receptors [β2-adrenergic (β2AR) and μ-opioid receptors] show preferential binding to βARR2 over βARR1, interact transiently with βARR, and usually do not colocalize with βARR in endosomes. Class B receptors [neurokinin 1 (NK1R) and calcitonin receptor-like (CLR) receptors] show equal affinity for βARR1 and βARR2, form sustained interactions, and internalize as a stable complex colocalizing in endosomes for extended periods. Class A receptors usually recycle and resensitize more rapidly than class B receptors (10, 13, 14). Conflicting evidence exists regarding the internalization behavior of the CRF1 and its association with βARR. CRF1 and βARR cointernalize into cytosolic vesicles, leading some investigators to classify the receptor as a class B GPCR (15, 16). Others found that internalization of CRF1 is independent of βARR-recruitment and classify the receptor as a class A GPCR (17, 18). Upon agonist activation, CRF1 couples to multiple G proteins, including Gsα (adenylyl cyclase/cAMP activation) (19, 20) and Gq (21–24), and activated receptors can initiate multiple signaling cascades, such as mobilization of intracellular Ca2+ (24), activation of kinase signaling pathways that include the protein kinase C, p44/p42, and p38 MAPK (25–28).
Activation of CRF1, by either CRF or Ucn1 or both, is critical in many stress-related pathophysiological and inflammatory conditions such as anxiety, depression, inflammatory bowel disease, irritable bowel syndrome, and at the onset of labor. CRF1 antagonism often attenuates disease symptoms in animal models of these diseases, but it is unclear how cells evoke distinct cellular responses in the face of the same receptor binding multiple ligands. The mechanisms by which CRF- or Ucn1-bound CRF1 traffics and signals differentially in the presence of its multiple agonists remains unknown. In this study, we tested the hypothesis that ECE-1 differentially regulates trafficking of agonist-bound CRF1 by differentially cleaving Ucn1 compared with CRF. We ascertained whether Ucn1 and CRF serve as substrates for ECE-1 by HPLC and determined the cleavage sites by mass spectrometry. We then determined the effect of inhibiting ECE-1 activity on CRF- or Ucn1-activated CRF1 trafficking, Ca2+, and cAMP signaling in human embryonic kidney (HEK) and human neuroblastoma SK-N-SH cells that endogenously express CRF1 receptors. We found that an ECE-1-mediated mechanism uncoupled the differential recycling and resensitization of CRF1 by its two agonists when agonist concentrations were high, but not at basal or low concentrations.
Results
CRF and Ucn1 differentially regulate CRF1 recycling and resensitization
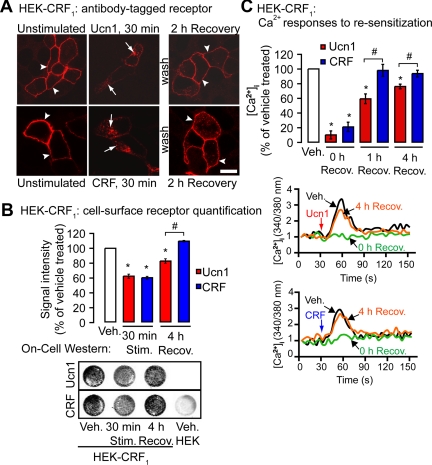
CRF1 has two known ligands: CRF and Ucn1; Ucn1 binds CRF1 with higher affinity than CRF. To determine whether higher binding affinity of Ucn1 translates to differential recycling and resensitization by Ucn1 compared with CRF, we directly compared the postendosomal trafficking of Ucn1- and CRF-activated CRF1. Coupling of CRF1 to G-proteins results in altered intracellular Ca2+ ([Ca2+]i) responses, and we also compared these downstream signaling responses of Ucn1- and CRF-activated CRF1. We stimulated HEK-CRF1 cells with equimolar concentrations of CRF and Ucn1. Initial dose-response experiments showed that at a low concentration (30 nm), the Ucn1-induced calcium signal was significantly higher than that induced by CRF (Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). At a high concentration (100 nm), CRF and Ucn1 elicited equal calcium responses (Supplemental Fig. 1A). Therefore, unless otherwise stated, we used an agonist concentration of 100 nm throughout our study to compare trafficking and signaling between CRF- and Ucn1-activated CRF1. In unstimulated cells, CRF1 was mostly localized to the plasma membrane (Fig. 1A, arrowheads), and activation of the receptor with Ucn1 or CRF resulted in receptor internalization to intracellular vesicles containing early endosomal antigen marker EEA1 (Fig. 1A, arrows, Merge). To confirm the endosomal localization of ligand-activated CRF1, we coexpressed CFR1 with cyan fluorescent protein (CFP)-tagged Rab5Q79L (CFP-Rab5Q79L), a constitutively active mutant of Rab5 that causes early endosomal fusion and facilitates detection of proteins in endosomes (29). Activated CRF1, as well as fluorescently tagged Ucn1 or CRF, prominently colocalized with Rab5Q79L in enlarged early endosomes (Fig. 1B, arrows).
Fig. 1.
Ucn1 and CRF induce internalization of CRF1 to early endosomes. A, In HEK-CRF1 cells, CRF1 translocated from the plasma membrane to intracellular vesicles containing EEA1 (arrows, merge) upon Ucn1 (upper panel) or CRF (lower panel) stimulation. B, In HEK cells coexpressing CRF1 and CFP-Rab5aQ79L, Rab5Q79L colocalized with internalized CRF1, 5FAM-Ucn1, or Rhodamine-CRF in enlarged endosomes (arrows). Scale, 10 μm. Rhod., Rhodamine.
To examine whether Ucn1 promotes different physiological responses than CRF, we determined activation of CRF1 under conditions in which cells are exposed to both agonists simultaneously at equimolar concentrations of ligands. We used green fluorescently tagged Ucn1 (5FAM-Ucn1) and red fluorescently tagged CRF (Rhodamine-CRF) and applied both to HEK-CRF1 cells at the same time (Supplemental Fig. 1B). At equimolar concentrations (100 nm) of each peptide, we detected no CRF binding. However, when we used 200 nm CRF and 10 nm Ucn1, the receptor bound to both peptides, and the internalized Ucn1/CRF-receptor complex strongly colocalized (Supplemental Fig. 1B). This suggests that CRF and Ucn1 activation results in internalization of CRF1 to the same endosomes, but that a much higher concentration of CRF is needed to access its receptor when Ucn1 is present at the same time.
To study postendocytic recycling of CRF1, we labeled the cell-surface CRF1 receptors by preincubating the cells with hemagglutinin (HA) antibody; the surface-tagged CRF1 (Fig. 2A) trafficked similarly to nontagged receptor (compare Fig. 1A with Fig. 2A), as has been observed with other GPCR (30, 31). Both Ucn1- and CRF-activated receptor internalized to a similar degree (Fig. 2B). After agonist washout and recovery for 2 h at 37 C, CRF1 stimulated by either agonist appeared to recycle to the plasma membrane (Fig. 2A). Quantification of CRF1 cell surface expression revealed that after 4 h of recovery, CRF-induced receptor recycling was complete, whereas in Ucn1-stimulated cells, 17.2 ± 3.0% of the receptor had not returned to the cell surface (Fig. 2B). To determine whether the above noted differences in agonist-induced receptor recycling to the cell surface resulted in altered signaling, we also compared resensitization of Ucn1- and CRF-activated CRF1 by measuring [Ca2+]i responses to repeated challenge with agonists 0–4 h after recovery. After 1 h recovery, responses were largely resensitized to CRF, but not to Ucn1 (Ucn1, 59.5 ± 6.5% of vehicle; CRF, 98.0 ± 8.2% of vehicle, Fig. 2C). After 4 h recovery, resensitization with Ucn1 remained incomplete (Ucn1, 76.2 ± 3.2% of vehicle; CRF, 93.7 ± 4.5% of vehicle; Fig. 2C). Thus, Ucn1 and CRF stimulation both resulted in similar internalization of CRF1, but the receptor recycled and resensitized more efficiently after CRF stimulation.
Fig. 2.
Ucn1- and CRF-induced internalization, recycling, and resensitization of CRF1. A, In HEK-CRF1 cells, surface-tagged CRF1 internalized to intracellular vesicles (arrows) upon Ucn1 or CRF stimulation and recycled to the plasma membrane at 2 h recovery (arrowheads). Scale, 10 μm. B, Receptors that remained on cell surface after ligand stimulation were quantified using On-cell Western assay, which revealed that Ucn1 and CRF treatment result in similar CRF1 loss from the cell surface. CRF-stimulated CRF1 fully recycled after 1 h or 4 h of recovery, whereas Ucn1-stimulated CRF1 partially recycled (representative On-cell Western assay showing loss of signal intensity due to receptor internalization after 30 min is shown below the histogram). C, Ucn1- and CRF-stimulated Ca2+ signals similarly desensitized after 0 h of recovery. Ucn1-stimulated Ca2+ signals partially resensitized, and CRF-stimulated Ca2+ signals fully resensitized after 4 h of recovery (representative traces of [Ca2+]i are shown below the histogram). (n ≥ 3 experiments; *, P < 0.05 compared with vehicle). Recov., Recovery; Stim., stimulated.
