Abstract
Mammalian male fertility depends on the epididymis, a highly segmented organ that promotes sperm maturation and protects sperm from oxidative damage. Remarkably little is known about how gene expression is controlled in the epididymis. A candidate to regulate genes crucial for epididymal function is reproductive homeobox gene on X chromosome (RHOX)5, a homeobox transcription factor essential for optimal sperm motility that is expressed in the caput region of the epididymis. Here, we report the identification of factors that control Rhox5 gene expression in epididymal cells in a developmentally regulated and region-specific fashion. First, we identify GATA transcription factor-binding sites in the Rhox5 proximal promoter (Pp) necessary for Rhox5 expression in epididymal cells in vitro and in vivo. Adjacent to the GATA sites are androgen-response elements, which bind to the nuclear hormone receptor androgen receptor (AR), and are responsible for the AR-dependent expression of Rhox5 in epididymal cells. We provide evidence that AR is recruited to the Pp in a region-specific and developmentally regulated manner in the epididymis that is dictated not only by differential AR availability but differential methylation of the Pp. Site-specific methylation of the Pp cytosine and guanine separated by one phosphate, most of which overlap with androgen-response elements, inhibited both AR occupancy at the Pp and Pp-dependent transcription in caput epididymal cells. Together, our data support a model in which DNA methylation, AR, and GATA factors collaborate to dictate the unique developmental and region-specific expression pattern of the RHOX5 homeobox transcription factor in the caput epididymis, which in turn controls the expression of genes critical for promoting sperm motility and function.
The generation of functional sperm requires that elongated spermatids from the testis undergo maturation in the epididymis. Several lines of evidence suggest that the testis-proximal region of the epididymis, the caput, is where some, if not all, of these maturation events occur (1–4). After undergoing maturation in the caput, spermatozoa travel through the corpus region to the testes-distal end of the epididymis, the cauda, where the spermatozoa are stored before ejaculation. Given the functional compartmentalization of events in the epididymis, there is a great interest in understanding the regulatory mechanisms that control the region-specific expression of genes in the epididymis. Toward understanding such regulatory mechanisms, it is important to define cis elements and factors that dictate gene transcription in the epididymis during its postnatal development. Although a large number of genes has been shown to exhibit developmentally regulated and region-specific expression in the epididymis (5–11), little is known regarding the molecular mechanisms responsible for their expression patterns. Likewise, although many epididymal genes have been shown to be androgen regulated (6, 12–15), the mechanism underlying this regulation has only just begun to be delineated (11, 16). Finally, although there has been progress in identifying 5′ regions upstream of transcription start sites that confer epididymal expression in transgenic mice in vivo (11, 16, 17), specific cis elements within these regions have not yet been discovered.
In this article, we report the identification of specific cis elements and regulatory factors that drive gene expression in caput epididymal cells in vitro and in vivo. The gene that we chose to perform this analysis on is homeobox gene on X chromosome (Rhox)5, the founding member of the mouse Rhox gene cluster, the largest homeobox gene cluster known to exist in any species (18–24). We chose Rhox5 for several reasons. First, we had previously narrowed the regulatory region required for the epididymal expression of Rhox5's proximal promoter (Pp) in vivo to a relatively short length: approximately 0.3 kb (25). To our knowledge, this is shorter than regulatory regions in other epididymal promoter so far defined. Second, we previously showed that Rhox5 is androgen-induced in the epididymis in both mice and rats (25, 26), indicating that Rhox5 has the potential to be a model system for understanding the molecular mechanisms by which androgens regulate gene transcription in the male reproductive tract and thereby promote fertility. This is particularly important given that androgens are necessary for both the formation and function of the epididymis (27–29). Androgens drive a wide variety of specific events in the epididymis, including epididymal cell proliferation, regulation of the transport of ions and small molecules across the epididymal epithelium, regulation of intermediary metabolism, and regulation of many proteins, including hormone receptors, oxidative-damage protective proteins, and junctional complexes (30). Finally, Rhox5 is preferentially expressed in the caput region of the epididymis (25), indicating that it is a candidate to regulate genes important for sperm maturation. Indeed, our analysis of Rhox5-null male mice revealed that they are subfertile and have sperm harboring a defect in forward motility (18).
Here, we address the mechanisms that control the developmentally regulated and region-specific expression of Rhox5 in the epididymis. We provide evidence that androgen receptor (AR) and androgen play an important role in regulating both Rhox5's temporal and caput-specific expression pattern. We find that juxtaposed with the positive regulation mediated by AR/androgen is negative regulation mediated by DNA methylation. This has implications for mammalian gene regulation in general, because it has been controversial whether DNA methylation controls tissue-specific and developmentally regulated gene expression in mammals (31). Together with our evidence that GATA transcription factors also participate in regulating Rhox5 expression in the epididymal cells in vitro and in vivo, it appears that multiple factors collaborate to dictate the unique expression pattern of Rhox5 in the epididymis.