Ucn1- and CRF-induced recycling and resensitization of CRF1 are Rab11 dependent
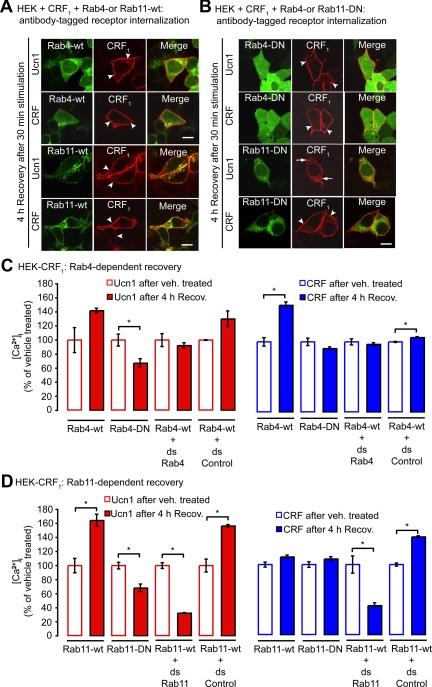
The movement of internalized receptors between various intracellular vesicular compartments is regulated by specific Rab GTPase (7). Rab4a and Rab11a GTPase are present in early and recycling endosomes and participate in recycling of receptors back to the plasma membrane (32). Rab4 regulates rapid recycling of receptors back to the cell surface, whereas Rab11 regulates slow recycling to the cell surface and to the trans-Golgi network. To determine their role in CRF1 recycling, we examined HEK cells coexpressing CRF1 and green fluorescent protein (GFP)-tagged Rab (Rab4a-GFP, Rab11a-GFP), or their GTP binding-deficient mutants Rab4aN121I-GFP (Rab4-DN) or Rab11aS25N-GFP (Rab11-DN), which act as dominant negative (DN) to inhibit function. After cell-surface labeling of CRF1 with HA antibody, cells were incubated with Ucn1 or CRF or vehicle for 30 min, washed, and allowed to recover for 4 h in agonist-free medium at 37 C. In vehicle-treated cells, CRF1 and Rab4a or Rab11a did not colocalize; Rab4a or Rab11a was detected in vesicles and CRF1 was at the cell surface (Supplemental Fig. 2, A and B). After 30 min of stimulation with Ucn1 or CRF, CRF1 predominantly colocalized in Rab4a- or Rab11a-positive endosomes (Supplemental Fig. 2, A and B). After 4 h of recovery, the bulk of the receptor had recycled to the cell surface, whereas Rab4a or Rab11a continued to exhibit intracellular vesicular staining, as well as some diffuse cytosolic staining (Fig. 3A). Coexpression of Rab4-DN did not affect this CRF1 trafficking pattern, and CRF1 still recycled normally to the cell surface (Fig. 3B). Coexpression of Rab11-DN, however, did affect CRF1 trafficking: after 4 h recovery, the CRF1 was still retained in intracellular vesicles, and recycling to the cell surface diminished in Ucn1-, but not in CRF-treated cells (Fig. 3B). We quantified CRF1 cell surface levels by On-cell Western assays and found that under basal (unstimulated) conditions, Rab4-DN or Rab11-DN coexpression resulted in increased cell-surface levels of CRF1 (Supplemental Fig. 2C). Because this indicated that the cell-surface CRF1 levels can vary between different transfections or batches of cells, we ensured that throughout our study, recycling and resensitization levels were calculated by normalizing against vehicle-treated cells from the same batch (i.e. equally transfected cells). Quantification by On-cell Western assays confirmed that coexpression of Rab4-DN had no effect on CRF1 recycling and coexpression of Rab11-DN inhibited Ucn1-induced CRF1 recycling by 22.3 ± 0.9%, while leaving CRF-induced receptor recycling unaffected (Supplemental Fig. 2D). Thus, studies with DN Rab suggests that activated CRF1 traffics to endosomes containing Rab4a and Rab11a, and whereas Rab4a activity is not required for CRF1 recycling, Rab11a activity is required for Ucn1-, but not CRF-induced CRF1 recycling.
Fig. 3.
Rab4a- and Rab11a-mediated recycling and resensitization of CRF1. HEK-CRF1 cells coexpressing the wild types (wt) Rab4-GFP or Rab11-GFP, or DN mutants Rab4aN121I-GFP or Rab11aS25N-GFP, were challenged with Ucn1 or CRF (100 nm) for 30 min, washed, and placed in agonist-free medium for 4 h for recovery. A, In cells coexpressing Rab4-wt or Rab11-wt, CRF1 recycled to the cell surface (arrowheads). B, In cells coexpressing Rab4-DN, CRF1 also recycled to the cell surface (arrowheads). In cells coexpressing Rab11-DN, the majority of Ucn1-stimulated CRF1 was retained in intracellular vesicles (arrows), whereas CRF-stimulated CRF1 still recycled to the cell surface (arrowheads). Scale, 10 μm. C, Quantification of Ucn1- (red) and CRF-stimulated (blue) Ca2+ signals after 4 h of recovery and Rab4 dependence. CRF- but not Ucn1-stimulated Ca2+ signals fully resensitized in HEK-CRF1 cells coexpressing Rab4-DN. Knockdown of Rab4 by coexpression of double-stranded RNA against Rab4 had no effects on resensitization of Ucn1- or CRF-stimulated Ca2+ signals. D, Rab11-dependent resensitization. CRF-stimulated, but not Ucn1-stimulated, Ca2+ signals fully resensitized in HEK-CRF1 cells coexpressing Rab11-DN. Knockdown of Rab11 inhibited resensitization of Ucn1- or CRF-stimulated Ca2+ signals. (n ≥ 3 experiments; *, P < 0.05 compared with vehicle). ds, Double stranded.
The effects of Rab4-DN or Rab11-DN coexpression on resensitization were examined by measuring Ca2+ mobilization in cells after 4 h recovery. In cells that coexpressed Rab4-DN, responses to CRF were largely resensitized, but responses to Ucn1 were not (Ucn1, 67.5 ± 6.0% of vehicle; CRF, 89.5 ± 2.3% of vehicle, Fig. 3C). Coexpression of Rab11-DN had no effect on responses to CRF, but resensitization with Ucn1 was inhibited (Ucn1, 68.4 ± 5.4% of vehicle; CRF, 108.9 ± 3.3% of vehicle, Fig. 3D). Interestingly, coexpression of Rab4-wt or Rab11-wt also had an effect on CRF1 resensitization. Rab4-wt coexpression increased CRF-mediated resensitization of CRF1, and Rab11-wt coexpression increased Ucn1-mediated resensitization of CRF1. To clarify the roles of Rab4 and Rab11 in CRF1 resensitization, we performed Rab4 and Rab11 knockdowns by RNAi against Rab4a or Rab11a. Efficient knockdown was confirmed by Western blot analysis (Supplemental Fig. 2E). Rab4 knockdown did not affect resensitization induced by either Ucn1 or CRF (Fig. 3C). Knockdown of Rab11 inhibited resensitization induced by both Ucn1 (32.7 ± 0.5% of vehicle) and CRF (41.9 ± 3.7% of vehicle; Fig. 3D). Taken together, these findings suggest that Rab4a activity is redundant, but that Rab11a activity is essential for receptor resensitization induced by either Ucn1 or CRF.
Ucn1 and CRF are differentially degraded by ECE-1
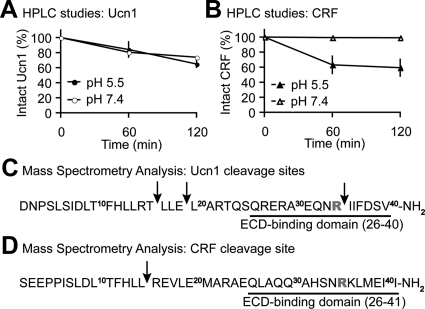
ECE-1 is known to hydrolyze a number of peptides just as efficiently as its first identified in vivo substrate big endothelin-1 (33), to regulate signaling (10, 11). ECE-1 degrades peptides at both neutral (pH 7.4) and acidic (pH 5.5) milieus. It degrades big endothelin-1 optimally at a neutral pH, consistent with generating endothelin-1 at the cell surface, whereas most other peptides (such as Substance P or neurokinin) are hydrolyzed optimally at acidic pH similar to that of endosomes (10). To test the notion that Ucn1 and CRF serve as substrates for ECE-1, each peptide was incubated with recombinant human (rh) ECE-1 at the acidity of extracellular (pH 7.4) and endosomal (pH 5.5) compartments for 0–120 min. In 2 h, rhECE-1 degraded Ucn1 at both pH 7.4 and pH 5.5 (Fig. 4A), whereas it degraded CRF at pH 5.5, but not at pH 7.4 (Fig. 4B). Thus, ECE-1 degrades Ucn1 at a broader pH range than CRF. We next determined amino acid residues cleaved by ECE-1 in Ucn1 and CRF peptides. Mass spectrometry identified several cleavage sites within Ucn1, indicating hydrolysis of T16-L17, E19-L20, and R34-I35 bonds (Fig. 4C) and one cleavage site within CRF, indicating hydrolysis of the L15-R16 bond (Fig. 4D). Thus, our data show that ECE-1 cleaves Ucn1 at three different sites but cleaves CRF at only one site. Importantly, we show that ECE-1 cleaves Ucn1 at Arginine-34′ (R-34), but not CRF (R-35), which was identified in a recent study (6) as the crucial residue in the N-terminal extracellular domain of CRF1-binding domain of both CRF and Ucn1 that allows these ligands to bind and activate CRF1.
Fig. 4.
ECE-1 differentially degrades Ucn1 and CRF. Degradation of Ucn1 (A) and CRF (B) by rhECE-1 at pH 5.5 and 7.4 assessed by HPLC. Ucn1 (panel C) and CRF (panel D) cleavage sites determined from mass spectrometry analysis of Ucn1 and CRF digests at pH 5.5 (n = 3); Arginine-34′ and -35′ present in Ucn1 and CRF, respectively, are crucial for interaction with CRF1 and highlighted in bold red. Note that ECE-1 cleaves Ucn1 but not CRF at this residue. Underlined residues in Ucn1 and CRF are the main residues that can bind the N-terminal extracellular domain (ECD) of CRF1 and activate it.
Activated CRF1 traffics with its ligands to intracellular vesicles containing ECE-1
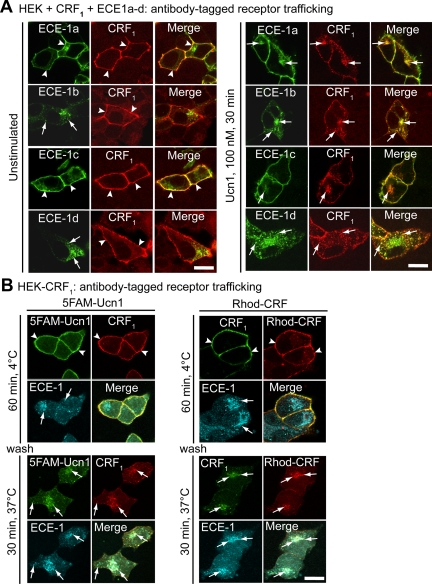
If ECE-1 can degrade Ucn1 at both the cell surface and in endosomes, we reasoned that Ucn1 and CRF1 should colocalize with ECE-1 at both these subcellular locales. HEK cells express all four ECE-1 isoforms (10), and we used transfected HEK cells to characterize subcellular colocalization of ECE-1 isoforms with CRF1. All four known ECE-1 isoforms are found in intracellular vesicles, but ECE-1a and -1c are predominantly expressed at the plasma membrane. In agreement with our hypothesis, we found that in unstimulated cells, CRF1 was detected at the plasma membrane, where it colocalized with ECE-1a and -1c (Fig. 5A, arrowheads). Incubation of cells with Ucn1 (or CRF) for 30 min resulted in prominent colocalization of CRF1 with ECE-1b or -1d and partial colocalization with ECE-1a and -1c in intracellular vesicles (Fig. 5A, arrows).
Fig. 5.
ECE-1 colocalizes with CRF1, Ucn1, and CRF in HEK cells. A, HEK-CRF1 cells coexpressing ECE-1a–d-GFP. In unstimulated cells, CRF1 colocalized with ECE-1a and ECE-1c at the plasma membrane (arrowheads). In Ucn1-stimulated cells, CRF1 prominently colocalized with ECE-1b and ECE-1d in intracellular vesicles and partially colocalized with intracellular ECE-1a and ECE-1c (arrows). B, HEK-CRF1 cells were incubated with 5FAM-Ucn1 (left) or Rhodamine-CRF (right) for 60 min at 4 C, washed, and incubated for 30 min at 37 C. After incubation at 37 C, CRF1-Ucn1 and CRF1-CRF trafficked from the plasma membrane (arrowheads) to intracellular vesicles where they colocalized with endogenous immunoreactive ECE-1 (arrows). Scale, 10 μm. Rhod., Rhodamine.
We investigated whether the subvesicular compartment to which CRF1-Ucn1 or CRF1-CRF traffic is different with respect to ECE-1 distribution. HEK cells stably expressing CRF1 (HEK-CRF1) were incubated with fluorescently tagged 5FAM-Ucn1 or Rhodamine-CRF for 1 h at 4 C, to allow binding of ligands to cell surface receptors, as seen in Fig. 5B (arrowheads). After 30 min incubation at 37 C, CRF1-Ucn1 or CRF1-CRF complex was prominently detected in endosomes with endogenous immunoreactive ECE-1b/d (Fig. 5B, arrows). Thus, Ucn1- or CRF-activated CRF1 internalizes to endosomes containing ECE-1, particularly ECE-1b and -1d.
We next determined whether ECE-1 is expressed in vivo in the same cells as CRF and Ucn1 under normal and pathological states. ECE-1 is expressed in many tissue types, including the colon (34). Ucn1 participates in the regulation of local inflammatory responses within the gastric mucosa (35) and can exert both anti- and proinflammatory effects (36, 37). We asked whether ECE-1 expression is altered during inflammation when Ucn1 levels are also affected. We found that in a rat model of trinitrobenzene sulfonic acid (TNBS)-induced colitis, Ucn1 and CRF levels were down-regulated in the colon 1 d after the inflammatory insult (Supplemental Fig. 3A). We also found that ECE-1c was significantly up-regulated 1 d after onset of TNBS colitis, coinciding with the drop in Ucn1 and CRF levels as determined by RT-PCR (Supplemental Fig. 3A). Immunoreactive ECE-1 was induced in the mucosal crypts, whereas Ucn1-IR was reduced, but expression of neither was changed from basal in the myenteric plexus (Supplemental Fig. 3, B and C). These in vivo observations that ECE-1 and CRF/Ucn1 show reciprocal changes during inflammation indicate that cleavage of CRF and Ucn1 by ECE-1 is of physiological relevance.
ECE-1 constitutively internalizes and traffics with Ucn1 or CRF to the same intracellular vesicles
To determine whether activation of the receptor results in spatiotemporal changes in ECE-1, we examined trafficking of an ECE-1 isoform from the plasma membrane by expressing ECE-1a with an extracellular herpes simplex virus (HSV)-tag in HEK cells. When incubated with HSV antibody for 1 h at 4 C, HSV-ECE-1a was detected at the plasma membrane (Supplemental Fig. 4A, arrowheads). After 30 min incubation at 37 C, ECE-1a was internalized to intracellular vesicles (Supplemental Fig. 4A, arrows). To determine whether HSV-ECE-1a cointernalizes with Ucn1 and CRF, we incubated HEK cells coexpressing CRF1 and HSV-ECE-1a with 5FAM-Ucn1 or Rhodamine-CRF. After incubation for 1 h at 4 C, HSV-ECE-1a colocalized with Ucn1 or CRF at the plasma membrane as shown in Supplemental Fig. 4B (arrowheads). After 30 min incubation at 37 C, HSV-ECE-1a colocalized with Ucn1 or CRF, along with CRF1, in intracellular vesicles as shown in Supplemental Fig. 4B (arrows). Thus, distribution of ECE-1 is dynamic, and all four ECE-1 isoforms constitutively shuttle between the plasma membrane and early endosomes with Ucn1 or CRF, as has been shown for other GPCR and ECE-1 (10).
ECE-1 inhibition disrupts Ucn1-, but not CRF-induced CRF1 recycling and resensitization
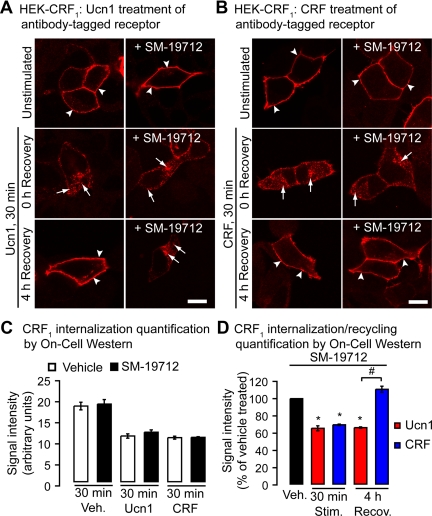
Given our data that ECE-1 degrades Ucn1 but not CRF at the critical Arg-34′ residue (Fig. 4, C and D), we predicted that ECE-1 cleavage will render Ucn1 nonfunctional and incapable of staying bound to CRF1, whereas CRF can remain bound to CRF1 even after ECE-1 cleavage. We tested the notion that ECE-1 inhibition will have an effect on Ucn1- but not CRF-activated CRF1 recycling. In HEK-CRF1 cells ECE-1 activity was inhibited, and the effects of ECE-1 inhibition on Ucn1- or CRF-induced receptor endocytosis and recycling were determined. To study postendocytic recycling of CRF1, we labeled cell-surface CRF1 in living cells using an antibody to an extracellular epitope tag (HA11). Cells were stimulated with Ucn1 or CRF for 30 min to initiate receptor internalization; CRF1 stimulated by either agonist appeared to recycle to the plasma membrane (Fig. 6, A and B). ECE-1-specific inhibitor SM-19712 that gives identical results to ECE-1 inhibition by small interfering RNA, as shown by us previously (10), did not affect internalization of CRF1 triggered by Ucn1 or CRF stimulation as detected by microscopy (Fig. 6, A and B). Internalization of the receptors was also quantified by On-cell Western assays (Fig. 6C), which revealed that ECE-1 activity is not required for agonist-activated internalization of CRF1. The recycling of CRF1 to the surface, however, was completely inhibited by SM-19712 in Ucn1- but not CRF-treated cells as detected by microscopy (Fig. 6, A and B) and as quantified by On-cell Western assays (Fig. 6D).