Results
AR and androgen activate Pp-dependent transcription in epididymal cells
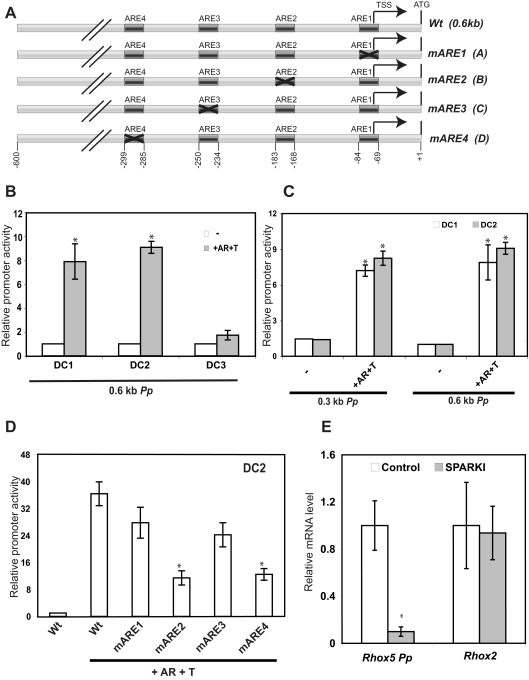
We previously showed that transcription from the Rhox5 Pp in the epididymis in vivo depends on AR and androgen (25, 26, 32). To determine whether this is a direct effect of AR and androgen acting on epididymal cells, we used distal caput (DC) epididymal cell lines (33). We transiently transfected these cells with a Pp-luciferase construct harboring approximately 0.6 kb of Pp 5′-flanking sequence (Fig. 1A). This 0.6-kb region includes four androgen-response elements (ARE) that we and others previously defined (34, 35) and is sufficient to recapitulate the androgen-inducible and region-specific expression characteristics of the endogenous Pp in the epididymis in vivo (25). Cotransfection with an AR expression plasmid and incubation with the androgen-analog R1881 led to increased luciferase expression from the 0.6-kb Pp-reporter construct in all three DC epididymal cell lines (Fig. 1B). The DC1 and DC2 cell lines exhibited dramatically induced reporter expression, whereas the DC3 cell line exhibited only a modest increase in reporter expression, so we did not further use the DC3 cell line.
Fig. 1.
Identification of AREs responsible for Pp-dependent transcription in epididymal cells. A, Schematic diagram of a wild-type (Wt) Pp construct and ARE-mutant derivatives (mAREs). All constructs harbor 0.3 or 0.6 kb of 5′-flanking sequence and have the Renilla luciferase gene downstream (not shown). B–D, Luciferase analysis performed using epididymal cells incubated with or without the synthetic testosterone R1881 (T) and transiently transfected with the constructs shown in A (100 ng) and a simian virus 40 promoter-driven firefly luciferase plasmid PGL3-E-V (50 ng), the latter of which serves as an internal control for normalization. Some cells were also cotransfected with an AR expression vector (100 ng). Shown are average values ± se from three experiments done in triplicate (values are relative to cells transfected with the empty Renilla luciferase expression vector, which was given a value of 1). E, Real-time PCR analysis of total cellular RNA from adult wild-type and SPARKI mice epididymides was done using at least four independent samples that were normalized to the level of L19 mRNA, which encodes a ribosomal protein. mRNA levels in control mice were given a value of 1. Average values ± se are shown. TSS, Transcription start site; m, mutant. An asterisk indicates statistically significant differences from the control (P ≤ 0.05).
To assess whether the Pp region housing the AREs is sufficient for AR/androgen-induced transcription, we removed the sequences upstream of the AREs (leaving only ∼0.3 kb of 5′-flanking sequence). We found that this 0.3-kb Pp-reporter construct responded to AR and R1881 in essentially the same manner as the 0.6-kb Pp construct in both the DC1 and DC2 cell lines (Fig. 1C). Inclusion of both AR and androgen was required for maximal induction of the 0.3-kb Pp-reporter construct (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). To directly examine the role of the AREs, we mutated each individually (Fig. 1A) and found that mutation of either ARE2 or ARE4 was sufficient to significantly reduce reporter activity from either DC1 or DC2 cells (Fig. 1D and Supplemental Fig. 2). We conclude that the epididymal DC cell lines recapitulate the ability of the Pp to drive transcription in the epididymis in vivo in an androgen- and AR-dependent manner.
To assess how AR promotes Pp-driven transcription in the epididymis in vivo, we evaluated the level of Rhox5 transcripts derived from the Pp (which we will henceforth refer to as Pp transcripts) in the specificity affecting AR knockin (SPARKI) mouse model. In this knockin mouse, the second zinc finger of AR is replaced with that of another nuclear hormone receptor, the glucocorticoid receptor, resulting in a chimeric protein deficient in binding to AR-specific AREs but that binds normally with classical AREs that do not discriminate between AR and glucocorticoid receptor (36). Using quantitative real-time PCR analysis, we found that the epididymides from SPARKI mice exhibited a more than 10-fold reduction in the level of Pp transcripts compared with littermate controls (Fig. 1E). As a negative control, we examined another Rhox family member, Rhox2 (24), and found that its expression was not significantly reduced in SPARKI epididymides (Fig. 1E). Together, this supports the notion that Pp-dependent Rhox5 expression in the epididymis in vivo depends on AR-specific AREs.
GATA-binding sites crucial for Pp-dependent Rhox5 transcription in epididymal cells in vitro and in vivo
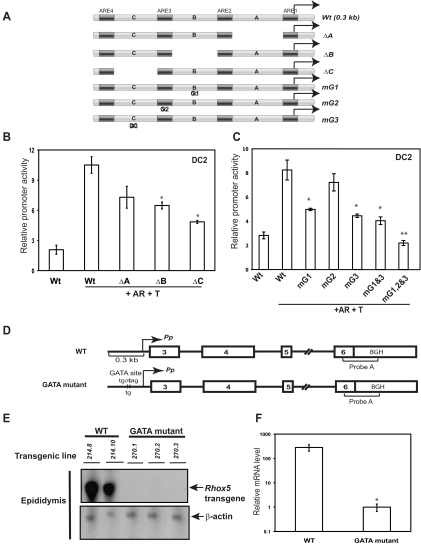
To determine whether other cis elements, in addition to the Pp AREs, have a role in driving Rhox5 transcription in epididymal cells, we deleted the regions between these AREs (regions A, B, and C in Fig. 2A). Deletion of either region B or C significantly decreased Pp-driven reporter levels, indicating that these two regions harbor cis elements important for Pp-dependent Rhox5 transcription in epididymal cells (Fig. 2B). In contrast, deletion of region A did not have a significant effect on reporter expression. Inspection of regions B and C revealed that they contain putative GATA transcription-factor-binding sites (labeled G1 and G3, respectively, in Fig. 2A), leading to the possibility that one or both of these sites have role in promoting Pp-dependent transcription. Indeed, mutation of either of these GATA sites reduced reporter activity (Fig. 2C). In contrast, mutation of a third GATA site (G2), one that overlaps with ARE3, did not significantly affect reporter expression (Fig. 2C). To assess whether the G1 and G3 have additive roles, we made a G1/G3 double mutant but found that it did not yield significantly reduced reporter activity compared with the G1- and G3-single mutants (Fig. 2C). To assess whether the G2 site had redundant activity, we made a G1/G2/G3 triple mutant. We found that this triple mutant generated significantly less reduced reporter activity than the G1/G3 double mutant (Fig. 2C), suggesting that the G2 site serves as a back-up (“shadow”) cis element when the G1 and G3 sites are crippled.