Fig. 6.
Effects of ECE-1 inhibition on internalization and recycling of CRF1. In HEK-CRF1 cells, Ucn1 (panel A) or CRF (panel B) induced internalization of CRF1 to endosomal vesicles (arrows). CRF1 recycled to the plasma membrane at 4 h recovery (arrowheads). SM-19712 caused retention of CRF1 in endosomes (arrows) and prevented recycling in Ucn1- but not CRF-treated cells. Scale, 10 μm. C, Quantification of CRF1 receptors expressed on the cell surface by On-cell Western assay shows that SM-19712 treatment has no effect on CRF1 loss from the cell surface compared with vehicle upon Ucn1 or CRF stimulation. D, In the presence of SM-19712, both Ucn1 and CRF induce a similar rate of CRF1 loss from the cell surface. CRF-stimulated CRF1 fully recycled after 4 h of recovery, whereas Ucn1-stimulated CRF1 recycling was diminished by SM-19712. Recov., Recovery; Stim., stimulated; Veh., vehicle.
CRF- and Ucn1-induced CRF1 recycling and resensitization is regulated by ECE-1 at low agonist doses, but only CRF-induced actions become independent of ECE-1 activity at a high agonist dose
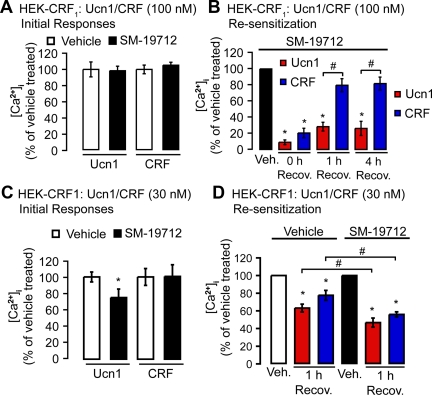
Because recycling can mediate resensitization, we compared Ucn1- and CRF-induced Ca2+ mobilization after recovery. HEK-CRF1 cells were treated with Ucn1 or CRF, or vehicle (control) for 30 min at 37 C, washed, and recovered in agonist-free medium for 0–4 h. The change in [Ca2+]i to a second Ucn1 or CRF challenge was measured. SM-19712 incubation had no significant effects on the initial [Ca2+]i response (Fig. 7A) induced by either agonist. When challenged at 0 h of recovery, Ucn1 or CRF-induced Ca2+ signaling was relatively minimal, indicating desensitization. Desensitization was not affected by ECE inhibition (Fig. 7B). Resensitization of CRF1 1 h after recovery was strongly inhibited by SM-19712 in cells treated with Ucn1 but not in cells treated with CRF (Fig. 7B). After recovery for 4 h, resensitization remained inhibited in cells treated with Ucn1 (Fig. 7B). We then tested whether these differences in Ucn1- and CRF-induced CRF1 resensitization could also be observed at a low agonist dose closer to the KD of the receptor. Interestingly, SM-19712 incubation reduced the initial [Ca2+]i response to 30 nm Ucn1 (Fig. 7C). However, we could still compare resensitization between cells treated with vehicle or with SM-19712, because we normalized the value at 1 h recovery against vehicle-treated cells that were under the same conditions (e.g. SM-19712 treated). In contrast to what we had observed at 100 nm agonist concentrations, resensitization of CRF1 1 h after recovery was inhibited by SM-19712 in both Ucn1- and CRF-treated cells at 30 nm agonist dose (Fig. 7D). Thus, ECE-1 activity regulates only Ucn1-, but not CRF-induced, CRF1 recycling and resensitization at high agonist concentrations and regulates both Ucn1- and CRF-induced CRF1 resensitization at low agonist concentrations.
Fig. 7.
Effects of ECE-1 inhibition on resensitization of CRF1. A, In HEK-CRF1 cells SM-19712 treatment had no effect on Ca2+ signals compared with vehicle upon Ucn1 or CRF (100 nm) stimulation. B, In the presence of SM-19712, both Ucn1 and CRF induced similar desensitization after 0 h of recovery. After 1 h or 4 h recovery, CRF-stimulated Ca2+ signals resensitized, whereas Ucn1-stimulated Ca2+ signals were inhibited by SM-19712. C, In HEK-CRF1 cells, SM-19712 treatment had no effect on 30 nm CRF-stimulated Ca2+ signals, but did reduce the 30 nm Ucn1-stimulated Ca2+ signals. D, At a CRF or Ucn1 concentration of 30 nm, SM-19712 treatment reduced both Ucn1 and CRF-mediated resensitization measured after 1 h recovery (n ≥ 3 experiments; *, P < 0.05 compared with vehicle). Recov., Recovery; Veh., vehicle.
To examine whether ECE-1 can regulate CRF1 activity in other cells of different etiologies, we repeated these experiments in neuronal cells (SK-N-SH cells), which endogenously express CRF1 and CRF2 (Supplemental Fig. 5A), as well as all four ECE-1 isoforms, with ECE-1b and ECE-1c showing strongest expression levels (Supplemental Fig. 5B). Despite robust CRF1 expression, these cells did not evoke Ca2+ responses after stimulation with CRF at varying doses; however, the response to Ucn1 was robust (Supplemental Fig. 5C). Similar to what we observed in HEK-CRF1 cells, resensitization of CRF1 1 h after recovery was inhibited by SM-19712 (Supplemental Fig. 5D). This inhibition was observed in the presence of CRF2 or CRF1 inhibitor (Supplemental Fig. 5D).
ECE-1 actions do not regulate cAMP signaling
Because coupling of CRF1 to G proteins not only results in Ca2+ signaling, but also in cAMP signaling (depending on what kind of G protein the receptor couples to), we also compared Ucn1- and CRF-induced cAMP responses after recovery. HEK-CRF1 cells were treated with Ucn1 or CRF, or vehicle (control) for 15 min at 37 C, washed, and recovered in agonist-free medium for 1 h. The cAMP response to a second Ucn1 or CRF challenge was measured. In contrast to our findings with Ca2+ signaling, SM-19712 did not inhibit cAMP responses after recovery in cells treated with either Ucn1 or CRF, and at either high or low agonist concentration (Supplemental Fig. 6, A and B). Thus, ECE-1 is not required for Ucn1- or CRF-induced cAMP signaling after recovery.
ECE-1 inhibition does not affect CRF1 association with βARR
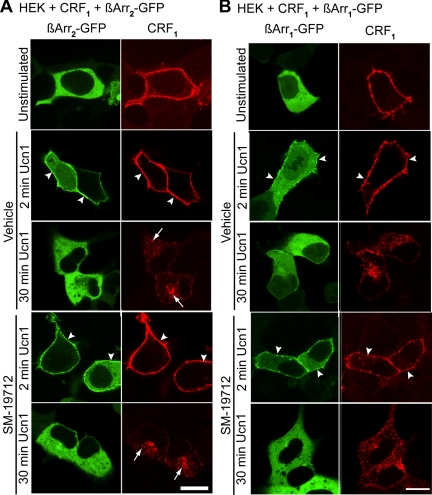
A recent study showed that CRF1 displays sex-based differences with respect to its interaction with β-arrestin2 during stress in locus ceruleus neurons of male, but not female, rats (38). We hypothesized that inhibition of Ucn1-activated CRF1 recycling by ECE-1 would prolong the association of CRF1 with βARR in endosomes. We therefore examined the nature of CRF1 interaction with βARR. Upon GPCR activation, βARR first translocate to the plasma membrane to couple the receptors to clathrin and adaptor protein 2 to mediate endocytosis after which this complex is internalized to the endosomes. Additionally, they uncouple the receptor from G proteins to mediate desensitization (39). Both Ucn1 and CRF stimulation for 2 min resulted in βARR1 and βARR2 translocation to CRF1 at the plasma membrane (Fig. 8, A and B, and Supplemental Fig. 7A). After 30 min of stimulation, the receptor had internalized and the βARR were redistributed to the cytosol and did not appear in CRF1-containing endosomes (Fig. 8, A and B, and Supplemental Fig. 7A). SM-19712 incubation had no effect on the association of CRF1 with βARR (Fig. 8, A and B, and Supplemental Fig. 7A). Expression of a DN mutant of βARR1 (318–419) (βARR1-DN) did not disrupt CRF1 internalization (Supplemental Fig. 7B). Thus, CRF1 acts like a class A GPCR in terms of its interaction with βARR, and ECE-1 inhibition does not prolong the association of CRF1 and βARR.