Fig. 2.
Identification of GATA sites crucial for Pp-dependent transcription in vitro and in vivo. A, Schematic of the wild-type (Wt) construct (also shown in Fig. 1A), deletion mutants lacking region A (72 nt), region B (39 nt), or region C (25 nt), and site-specific mutants that lacked GATA consensus site G1, G2, or G3. B and C, Transient transfection analysis performed and quantified as described in the Fig. 1 legend with the constructs indicated. D, Schematic of the wild-type Pp transgene (Pem-214) previously described (25) and a derivative harboring the 2-nt mutation indicated (Pem-270). E, RNase protection analysis of whole epididymis total cellular RNA (10 μg) from adult transgenic mice containing either the Pem-214 or Pem-270 transgene. The transgene-specific probe (probe A) contains bovine GH (BGH) 3′ UTR sequences. A band of the expected size (∼200 nt) was protected by epididymis RNA. A β-actin probe was included in all assays as a loading control (the protected band was ∼35 nt). F, Transgene expression from five Pem-270 and two Pem-214 transgenic mouse lines (average values ± se, an asterisk indicates P ≤ 0.05). The average mRNA signal in GATA mutant mice was set as 1. T, Synthetic testosterone analog.
To assess the role of GATA factors in driving Pp expression in vivo, we generated transgenic mice harboring a Pp construct with a mutation in GATA site G1 (Fig. 2D). We chose to examine the in vivo role of this particular GATA site because it perfectly matches the GATA-binding site consensus sequences (37). We mutated the G1 site by making a AT-to-TG mutation, because this is known to render GATA sites incapable of binding to GATA factors (38, 39). We analyzed five independent transgenic mice lines containing this G1-mutant construct, none of which expressed Pp transcripts detectable by ribonuclease (RNase) protection assay (Fig. 2, E and F). In contrast, transgenic lines harboring the wild-type construct expressed high levels of Pp transcripts (Fig. 2, E and F). We conclude that the G1 GATA site promotes Pp-dependent transcription in epididymal cell lines and is necessary for Pp mRNA expression in the epididymis in vivo.
Consistent with GATA factors being important for Pp-dependent transcription, we found that all six of the Gata factors are expressed in the DC cell lines (Supplemental Fig. 3). Overexpression of any one of the six GATA factors did not significantly increase expression from the 0.3-kb Pp-reporter construct in DC2 cells (Supplemental Fig. 4), presumably because GATA factor levels are so high in epididymal cells that it is not rate limiting for Pp-dependent transcription (see below). For feasibility reasons, we did not attempt loss-of-function experiments (i.e. depletion of GATA factors by RNA interference), because GATA factors are known to be highly redundant (37, 40), and thus, if we knocked down any one of the GATA factors, the remaining GATA factors would likely compensate its role. Furthermore, GATA factors are required for a wide range of functions (37), and thus their knockdown could have widespread pleiotropic effects that are difficult to interpret.
To determine which of the six known GATA factors might regulate the Pp in vivo, we examined their expression pattern in the three main regions of the epididymis. We found that transcripts encoding five of the six GATA factors are expressed in the epididymis (Supplemental Figs. 3 and 5). The only exception was GATA5, whose corresponding mRNA was undetectable in the epididymis, even though it was expressed by all three epididymal cell lines (Supplemental Fig. 3). Importantly, several of the GATA family members are expressed in the caput region of the epididymis, where the Pp transcripts are selectively expressed. Of particular interest was GATA3, which is the only GATA factor whose mRNA is expressed at higher levels in the caput than the other regions (Supplemental Fig. 5), making GATA3 a candidate to contribute to the region-specific expression of Rhox5 in the epididymis. To further evaluate which GATA factors are candidates to regulate Pp-dependent transcription, we examined their developmental expression pattern during postnatal development. We found that all five of the GATA factors expressed in the adult epididymis were also expressed postnatally (Supplemental Fig. 6). Interestingly, only Gata3 levels were maintained postnatally; the other four Gata mRNA dramatically decreased in level during this time period (note that the data are presented on a log scale). However, none of the GATA family member had a developmental expression pattern that precisely mirrored that of the Pp transcripts (Supplemental Fig. 6). We conclude that although three GATA sites are important for Pp-dependent transcription in epididymal cells and that GATA factors are available in epididymal cells to act through these sites, it is unlikely that GATA factors are sufficient to dictate the temporal and region-specific expression pattern of the Pp in vivo.
Region-specific and developmentally regulated AR expression and recruitment
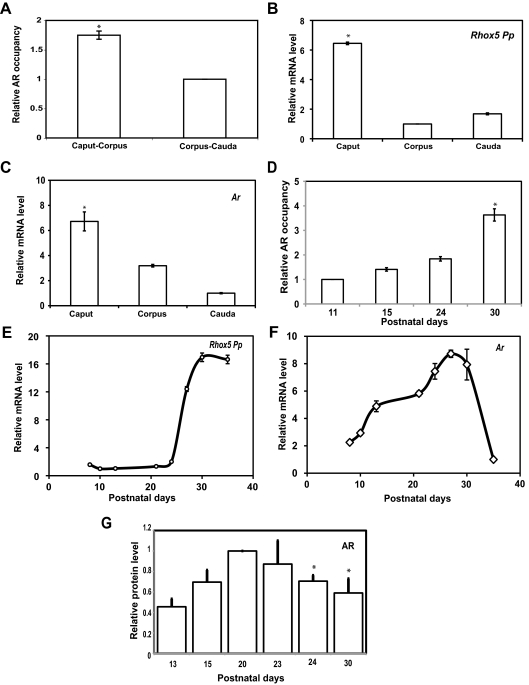
Because our data indicated that GATA factors are necessary but unlikely to be rate limiting for Pp-driven Rhox5 transcription in the epididymis in vivo, we evaluated whether AR serves in this role instead. We first determined whether AR has a pattern of recruitment to the Pp that is consistent with it promoting the region-specific expression of Rhox5. Indeed, using chromatin immunoprecipitation (ChIP) analysis with PCR primers that detect DNA fragments harboring all four AREs in the Pp, we found that AR was more highly recruited to the Pp in the caput than the cauda (Fig. 3A), consistent with the higher levels of the Pp transcripts in the caput than in the cauda (Fig. 3B). Ar mRNA levels were also higher in the caput region than the cauda region (Fig. 3C). Together with our direct evidence that AR promotes Pp-driven transcription in epididymal cells in vitro and in vivo (Fig. 1 and Supplemental Figs. 1 and 2), this strongly suggests that AR availability controls, at least in part, the region-specific transcription driven by the Pp in the epididymis.
Fig. 3.
Developmental and region-specific pattern of Ar and Pp expression and AR occupancy at the Pp. A and D, ChIP analysis of AR occupancy in adult epididymides segments or postnatal whole epididymides at the indicated time points. The values shown are mean ± se, obtained by real-time PCR from at least three pooled samples run in triplicate from three different experiments. AR occupancy is relative to the corpus-cauda region, which was given a value of 1. B, C, E, and F, Real-time PCR analysis of total cellular RNA from adult epididymides segments or whole postnatal epididymides at the indicated postnatal time points. The values shown are average fold (±se) from at least four independent samples, quantified as described in the Fig. 1E legend (the lowest value in each panel is set to 1). G, Western blot analysis of AR protein level using whole-cell lysates from the epididymides of mice from the indicated postnatal time points. The values shown are average fold (±se) from three individual blots that were each normalized to a loading control (β-actin and β-tubulin). The relative level of AR at d 20 was set to 1; an asterisk indicates statistically significant (P ≤ 0.05) differences from d 20. Supplemental Fig. 7 shows a representative blot.
We next examined whether AR availability controls the developmental pattern of Pp-driven Rhox5 expression in the epididymis. ChIP analysis showed that there is a dramatic increase in AR recruitment to the Pp during postnatal development, such that there was approximately 4-fold increase in AR recruited between postnatal day (P)11 and P30 (Fig. 3D). This developmental pattern of AR recruitment to the Pp largely mirrored that of Pp transcript levels, indicating that AR is also likely to be a key rate-limiting factor that controls Rhox5 expression in the epididymis postnatally (Fig. 3E). However, it is unlikely that AR recruitment is dictated only by availability, because Ar mRNA levels only increased by approximately 2-fold during the time interval when Pp transcripts increased by approximately 10-fold (between P21 and P27) (Fig. 3F). Furthermore, Ar mRNA levels reproducibly decrease in level between P30 and P36. Finally, Western blot analysis revealed that AR protein levels largely mirrored that of Ar mRNA levels, because AR protein levels modestly increased initially and then modestly decreased later during postnatal development (Fig. 3G and Supplemental Fig. 7). Together, these data suggest that another mechanism in addition to AR availability dictates AR recruitment to the Pp, a subject that we deal with in the next section.
DNA methylation regulates AR recruitment and Pp-dependent Rhox5 transcription
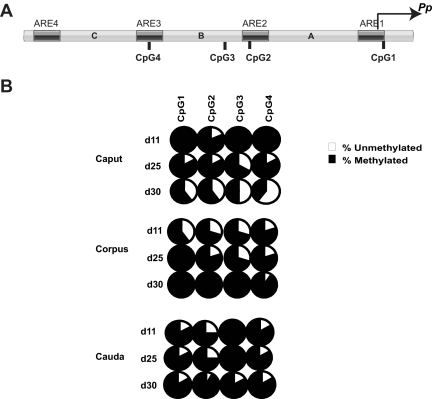
We considered the possibility that AR recruitment to the Pp, and hence the rate of Pp-dependent transcription, is controlled by DNA methylation, a key event that controls the competency of chromatin to respond to many transcription factors (41). We deemed the Pp to be a good candidate to be regulated by DNA methylation, because blockade of DNA methylation by various means has been shown to up-regulate the expression of several Rhox family members (24). Furthermore, the Pp has an intermediate density of cytosine and guanine separated by one phosphate (CpGs) (Fig. 4A), which has been shown to be ideal for DNA methylation control (42). As a first step to assess whether DNA methylation might regulate Pp-dependent transcription in the epididymis, we examined the methylation status of the Pp in the epididymis in vivo. We found that the Pp is differentially methylated in the epididymis in a pattern consistent with both its region-specific and developmentally regulated expression being directed by DNA methylation. In particular, we found that the Pp is hypermethylated in the caput region at an early time point when it is hardly expressed and then undergoes progressive demethylation as its expression increases during development (Figs. 3, B and E, and 4B). In contrast, the corpus and cauda regions, both of which express much lower levels of Pp than the caput (Fig. 3B), did not exhibit reduced Pp methylation during postnatal development (Fig. 4B). In fact, the corpus exhibited increased Pp methylation postnatally. We conclude that the Pp is demethylated in a developmentally regulated and region-specific manner in the epididymis.
Fig. 4.
Demethylation of the Pp in a region-specific and developmentally regulated manner in the mouse epididymis. A, Schematic diagram of the Pp showing the location of 4 CpG sites with respect to the ARE. B, Percent of methylation level at each CpG site in the Pp determined from genomic DNA obtained from three segments of postnatal epididymides at the indicated postnatal time points. The data comes from at least 10 clones per time point.