Fig. 8.
Effects of ECE-1 inhibition on CRF1 association with βARR. HEK cells coexpressing CRF1 and βARR2-GFP (panel A) or βARR1-GFP (panel B) were challenged with Ucn1 for 2 or 30 min. In cells treated with vehicle or with SM-19712, at 2 min, Ucn1 induced translocation of βARR2 or βARR1 to the plasma membrane (arrowheads). At 30 min, Ucn1 induced trafficking of CRF1 to intracellular vesicles (arrows), whereas βARR2 or βARR1 had returned to a diffuse cytosolic distribution. Scale, 10 μm.
Discussion
Activation of CRF1 by its two known ligands, Ucn1 and CRF, often results in paradoxical effects on cellular signaling and physiological function (40, 41). The cellular mechanisms that regulate and mediate this differential action of CRF1 remain unknown. Our results show that ECE-1 cleaves Ucn1 at three different sites but cleaves CRF at only one site. Importantly, we show that ECE-1 cleaves Ucn1 at Arginine-34′ (R-34), which was identified in a recent study (6) as the crucial residue in both CRF (R-35) and Ucn1 that allows these ligands to bind and activate CRF1. HPLC studies confirmed that ECE-1 degrades Ucn1 at both extracellular (pH 7.4) and endosomal (pH 5.5), whereas CRF is degraded at acidic pH alone. Thus, at a low or basal level, ECE-1 can disrupt association of Ucn1 or CRF with CRF1 in endosomes and free the receptor to promote recycling and resensitization. CRF1 activation is critical during the onset of labor and in many inflammatory conditions, and its antagonism ameliorates many inflammatory symptoms in animal models of irritable bowel syndrome (42) and Clostridium difficile-induced ileitis (43, 44). Here, we show that a membrane-specific isoform of ECE-1 is preferentially up-regulated in a rat model of Crohn's colitis at d 1. Concurrent with this up-regulation of ECE-1, Ucn1 and CRF expression were down-regulated. In contrast to these findings for Ucn1, we have previously shown that Ucn2 expression is up-regulated whereas CRF2 expression is down-regulated in this acute phase (45). Consistent with this observation, CRF1 colocalized with all four ECE-1 isoforms both at the cell surface and in endosomes. Furthermore, at high doses of CRF or Ucn1, CRF-stimulated CRF1 signaling becomes independent of ECE-1 activity, whereas Ucn1-stimulated signaling remains ECE-1 dependent. We thus hypothesize that CRF receptors are protected from being overstimulated by their high affinity agonists during inflammation by either regulating expression of agonist/receptor or by another mechanism, such as ECE-1, which can potentially degrade both Ucn and CRF.
Although CRF1 trafficked to endosomes at a similar degree after challenge with either agonist, resensitization of CRF1 was significantly greater with CRF challenge than with Ucn1, as determined by measuring [Ca2+]i responses to repeated challenge with agonists 0–4 h after recovery. Inhibition of ECE-1 activity with the specific inhibitor SM-19712 (10) prevented CRF1 recycling and resensitization at low doses, but at higher doses, ECE-1 activity regulated CRF1 trafficking stimulated by Ucn1, but not by CRF. Interestingly, ECE-1 activity was not required for cAMP signaling mediated by CRF1. Inhibition of ECE-1 activity by using small interfering RNA against ECE-1 has previously given results similar to those seen after using SM-19712 (10), thereby confirming specificity of this inhibitor. Thus, ECE-1 is a key determinant of postendocytic recycling and the resensitization fate of CRF1.
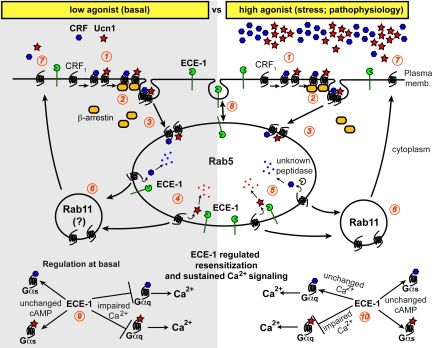
Two scenarios in which ancillary factors are key determinants of downstream signaling outcomes are 1) the existence of multiple receptors for a given agonist, or 2) a receptor being activated by multiple agonists. CRF1, a GPCR, falls into the latter category and is activated by at least two agonists, CRF and Ucn1. Although Ucn1 binds to CRF1 with a much greater affinity than CRF, it is not known whether Ucn1 preferentially activates CRF1 and whether Ucn1-bound CRF1 traffics, recycles, and signals in a manner similar to that bound to CRF, or traffics via overlapping, but divergent pathways. Our results show that CRF1 traffics from the plasma membrane to endosomes containing ECE-1 at similar efficiency (both CRF or Ucn1 stimulation result in internalization of ∼40% of the total cell surface CRF1). At low doses of agonists, ECE-1 activity is required for CRF1 recycling and resensitization upon stimulation with Ucn1 or CRF. Activation of CRF1 may be dependent on context or tissue type; as seen in SK-N-SH neuronal cells, we did not observe any detectable Ca2+ responses after stimulation with CRF. The availability of Ucn1 in endosomes may be controlled by ECE-1 and could be a critical factor in controlling recycling and resensitization of CRF1. Here we report a novel mechanism by which an endosomal peptidase, ECE-1, differentially regulates signaling of the two known agonists for CRF1 and mediates differential regulation of receptor trafficking, resensitization, and signaling. Cleavage of Ucn1 by ECE-1 at R-34 will disrupt the salt bridge that Ucn1 forms with glutamic acid (E-104) in the extracellular ligand-binding domain of CRF1, but this salt bridge will remain intact in the CRF-CRF1 complex even after ECE-1 cleaves CRF because the critical R-35 residue in CRF is not cleaved by ECE-1. Our data show that CRF-stimulated CRF1 recycles by a mechanism that does not require ECE-1 degradation of CRF, whereas Ucn1-stimulated CRF1 recycles by a mechanism that does require ECE-1 degradation of Ucn1 (Fig. 9).
Fig. 9.
ECE-1 activity determines differential recycling fate of CRF1 upon Ucn1- or CRF-stimulation depending on agonist dose. 1, CRF or Ucn1 binds and activates CRF1. 2, βARR transiently associate with the receptor to mediate desensitization. 3, CRF1 is endocytosed to Rab5 containing early endosomes. 4, Under low agonist conditions, ECE-1 facilitates dissociation of the ligands from CRF1, allowing CRF1 to recycle and resensitize. 5, Under high agonist conditions, ECE-1 facilitates removal of Ucn1 from CRF1, thereby causing Ucn1-mediated receptor to recycle and resensitize. CRF then dissociates from CRF1 by an ECE-1 independent mechanism (presumably by an unknown peptidase?) that allows the freed receptor to recycle and resensitize. 6, Rab11 knockdown studies suggest that CRF1 recycles and resensitizes via a Rab11-dependent pathway. The question mark (?) indicates that we did not test this at a low agonist concentration. 7, Recycled CRF1 is available for resensitization by CRF or Ucn1. 8, ECE-1 is also present at the plasma membrane and gets constitutively shuttled between the plasma membrane and endosomes. 9, At low/basal agonist concentrations, ECE-1 activity regulates both Ucn1- and CRF-mediated Ca2+ signaling, whereas ECE-1 does not appear to affect Gαs-coupled CRF1 and downstream cAMP signaling. 10, At high/pathophysiological agonist concentrations, ECE-1 activity regulates Ucn1-, but not CRF-mediated Ca2+ signaling, whereas ECE-1 does not appear to affect CRF1-mediated cAMP signaling. memb., Membrane.
Rab GTPase participate in multiple steps of vesicular transport. The role of Rab GTPase in intracellular trafficking of the CRF1 activated by CRF has been studied previously, but their role in Ucn1-activated CRF1 has not been delineated. CRF-stimulated CRF1, in both HEK239 and primary cortical neurons, has been shown to transit from Rab5a-positive early endosomes to Rab4a-positive recycling endosomes (46). In agreement with that report, we found that not only CRF, but Ucn1, induced translocation of CRF1 from the cell surface to endosomes containing Rab5a, Rab4a, and Rab11a. Expression of a DN, GTP-binding deficient Rab4aN121I or wild-type Rab4a did not markedly influence endocytosis or recycling of the CRF1, as detected by immunofluorescence. Rab4aN121I, however, did inhibit resensitization of Ucn1-stimulated CRF1. Resensitization and trafficking can be independent of each other, as shown previously for another GPCR receptor, Neurokinin-1 (NK1) receptor (13). DN Rab4a mutant does not affect NK1 receptor recycling but inhibits receptor resensitization. A recently published study showed that Angiotensin II type I receptor, another GPCR, is dephosphorylated and desensitized by Rab4a (47); thus dephosphorylation of GPCR in Rab4 endosomes may be another step that might decouple resensitization and recycling. DN mutants of Rab can interfere with proper functioning of endogenously expressed Rab by two other mechanisms: they can reduce coupling of GTP to endogenously expressed Rab, and the DN Rab are not necessarily targeted to the same subcellular location as wild-type or endogenously expressed ones. To evaluate whether Rab4a indeed plays a role in CRF1 trafficking, we used RNAi to silence Rab4a expression. To our surprise, unlike the DN Rab4a, silencing of Rab4a had no effect on trafficking or signaling of CRF1 after either CRF or Ucn1 stimulation. Thus, mistargeting of DN Rab4a might have led to impaired Ucn1-mediated trafficking of CRF1. We propose that Rab4a is redundant and not necessary for normal processing of CRF1.