To examine factors that might have a role in the demethylation and subsequent transcriptional repression of Pp-dependent transcription in the caput, we performed ChIP analysis on the maintenance methylase DNA methyltransferase (DNMT)1, and a methyl binding CpG protein 2 (MeCP2), recruits transcriptional repressor complexes. In the immature caput epididymis, we observed DNMT1 and MeCP2 occupancy at the Pp, which dwindled to background levels later in epididymis development (Supplemental Fig. 8), mirroring the reduction in Pp methylation that occurs as caput epididymal development proceeds (Fig. 4B). We also examined Pp occupancy of the de novo methylase, DNMT3B, but were not able to detect a ChIP signal above background at any of the developmental time points we tested (data not shown).
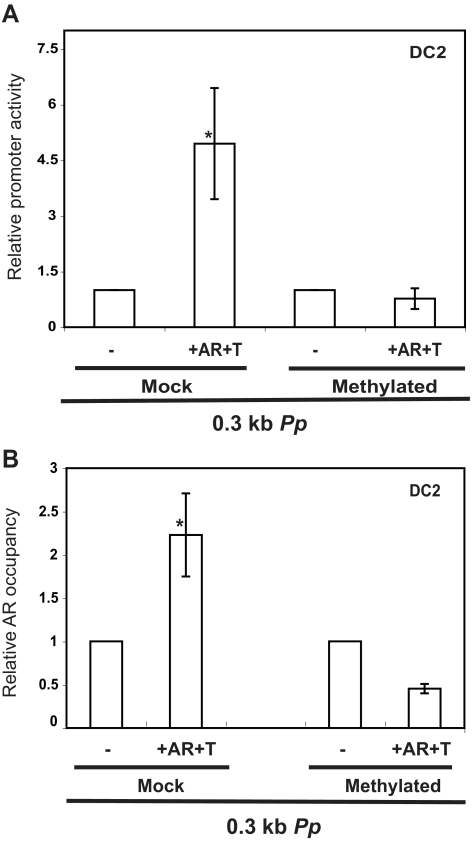
To directly determine whether DNA methylation has a causal role in controlling Pp-dependent transcription, we in vitro methylated the four CpGs in the Pp region in a luciferase reporter construct using the cassette methylation procedure, which specifically methylates cytosines in a CpG context in the region of interest but not the vector (see Materials and Methods). We found that methylation of the four CpGs in the Pp reduced its ability to respond AR and R1881 in transfected DC2 cells (Fig. 5A), indicating that DNA methylation of the Pp is sufficient to repress Pp-dependent transcription. Three of the four CpGs in the Pp overlap or are directly adjacent to ARE (Fig. 4A), raising the possibility that DNA methylation represses Pp-dependent transcription by inhibiting its ability to recruit AR. To address this, we used ChIP analysis on cells transfected with the Pp-reporter plasmid. Comparison of DC2 cells transfected with in vitro-methylated vs. mock-methylated Pp-reporter plasmid revealed that DNA methylation did indeed inhibit AR recruitment (Fig. 5B). Together with the results described above, this suggests that DNA methylation inhibits the recruitment of AR to the Pp, which, in turn, inhibits Pp-dependent transcription.
Fig. 5.
Methylation inhibits AR recruitment and Pp-dependent transcription. A, Luciferase analysis of epididymal cells transiently transfected with mock and methylated Pp construct, performed as described in the legend of Fig. 1B. Cells incubated with the synthetic testosterone R1881 (T) and cotransfected with AR expression vector are indicated. B, ChIP analysis of DC2 cells transiently transfected with mock and methylated Pp construct using AR and a negative control antiserum. The values shown are mean (±se) obtained by real-time PCR from three samples run in triplicate. An asterisk indicates statistically significant differences from the control (P ≤ 0.05).
Discussion
Understanding the molecular mechanisms driving male reproduction is crucial for creating new therapies for infertility and novel male contraceptive approaches. In this article, we address this topic using the Rhox5 homeobox gene, which encodes a transcription factor expressed in somatic cells in the epididymis in mice and rats (18, 25, 26, 43). Rhox5 is functionally important for male reproduction, because mutational inactivation of Rhox5 in mice causes male subfertility accompanied by increased germ cell apoptosis, reduced sperm count, and reduced sperm motility (18). Rhox5 is most highly expressed in the caput, the region of epididymis that sperm must pass through to acquire full motility (3, 14, 44, 45). This suggests that Rhox5's ability to promote sperm motility derives from its expression in the caput; however, because Rhox5 is also expressed in Sertoli cells (18, 32, 34, 43, 46), we cannot rule out that Rhox5 also (or instead) promotes sperm motility indirectly by acting upstream in Sertoli cells. Here, we focus on Rhox5's transcriptional regulation in the epididymis. We demonstrate that the Rhox5 promoter active in the epididymis, the Pp, depends on cis elements binding to AR and GATA transcription factors in epididymal cell lines (Figs. 1, 2, and 5 and Supplemental Figs. 1 and 2). In contrast, a recent study by Hu et al. (47) failed to observe significant recruitment of AR to the Pp in the epididymis. Although we do not know the reason for this apparent discrepancy, it may be because we used the caput region of the epididymis from postnatal stages that express high levels of Pp transcripts (rather than caput epididymis from the adult stage, as used by Hu et al.). Additional in vivo evidence that Pp-dependent transcription is regulated by AR came from our finding that knockin mice that express a form of AR that only binds classical AREs, not AR-specific AREs, had dramatically reduced levels of Pp transcripts (Fig. 1E). Using transgenic mice, we found that one of the GATA factor-binding sites in the Pp is also critical for Pp mRNA expression in the epididymis in vivo (Fig. 2E). Finally, we provide several lines of evidence that an additional layer of Pp regulation is conferred by DNA methylation. We show that Pp transcripts have a regional and temporal pattern of DNA methylation in the epididymis in vivo that is consistent with it being generated as a result of DNA methylation (Fig. 4). Coupled with our finding that DNA methylation inhibits recruitment of AR to the Pp (Fig. 5B) and is sufficient to repress Pp-dependent transcription in epididymal cells (Fig. 5A), this suggests that DNA methylation inhibits AR recruitment to the Pp, which, in turn, inhibits its transcriptional activation.