In agreement with the above observations, Rab11aS25N also did not affect endocytosis of the CRF1, but inhibited CRF1 recycling in cells treated with Ucn1 but not those treated with CRF. This suggests that, like Rab4a, DN Rab11 is also mistargeted, resulting in changes in trafficking of Ucn1-activated, but not CRF-activated CRF1 from recycling endosomes to the plasma membrane. In contrast to silencing of Rab4, silencing of Rab11 by RNAi impaired trafficking of both CRF- and Ucn1-induced CRF1. A previous study found that CRF-activated CRF1 recycling is dependent on Rab4 but not on Rab11 (46). The discrepancy may be due to the higher agonist concentration used in that study (500 nm vs. 100 nm in our study), and unlike our in our study, the results were based on nonquantitative immunostaining only. Thus, CRF-activated receptors appear to require the Rab11 pathway, but not the Rab4 pathway, and requirement of different Rab may be dependent on cell type, context, or ligand concentration.
CRF-activated CRF1 recruits both βARR1 and βARR2 in multiple cell lines and primary neurons, but activated CRF1 associates with βARR transiently and does not internalize with either βARR to endosomes (17). We also found that CRF1 activation with either CRF or Ucn1 resulted in transient association with both βARR and no colocalization to endosomes; thus, with respect to its association with βARR, CRF1 behaves like a class A GPCR (17). Recently, basal levels of Gs coupled to CRF1 were shown to be higher in brains of female rats than in male rats; this enhanced Gs coupling translated to a higher basal cAMP response in locus ceruleus neurons of female, but not male, rats (38). Interestingly, the same study noted no sex-based differences in levels of Gq or Go coupled to CRF1; Gq- or Go-coupled CRF1 mediates non-cAMP responses such as Ca2+ or inositol 1,4,5-triphosphate mobilization. The same study showed that stress induced greater Gs coupling to CRF1 in neurons in male brains, whereas neurons in the female brains did not show increased coupling to Gs during conditions of stress. Furthermore, stress induced interaction of βARR2 in males, but not in females. In our study, we found only transient association of CRF1 with βARR2 and no effect of ECE-1 inhibition on cAMP levels, suggesting that ECE-1 may specifically target Gq- or Go-coupled CRF1, thereby specifically affecting a signaling pathway that is Ca2+ dependent. Thus, our results uncouple the differential processes of recycling and resensitization of one GPCR triggered by two different agonists, and high, but not basal, levels of agonist concentrations.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA), weighing about 260–280 g, were used for all experiments. They were individually housed in hanging wire cages and handled daily. They had ad libitum access to food and water and were given at least 3–5 d to acclimate to the housing facility before colitis was induced. All procedures were in accordance with the Institutional Animal Care and Use Committee at the University of California, San Francisco. A rectal enema of 30 mg TNBS in 50% ethanol (EtOH) in a final volume of 250 μl was used to induce an immunologically mediated colitis as described previously (45). A 50% ethanol enema served as a vehicle control. Rats were then killed 24 h after TNBS enema, and tissue was collected for RNA and immunohistochemical analyses.
Antibodies
Antibodies were from the following sources: rabbit anti-Ucn1 (1:1000) and rabbit anti-HA11 (1:500) from Sigma-Aldrich (St. Louis, MO); rat anti-HA11 (1:500) from Roche (Indianapolis, IN); mouse anti-HSV from Novagen (Madison, WI); rabbit anti-ECE-1 (1:200) from Invitrogen (catalog no. 52–6497, which cross-reacts with ECE-1b and ECE-1d, but not with ECE-1a or ECE-1c (10); mouse anti-early endosomal antigen-1 (EEA1) (1:500) from BD Transduction Laboratories (Lexington, KY); mouse anti-GFP (1:3000) from Santa Cruz; rabbit anti-γ-tubulin (1:3000) from Sigma; goat antimouse, or -rabbit, or -rat IgG coupled to fluorescein isothiocyanate, rhodamine red-X, or cyanine 5 from Jackson ImmunoResearch Laboratories (West Grove, PA); goat antimouse or -rabbit IgG coupled to Alexa Fluor 680 from Invitrogen (Carlsbad, CA), and coupled to IRDye 800 from Rockland Immunochemicals, Inc. (Philadelphia, PA).
Reagents
Ucn1, CRF, 5FAM-Ucn1, and Rhodamine-CRF were from American Peptide (Sunnyvale, CA). rhECE-1 was from R&D Systems (Minneapolis, MN). The ECE-1 inhibitor SM-19712 (4-chloro-N-[(4-cyano-3-methyl-1-phenyl-1H-pyrazol-5-yl)amino]carbonyl] benzenesulfonamide, monosodium salt) was from Sigma-Aldrich. All other reagents were from Sigma-Aldrich.
Semiquantitative RT-PCR
Total RNA was isolated from snap-frozen tissue using RNA Stat-60 (Tel-Test, Friendswood, TX) according to the manufacturer's protocol. First-strand cDNA was synthesized from 2 μg of total RNA using random hexamers. RT-PCR was performed using 5 μl of RT and gene-specific primers. The sequences of the gene-specific primers and annealing temperatures used in this study are summarized in Table 1. Primer sequences for human CRF1 and CRF2 have been described previously elsewhere (48). Cyclophilin was selected as an unrelated housekeeping gene for normalization. PCR products were analyzed using agarose gel electrophoresis, and band intensities of rat ECE-1c, Ucn1, and CRF were quantified relative to cyclophilin using ImageJ64 (National Institutes of Health).
Table 1.
Gene-specific PCR primers used in this study
| Gene | Primer sequence (5′ to 3′) | Annealing temperature | Product size (bp) |
|---|---|---|---|
| rCRF | Sense: GAGCCCAAGTACGTTGAG | 61 C | 331 |
| Antisense: AATCGGCTGAGGTTGCTG | |||
| hCRF1 | Sense: CAAACAATGGCTACCGGGAG | 62 C | 475 |
| Antisense: ACACCCCAGCCAATGCAGA | (Ref. 48) | ||
| hCRF2 | Sense: CCTCACCAACCTCTCAGGTCC | 62 C | 247 |
| Antisense: CAGGTCATACTTCCTCTGCTTGTC | (Ref. 48) | ||
| rCyclophilin | Sense: TGCAGACGCCGCTGTCTC | 66 C | 594 |
| Antisense: TGCTCTCCTGAGCTACAG | |||
| hECE-1a | Sense: GGCTGAATCTGTGGGAACCAGA | 66 C | 505 |
| Antisense: GAGGAGGTGCTTGATGATTGCTTG | |||
| hECE-1b | Sense: CGGTGTCCGCCCTGCTGT | 60 C | 470 |
| Antisense: GAGGAGGTGCTTGATGATTGCTTG | |||
| hECE-1c | Sense: GCGGAGCACGCGAGCTAT | 66 C | 460 |
| Antisense: GAGGAGGTGCTTGATGATTGCTTG | |||
| rECE-1c | Sense: CCTTAGCGGGAGGTGCAT | 62 C | 288 |
| Antisense: GGCGTTCTTGTCCGGTACT | |||
| hECE-1d | Sense: GAGGGAGTCCGTGCTGCAT | 60 C | 472 |
| Antisense: GAGGAGGTGCTTGATGATTGCTTG | |||
| hRab4a | Sense: GGTTATTGGAAATGCAGGAACTGG | 63 C | 506 |
| Antisense: CTGGGTCCAGCTCACCTGATTC | |||
| hRab11a | Sense: CGACGACGAGTACGACTACCTC | 60 C | 408 |
| Antisense: CTTGCTTCATCTGTAGGAACTGC | |||
| rUcn1 | Sense: CTCCTGGTAGCGTTGCTGCTTCTG | 60 C | 339 |
| Antisense: GCCCACCGAATCGAATATGATGC |
h, Human sequences; r, rat sequences.
cDNA
HA11-tagged CRF1 cDNA was purchased from Missouri S&T cDNA Resource Center (www.cdna.org) and subcloned into a pcDNA5.1/FRT vector for stable expression. cDNA encoding ECE-1a–d with intracellular N-terminal GFP have been described elsewhere (11). CFP-Rab5Q79L, Rab4a-GFP, Rab11a-GFP, Rab4aN121I-GFP, Rab11aS25N-GFP, HSV-ECE-1a, β-arrestin1, and β-arrestin2 have been described previously (10, 13). Long double-stranded RNA were designed for Rab4a and Rab11a from the N-terminal coding region of the gene. Unique sequences for human Rab4a and Rab11a were amplified using gene-specific primers (Table 1), cloned in TOPO vector (Invitrogen), and sequenced. Sense and antisense RNA were transcribed in vitro using RNA polymerase from linearized templates (MEGAscript, Ambion) as described previously (44, 49). Sequences against globin were used as a nonspecific control for this study (44, 49).