Our finding that Rhox5 is induced by androgen and AR in epididymal cells (Fig. 1 and Supplemental Figs. 1 and 2) indicates that it is a candidate to mediate androgen action in the epididymis. A multitude of studies have shown that the epididymis requires androgen, not only for its maintenance but also for its genesis and function (14, 30, 48). One of the major sites of androgen action is the caput region. One line of evidence for this is the finding that selective loss of AR in the proximal region of developing epididymis leads to major defects in the caput region, including failure of principal cell maturation, reduction in the number of principal cells, and reduced lumen diameter (28, 29). Furthermore, most of the androgen-regulated genes that have been identified in the mouse epididymis are preferentially expressed in the caput region. Genes shown to be androgen regulated in the caput region of the epididymis include those encoding glutathione peroxidase-5, glutamine synthetase, myoinositol synthase-A1, aflatoxin B1 aldehyde reductase, amino acid transporter N2, and alkaline phosphatase 2 (15, 49). Although the functional significance of this is not known, it is tempting to speculate that this is because the caput is the focal point of androgen regulation in the epididymis (2). In the future, it will be important to define the transcriptional network downstream of AR that drives androgen-dependent events in the caput epididymis. RHOX5 may act a proximal conveyer of information in such a network, as we provided several lines of evidence here that the Rhox5 gene is a direct target of AR in the epididymis (Fig. 1 and Supplemental Figs. 1 and 2). As further support, we recently obtained direct evidence that AR regulates several genes in the testis through the action of RHOX5 (19, 24). While it is not known if these genes are regulated by RHOX5 in the epididymis, recently it was shown that RHOX5 regulates the expression of several other RHOX family members in the epididymis (54). This suggests the existence of a complex hierarchical network of RHOX transcription factors that drive androgen-dependent events in the epididymis.
Our evidence that the temporal and region-specific expression of Rhox5 is regulated by DNA methylation (Fig. 4) is important in light of the uncertain role of DNA methylation as a gene regulator in some contexts. Although DNA methylation has been clearly established as being crucial for genomic imprinting, X chromosome inactivation, transposon silencing, and maintaining the transcriptionally silent state in heterochromatin, its role in directing tissue-specific and developmentally regulated expression of genes has been controversial (50). In principal, DNA methylation is ideal for driving such regulation, because it provides a “heritable” transcriptional state that can be maintained after cell division in developing tissues through the action of DNMT1. However, its role has been questioned, because methylated cytosines are deaminated to form inosines and then repaired to make thymidine, leading to the loss of methylated CpG in the germline over evolutionary time. We note that this concern does not apply to genes expressed in male and female germ cells, because such genes would be expected to harbor an unmethylated version of the promoter in the germ cells (the only cells that transmit genetic material to the next generation) to allow expression in these cells (19). Indeed, both the Pp and Pd are hypomethylated and expressed in the testis (32), explaining why both of these Rhox5 promoters have been able to maintain their CpGs over evolutionary time. Consistent with the notion that Rhox5 is regulated by DNA methylation, previous studies have shown that Rhox5 transcripts are up-regulated in response to the DNA methylation inhibitor 5AzaC in mesenchymal stem cells and in response to the loss of DNMT in fibroblasts and mouse embryos (19, 24). Although suggestive, these studies did not determine whether DNA methylation has a direct causal role in repressing Rhox5, nor did they identify which of Rhox5's alternative promoters are regulated by DNA methylation. Here, we show that methylation of the four CpGs in the Pp is sufficient to repress the Rhox5 expression in transfected epididymal cells (Fig. 5A). This is likely to be physiologically relevant, because we found that DNA methylation at the Pp is developmentally and spatially regulated in a manner consistent with Pp RNA expression in the epididymis (Figs. 3 and 4).
Our finding that DNA methylation inhibits AR recruitment to the Pp suggests that DNA methylation inhibits Pp-dependent transcription by preventing AR from binding to the Pp AREs. In support of this, three of the four AREs in the Pp overlap or are directly adjacent to CpGs (Figs. 4 and 5). Although we believe this interpretation most simply explains our results, we cannot rule out that DNA methylation acts indirectly to inhibit AR binding, e.g. through an intermediary factor. We also do not know whether AR recruitment to the endogenous Rhox5 gene is inhibited by DNA methylation. Our ChIP experiments were performed with transiently transfected templates, because there is currently no reliable method to specifically alter the methylation status of endogenous genes.
Together, our data support a model in which DNA methylation-dependent recruitment of AR dictates Rhox5's region-specific and developmentally regulated expression pattern in the epididymis. Future studies will be required to identify factors upstream and downstream of this circuit. For example, what elicits the demethylation of the Pp during postnatal development in a tissue-specific manner? Does the Pp recruit DNA-binding factors and/or noncoding RNA, which, in turn, recruit DNMT? Do specific Pp cis elements perform this function, and if so, what trans-acting factors do they recruit to elicit demethylation? The future discovery of such upstream regulatory factors, coupled with the identification of AR-regulated genes directly controlled by RHOX5 in the epididymis, may explain the molecular basis for some cases of male infertility and may identify focal points for future male contraceptive methods.
Materials and Methods
Plasmids, cell culture, transfection, and reporter analysis
The Pp-reporter constructs (0.3- and 0.6-kb 5′-flanking Pem-250 and Pem-124, respectively), ARE mutant constructs, and GATA mutant constructs were generated by subcloning, site-directed mutagenesis, and/or deletion PCR using the primers listed in Supplemental Table 1, as described in Ref. 34. The human AR pcDNA 3.1 plasmid (G541) was kindly provided by Zhengxin Wang (The University of Texas M.D. Anderson Cancer Center). The DC epididymal cell lines were developed in the laboratory Marie-Claire Orgebin-Crist by serial cloning of primary cultures from the caput epididymidis of transgenic mice overexpressing the ts simian virus 40 large T-antigen gene. These cells were cultured in Iscove's Modified Dulbecco's medium (GIBCO, Rockville, MD) supplemented with 10% fetal calf serum, 1 nm 5 ∝-androstan-17β-ol-3-one, and 2.5 ml of penicillin-streptomycin/500 ml medium, as described (33, 34). All three DC cell lines that we used in our study (DC1, DC2, and DC3) expressed similar levels of markers found on normal epididymal epithelial cells, including phosphatidyl ethanolamine binding protein-like protein, mouse epididymal retinoic acid-binding protein, cytokeratin, and AR (33, 34). We transiently transfected these cell lines using lipofectamine 2000 (Invitrogen, Carlsbad, CA) and analyzed for luciferase activity using Promega (Madison, WI) dual luciferase kit, as previously described (34).
Generation of transgenic mice
A 4.6-kb Rhox5 gene fragment (Pem-270) containing a mutated G2 GATA-binding site in the Pp [generated using primers MDA-1858 and MDA-1859 (Supplemental Table 1) as previously described (34)], was excised using the restriction enzymes EcoRV and NotI. This gene fragment was gel purified and injected into the male pronuclei of C57BL/6 mouse embryos by the M.D. Anderson Cancer Center transgenic mouse core laboratory. Six founder transgenic lines containing the Pem-270 transgene DNA (detected by PCR) were obtained (Pem-270.1 to Pem-270.6). All the experiments with mice were performed in accordance with institutional guidelines that are dictated by the American Association for the Accreditation of Laboratory Animal Care.
RNA isolation and analysis
Total tissue RNA was isolated using TRIzol (Invitrogen) following the manufacturer' instructions. RNase protection analysis was performed as described (51) using a Rhox5 transgene-specific riboprobe that contains 61 nt of Rhox5 exon 6 and 250 nt of the bovine GH 3′ untranslated region; it was transcribed from a plasmid containing Pp-driven Rhox5 (from exon 2-exon 6) in pBluescript KS (+), called Pem-121, digested with NdeI, as previously described (34). The β-actin riboprobe (34 nt of β-actin exon 3) was prepared by linearizing a β-actin plasmid (G-98) with BanI, as previously described (26, 34). For quantitative real-time PCR analysis, cDNA were generated using an iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA); amplication was done using SYBR Green fluorescence, and analysis was done using the ΔCT method (where CT is the cycle number at which the PCR signal crosses the threshold) that takes primer set efficiencies into consideration (34). All primer pairs selected for use had efficiencies ranging from 98 to 102%, as determined using the series dilution method of standard curve analysis, as described (52).
DNA methylation analysis
Genomic DNA extraction and bisulphite modification of the DNA were carried out as described elsewhere (53). Bisulfite-modified DNA (100 ng) was PCR amplified with the Pp-specific primers MDA-1838 and MDA-1839 (Supplemental Table 1). Gel-purified PCR products were cloned using the TOPO-TA cloning kit (Stratagene, La Jolla, CA) and sequenced by the M.D. Anderson Cancer Center Sequencing Core facility. For cassette DNA methylation analysis, the Pem-250 plasmid was in vitro methylated with the SssI methylase following the manufacturers instructions (Promega). A negative control was incubated under the same conditions without SssI (“mock methylated”). The 342-bp HindIII-PstI fragment containing the Pp (−321 to +1 relative to the translation start site) was liberated from methylated and mock-methylated Pem-250 and ligated in bulk into unmethylated PRL-null, a promoterless plasmid containing the firefly luciferase gene. The ligation products were gel purified and used for transfection.
ChIP analysis
Epididymis tissue from C57BL/6 mice were isolated and homogenized, and samples were cross-linked and processed for ChIP analyses as described elsewhere (34). To the precleared chromatin, we incubated AR (catalog no. 06-680; Millipore, Bedford, MA), MeCP2 (catalog no. AB2828; Abcam, Cambridge, MA), DNMT3B (catalog no. NB300-516; Novus Biologicals, Littleton, CO), or DNMT1 (catalog no. IMG-261A; Imgenex, San Diego CA) antiserum (200 ng) overnight at 4 C. The extracts were incubated with a slurry of protein A/G bound to agarose containing salmon sperm DNA (Upstate, Charlottesville, VA) for 3–4 h at 4 C and then washed following the manufacturer's instructions. Immune complexes were disrupted with 1% sodium dodecyl sulfate and 0.1 m NaHCO3, and the DNA was reverse cross-linked by incubation with 200 mm NaCl at 65 C for 4 h, deproteinated with proteinase K for 1 h, extracted with phenol-chloroform, ethanol precipitated, and resuspended in 30 μl of H2O. PCR amplification was performed with 3 μl of DNA and a 6-carboxyfluorescein-labeled TaqMan probe specific for Pp transcripts (Applied Biosystems, Foster City, CA) and oligonucleotides MDA-2117 and MDA-2118 (corresponding to nucleotides −96 to −114 and nucleotides −59 to −75, respectively, with respect to the ATG start codon) (see Supplemental Table 1) to specifically amplify the Pp region. The primer set spans only ARE1, but ChIP analysis should detect factors binding to the region encompassing all four AREs in the Pp, because we sheared the genomic DNA to the size of approximately 500 bp; ARE2 to ARE4 are between 95 and 215 bp, respectively, from ARE1. The magnitude of occupancy was calculated as the percentage enrichment for the factor studied (using a specific antibody) subtracted from percentage of background enrichment (nonspecific antibody negative control). For calculating enrichment, we first calculated the percent recovery by using the formula, recovery (% input) = 2^(Ct input−Ct ChIP)* dilution factor of input*100%, and then we calculated occupancy by taking a ratio of specific signal above the background.