Tranfected cells and cell lines
HEK293 cells were grown in DMEM containing 10% heat-inactivated fetal bovine serum in 95% air, 5% CO2 at 37 C. HEK293 cells are know to endogenously express low levels of CRF1 (50). The generation and maintenance of HEK-FLP cells (Invitrogen) stably expressing CRF1 with an N-terminal HA11 epitope were as described previously for other GPCR (10, 51). In some experiments (involving coexpression of two proteins), HEK cells were transiently transfected using Lipofectamine 2000 according to the manufacturer's guidelines (Invitrogen). For analysis of ERK2 activation, cells were starved for 12–16 h (DMEM, 0.1% BSA) before onset of experiments. Human neuroblastoma cell line SK-N-SH (American Type Culture Collection, Manassas, VA) was grown in Eagle's MEM with Earles Balanced Salt Solution, supplemented with nonessential amino acids, 2 mm l-glutamine, 1 mm sodium pyruvate, 1500 mg/liter sodium bicarbonate, and 10% heat-inactivated fetal bovine serum in 95% air, 5% CO2 at 37 C. Cells were plated in 96-well plates 48 h before the experiments.
SDS-PAGE and Western blotting
Cells were lysed in 50 mm Tris/HCl, pH 7.4, 1% sodium dodecyl sulfate, boiled, and centrifuged. Lysates (15 μg of protein) were separated by SDS-PAGE (12% acrylamide gels), transferred to polyvinylidene difluoride membranes (Immobilon-FL, Millipore Corp., Billerica, MA), and analyzed by Western blotting using mouse anti-GFP (1:3000) and rabbit anti-γ-tubulin (1:3000) (overnight at 4 C) (52). Membranes were incubated with secondary antibodies conjugated to Alexa Fluor 680 or IRDye 800 (1:20,000, 1 h at room temperature), and blots were analyzed with the Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE). Rab4-GFP or Rab11-GFP levels were normalized to γ-tubulin levels.
Immunofluorescence, 3,3′-diaminobenzidine staining, and confocal microscopy
HEK-CRF1 cells were incubated with 100 nm Ucn1 or CRF (0–30 min, 37 C), unless otherwise stated. Cells were fixed and incubated with primary antibodies as previously described by us (30, 45). Cells were observed with a Zeiss laser-scanning confocal microscope (LSM Meta 510; Carl Zeiss, Thornwood, NY) using a Fluar Plan Apochromat ×63 oil immersion objective (NA 1.4) (30).
Internalization and recycling of antibody-tagged CRF1 or ECE-1
Surface CRF1 or ECE-1a was labeled by incubating live HEK cells expressing HA-CRF1 or HSV-ECE-1a with an antibody to the N-terminal HA11 or HSV-epitope tag: 1:100 (45 min, 37 C for CRF1 and 60 min, 4 C for ECE-1a). Cells were incubated with 100 nm Ucn1 or CRF (30 min, 37 C) unless specified otherwise, washed, and incubated in Ucn1- and CRF-free medium (0–4 h, 37 C). Cells were fixed, and antibody-tagged CRF1 or ECE-1a was localized by indirect immunofluorescence.
Quantification of cell-surface receptors using On-cell Western assays
Receptors that remain on the cell surface before and after agonist treatment were quantified using On-cell Western assays as described previously (53). Briefly, HEK-CRF1 cells were grown on 96-well plates (25,000 cells were seeded per well; three wells were used per condition) and incubated with 100 nm Ucn1 or CRF or vehicle. This experiment was repeated three times on 3 different days, i.e. n =3 (or nine wells per condition). For recycling, cells were subsequently washed and incubated in Ucn1- and CRF-free medium (4 h, 37 C). Cells were fixed, washed with PBS, and blocked with Li-COR blocking buffer. Cells were then incubated with an antibody to the N-terminal HA11-epitope tag: 1:400 at 4 C overnight, washed with PBS followed by PBS/0.1% Tween washes. Cells were incubated with secondary antirabbit AlexaFluor 680 (1:800, 1 h, room temperature), and densitometric analysis was obtained using the Odyssey IR Imaging System (Li-COR).
Whole-cell cAMP measurements
HEK-CRF1 cells grown on 12-well plates (200,000 cells were seeded per well; three wells were used per condition giving an n = 1, and experiments were repeated two more times on different days, giving an n =3) were challenged with Ucn1 or CRF (100 nm) or vehicle (control). Cells were then rapidly washed with ice-cold PBS and solubilized with 0.1 m HCl/0.1% Triton X-100. The lysates were used to measure levels of cAMP with a competitive immunoassay kit (Assay Designs, Inc., Ann Arbor, MI) according to the manufacturer's guidelines. All cAMP concentrations were corrected for protein levels. Results are expressed as fold increase over vehicle.
Measurement of [Ca2+]I
HEK-CRF1 or SK-N-SH cells were grown on 96-well plates as described for On-cell Western assays, and [Ca2+]i was measured using Fura-2AM as described previously (11). To examine responses to a single stimulus, cells were challenged with Ucn1 or CRF (10–100 nm). Results are expressed as increase above basal values. To assess desensitization and resensitization, cells were incubated with Ucn1 or CRF (30 min, 30 or 100 nm) or vehicle, washed, and recovered in agonist-free medium for 0–4 h at 37 C. Cells were challenged with Ucn1 or CRF (30 or 100 nm), and [Ca2+]i was measured. Results are expressed as percent of controls (100%).
HPLC and mass spectrometry
Ucn1 or CRF peptides (250 μm) were incubated with rhECE-1 (100 nm) in 50 mm Mes/KOH (pH 5.5) or 50 mm Tris HCl (pH 7.4) for 0–120 min at 37 C. All reactions were stopped by boiling for 5 min. To assess cleavage by ECE-1, samples were run on a reversed-phase HPLC. The samples for mass spectrometry were prepared as follows: Ucn1 or CRF incubated with rhECE-1 at 37 C for 0 or 90 min. Control samples included Ucn1 or CRF without rhECE-1 at 37 C for 90 min. All reactions were stopped by boiling for 5 min. The samples were then submitted to University of California San Francisco Mass Spectrometry Core and were analyzed using an ABI 4700 MALDI TOF/TOF mass spectrometer. No ECE-1 cleavage resulting in intact peptides was found for the following samples: Ucn1 or CRF plus rhECE-1 at pH 5.5 or pH 7.4 that were incubated for 0 min; Ucn1 or CRF without rhECE-1 at pH 5.5 or pH 7.4 that were incubated for 90 min; CRF plus rhECE-1 at pH 7.4 that was incubated for 90 min. For the samples Ucn1 or CRF plus rhECE-1 at pH 5.5 that were incubated for 90 min the mass spectrometry provided several cleaved peptides. These cleaved products corresponded to residues 17–19, 35–40, 17–34, 20–40, 17–40 for Ucn1, and 16–41 for CRF.
Drug treatments
SM-19712 (10 μm) or vehicle (control) was preincubated with cells for 60 min and included in buffers throughout the experiment. Cells were preincubated for 30 min with CRF2 antagonist Astressin2B (Sigma) or CRF1 antagonist CP-154526 (Sigma) or vehicle (control) in 10-fold excess relative to the concentration of the ligand used.
Statistics
Data are presented as mean ± sem from n ≥ 3 experiments. Prism (GraphPad Software, San Diego, CA) was used for statistical analysis. Differences were assessed by Student's t test for two comparisons, with P < 0.05 (*) considered significant.
Supplementary Material
Acknowledgments
We thank J. Chang and G.S. Cottrell for help in generating the pcDNA5/FRT-CRF1 construct, and Pamela Derish (University of California, San Francisco) for critical reading of the manuscript.
This work was supported in part by National Institutes of Health (NIH) Grants DK080787 (to A.B.) and a Hellman Family Foundation grants (to A.B). N.W.B. was supported by NIH Grants DK39957, DK43207, and DK57840. Mass spectrometry study was conducted at UCSF Mass Spectrometry Facility and was supported by National Center for Research Resources (NCRR) 5P41RR001614 (Bio-organic Bio-medical Mass Spectrometry Resource Grant) to Dr. Al Burlingame.
Current address for N.W.B.: Monash Institute of Pharmaceutical Sciences, 380 Royal Parade, Parkville, Victoria 3052, Australia.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- βARR
- β-Arrestin
- [Ca2+]i
- intracellular Ca2+
- CFP
- cyan fluorescent protein
- CRF
- corticotropin-releasing factor
- CRF1
- corticotropin-releasing factor receptor 1
- DN
- dominant-negative
- ECE-1
- endothelin-converting enzyme 1
- GFP
- green fluorescent protein
- GPCR
- G protein-coupled receptor
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- HSV
- herpes simplex virus
- NK1
- Neurokinin-1
- rh
- recombinant human
- TNBS
- trinitrobenzene sulfonic acid
- Ucn1
- urocortin 1.
References
- 1. Rivier C, Vale W. 1983. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305:325–327 [DOI] [PubMed] [Google Scholar]
- 2. Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. 1998. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20:1093–1102 [DOI] [PubMed] [Google Scholar]
- 3. Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. 2000. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24:410–414 [DOI] [PubMed] [Google Scholar]
- 4. Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee KF. 2002. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet 31:363–369 [DOI] [PubMed] [Google Scholar]
- 5. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. 1995. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378:287–292 [DOI] [PubMed] [Google Scholar]
- 6. Pal K, Swaminathan K, Xu HE, Pioszak AA. 2010. Structural basis for hormone recognition by the Human CRFR2α G protein-coupled receptor. J Biol Chem 285:40351–40361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seachrist JL, Ferguson SS. 2003. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74:225–235 [DOI] [PubMed] [Google Scholar]
- 8. Sipe DM, Murphy RF. 1987. High-resolution kinetics of transferrin acidification in BALB/c 3T3 cells: exposure to pH 6 followed by temperature-sensitive alkalinization during recycling. Proc Natl Acad Sci USA 84:7119–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. 1984. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell 37:789–800 [DOI] [PubMed] [Google Scholar]
- 10. Padilla BE, Cottrell GS, Roosterman D, Pikios S, Muller L, Steinhoff M, Bunnett NW. 2007. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and β-arrestins. J Cell Biol 179:981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roosterman D, Cottrell GS, Padilla BE, Muller L, Eckman CB, Bunnett NW, Steinhoff M. 2007. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci USA 104:11838–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cottrell GS, Padilla BE, Amadesi S, Poole DP, Murphy JE, Hardt M, Roosterman D, Steinhoff M, Bunnett NW. 2009. Endosomal endothelin-converting enzyme-1: a regulator of β-arrestin-dependent ERK signaling. J Biol Chem 284:22411–22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. 2004. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem 279:30670–30679 [DOI] [PubMed] [Google Scholar]
- 14. Shenoy SK, Lefkowitz RJ. 2003. Multifaceted roles of β-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J 375:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Markovic D, Papadopoulou N, Teli T, Randeva H, Levine MA, Hillhouse EW, Grammatopoulos DK. 2006. Differential responses of corticotropin-releasing hormone receptor type 1 variants to protein kinase C phosphorylation. J Pharmacol Exp Ther 319:1032–1042 [DOI] [PubMed] [Google Scholar]
- 16. Perry SJ, Junger S, Kohout TA, Hoare SR, Struthers RS, Grigoriadis DE, Maki RA. 2005. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem 280:11560–11568 [DOI] [PubMed] [Google Scholar]
- 17. Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. 2007. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and β-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol 293:R209–R222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasmussen TN, Novak I, Nielsen SM. 2004. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of β-arrestin 1 recruitment. Eur J Biochem 271:4366–4374 [DOI] [PubMed] [Google Scholar]
- 19. Hoare SR, Sullivan SK, Fan J, Khongsaly K, Grigoriadis DE. 2005. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides 26:457–470 [DOI] [PubMed] [Google Scholar]
- 20. Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. 2005. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol 19:474–490 [DOI] [PubMed] [Google Scholar]
- 21. Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. 2001. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem 76:509–519 [DOI] [PubMed] [Google Scholar]
- 22. Hillhouse EW, Grammatopoulos DK. 2006. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27:260–286 [DOI] [PubMed] [Google Scholar]
- 23. Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. 2004. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1α receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol 18:624–639 [DOI] [PubMed] [Google Scholar]
- 24. Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. 2009. Molecular mechanisms of corticotropin-releasing factor receptor-induced calcium signaling. Mol Pharmacol 75:648–657 [DOI] [PubMed] [Google Scholar]
- 25. Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW. 2000. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1α and R2β CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol 14:2076–2091 [DOI] [PubMed] [Google Scholar]
- 26. Brar BK, Chen A, Perrin MH, Vale W. 2004. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2β by the CRF/urocortin family of peptides. Endocrinology 145:1718–1729 [DOI] [PubMed] [Google Scholar]
- 27. Punn A, Levine MA, Grammatopoulos DK. 2006. Identification of signaling molecules mediating corticotropin-releasing hormone-R1α-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol 20:3179–3195 [DOI] [PubMed] [Google Scholar]
- 28. Hemley CF, McCluskey A, Keller PA. 2007. Corticotropin releasing hormone–a GPCR drug target. Curr Drug Targets 8:105–115 [DOI] [PubMed] [Google Scholar]
- 29. Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13:1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasdemir B, Bunnett NW, Cottrell GS. 2007. Hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) mediates post-endocytic trafficking of protease-activated receptor 2 and calcitonin receptor-like receptor. J Biol Chem 282:29646–29657 [DOI] [PubMed] [Google Scholar]
- 31. Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. 2007. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J Biol Chem 282:12260–12271 [DOI] [PubMed] [Google Scholar]
- 32. Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149:901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson GD, Stevenson T, Ahn K. 1999. Hydrolysis of peptide hormones by endothelin-converting enzyme-1. A comparison with neprilysin. J Biol Chem 274:4053–4058 [DOI] [PubMed] [Google Scholar]
- 34. Korth P, Bohle RM, Corvol P, Pinet F. 1999. Cellular distribution of endothelin-converting enzyme-1 in human tissues. J Histochem Cytochem 47:447–462 [DOI] [PubMed] [Google Scholar]
- 35. Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. 2000. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 21:1799–1809 [DOI] [PubMed] [Google Scholar]
- 36. Chatzaki E, Charalampopoulos I, Leontidis C, Mouzas IA, Tzardi M, Tsatsanis C, Margioris AN, Gravanis A. 2003. Urocortin in human gastric mucosa: relationship to inflammatory activity. J Clin Endocrinol Metab 88:478–483 [DOI] [PubMed] [Google Scholar]
- 37. Kohno M, Kawahito Y, Tsubouchi Y, Hashiramoto A, Yamada R, Inoue KI, Kusaka Y, Kubo T, Elenkov IJ, Chrousos GP, Kondo M, Sano H. 2001. Urocortin expression in synovium of patients with rheumatoid arthritis and osteoarthritis: relation to inflammatory activity. J Clin Endocrinol Metab 86:4344–4352 [DOI] [PubMed] [Google Scholar]
- 38. Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. 2010. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Ménard L, Caron MG. 1996. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366 [DOI] [PubMed] [Google Scholar]
- 40. Chang J, Adams MR, Clifton MS, Liao M, Brooks JH, Hasdemir B, Bhargava A. 2011. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol 300:G884–G894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gourcerol G, Wu SV, Yuan PQ, Pham H, Miampamba M, Larauche M, Sanders P, Amano T, Polak A, Im E, Pothoulakis C, Rivier J, Tache Y, Million M. 2011. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology 140:1586–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larauche M, Kiank C, Tache Y. 2009. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol 60(Suppl 7):33–46 [PMC free article] [PubMed] [Google Scholar]
- 43. Wlk M, Wang CC, Venihaki M, Liu J, Zhao D, Anton PM, Mykoniatis A, Pan A, Zacks J, Karalis K, Pothoulakis C. 2002. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology 123:505–515 [DOI] [PubMed] [Google Scholar]
- 44. la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. 2005. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci USA 102:7647–7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang J, Hoy JJ, Idumalla PS, Clifton MS, Pecoraro NC, Bhargava A. 2007. Urocortin 2 expression in the rat gastrointestinal tract under basal conditions and in chemical colitis. Peptides 28:1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. 2006. Differential regulation of corticotropin releasing factor 1α receptor endocytosis and trafficking by β-arrestins and Rab GTPases. J Neurochem 96:934–949 [DOI] [PubMed] [Google Scholar]
- 47. Esseltine JL, Dale LB, Ferguson SS. 2011. Rab GTPases bind at a common site within the angiotensin II type I receptor carboxyl-terminal tail: evidence that Rab4 regulates receptor phosphorylation, desensitization, and resensitization. Mol Pharmacol 79:175–184 [DOI] [PubMed] [Google Scholar]
- 48. Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. 2002. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab 87:340–346 [DOI] [PubMed] [Google Scholar]
- 49. Bhargava A, Dallman MF, Pearce D, Choi S. 2004. Long double-stranded RNA-mediated RNA interference as a tool to achieve site-specific silencing of hypothalamic neuropeptides. Brain Res Brain Res Protoc 13:115–125 [DOI] [PubMed] [Google Scholar]
- 50. Dautzenberg FM, Higelin J, Teichert U. 2000. Functional characterization of corticotropin-releasing factor type 1 receptor endogenously expressed in human embryonic kidney 293 cells. Eur J Pharmacol 390:51–59 [DOI] [PubMed] [Google Scholar]
- 51. Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. 2006. Ubiquitin-dependent down-regulation of the neurokinin-1 receptor. J Biol Chem 281:27773–27783 [DOI] [PubMed] [Google Scholar]
- 52. Hasdemir B, Murphy JE, Cottrell GS, Bunnett NW. 2009. Endosomal deubiquitinating enzymes control ubiquitination and down-regulation of protease-activated receptor 2. J Biol Chem 284:28453–28466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Markovic D, Punn A, Lehnert H, Grammatopoulos DK. 2008. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2β endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol 22:689–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.