Statistical analysis
Statistical analysis was performed using the Student's unpaired t test. P values less than or equal to 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Xiaodi Hu for her instrumental help with the ChIP assay and cassette methylation.
This work was supported by National Institutes of Health Grant HD045595.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- ARE
- androgen-response element
- ChIP
- chromatin immunoprecipitation
- CpG
- cytosine and guanine separated by one phospate
- DC
- distal caput
- DNMT
- DNA methyltransferase
- MeCP2
- methyl binding CpG protein 2
- P
- postnatal day
- Pp
- proximal promoter
- RNase
- ribonuclease
- Rhox
- homeobox gene on X chromosome
- SPARKI
- specificity affecting AR knockin.
References
- 1. Bedford JM, Calvin H, Cooper GW. 1973. The maturation of spermatozoa in the human epididymis. J Reprod Fertil Suppl 18:199–213 [PubMed] [Google Scholar]
- 2. Olson GE, Orgebin-Crist MC. 1982. Sperm surface changes during epididymal maturation. Ann NY Acad Sci 383:372–392 [DOI] [PubMed] [Google Scholar]
- 3. Hinton BT, Palladino MA, Rudolph D, Lan ZJ, Labus JC. 1996. The role of the epididymis in the protection of spermatozoa. Curr Top Dev Biol 33:61–102 [DOI] [PubMed] [Google Scholar]
- 4. Gerena RL, Eguchi N, Urade Y, Killian GJ. 2000. Stage and region-specific localization of lipocalin-type prostaglandin D synthase in the adult murine testis and epididymis. J Androl 21:848–854 [PubMed] [Google Scholar]
- 5. Douglass J, Garrett SH, Garrett JE. 1991. Differential patterns of regulated gene expression in the adult rat epididymis. Ann NY Acad Sci 637:384–398 [DOI] [PubMed] [Google Scholar]
- 6. Cornwall GA, Hann SR. 1995. Specialized gene expression in the epididymis. J Androl 16:379–383 [PubMed] [Google Scholar]
- 7. Kirchhoff C. 1999. Gene expression in the epididymis. Int Rev Cytol 188:133–202 [DOI] [PubMed] [Google Scholar]
- 8. Ezer N, Robaire B. 2003. Gene expression is differentially regulated in the epididymis after orchidectomy. Endocrinology 144:975–988 [DOI] [PubMed] [Google Scholar]
- 9. Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. 2005. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod 73:404–413 [DOI] [PubMed] [Google Scholar]
- 10. Sipilä P, Pujianto DA, Shariatmadari R, Nikkilä J, Lehtoranta M, Huhtaniemi IT, Poutanen M. 2006. Differential endocrine regulation of genes enriched in initial segment and distal caput of the mouse epididymis as revealed by genome-wide expression profiling. Biol Reprod 75:240–251 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki K, Yu X, Chaurand P, Araki Y, Lareyre JJ, Caprioli RM, Orgebin-Crist MC, Matusik RJ. 2007. Epididymis-specific lipocalin promoters. Asian J Androl 9:515–521 [DOI] [PubMed] [Google Scholar]
- 12. Kohane AC, Piñeiro L, Blaquier JA. 1983. Androgen-controlled synthesis of specific proteins in the rat epididymis. Endocrinology 112:1590–1596 [DOI] [PubMed] [Google Scholar]
- 13. Hall JC, Killian GJ. 1989. Two-dimensional gel electrophoretic analysis of rat sperm membrane interaction with cauda epididymal fluid. J Androl 10:64–76 [DOI] [PubMed] [Google Scholar]
- 14. Orgebin-Crist MC. 1996. Androgens and epididymal function. In: Bhasin DGH, Spieler JM, Swerdloff RS, Wang C, eds. Pharmacology, biology, and clinical applications of androgens. New York: Wiley-Liss, Inc.; 27–38 [Google Scholar]
- 15. Chauvin TR, Griswold MD. 2004. Androgen-regulated genes in the murine epididymis. Biol Reprod 71:560–569 [DOI] [PubMed] [Google Scholar]
- 16. Yu X, Suzuki K, Wang Y, Gupta A, Jin R, Orgebin-Crist MC, Matusik R. 2006. The role of forkhead box A2 to restrict androgen-regulated gene expression of lipocalin 5 in the mouse epididymis. Mol Endocrinol 20:2418–2431 [DOI] [PubMed] [Google Scholar]
- 17. Lareyre JJ, Thomas TZ, Zheng WL, Kasper S, Ong DE, Orgebin-Crist MC, Matusik RJ. 1999. A 5-kilobase pair promoter fragment of the murine epididymal retinoic acid-binding protein gene drives the tissue-specific, cell-specific, and androgen-regulated expression of a foreign gene in the epididymis of transgenic mice. J Biol Chem 274:8282–8290 [DOI] [PubMed] [Google Scholar]
- 18. Maclean JA, 2nd, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, Macleod C, Wilkinson MF. 2005. Rhox: a new homeobox gene cluster. Cell 120:369–382 [DOI] [PubMed] [Google Scholar]
- 19. Maclean JA, 2nd, Wilkinson MF. 2005. Gene regulation in spermatogenesis. Curr Top Dev Biol 71:131–197 [DOI] [PubMed] [Google Scholar]
- 20. Jackson M, Watt AJ, Gautier P, Gilchrist D, Driehaus J, Graham GJ, Keebler J, Prugnolle F, Awadalla P, Forrester LM. 2006. A murine specific expansion of the Rhox cluster involved in embryonic stem cell biology is under natural selection. BMC Genomics 7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacLean JA, 2nd, Lorenzetti D, Hu Z, Salerno WJ, Miller J, Wilkinson MF. 2006. Rhox homeobox gene cluster: recent duplication of three family members. Genesis 44:122–129 [DOI] [PubMed] [Google Scholar]
- 22. Morris L, Gordon J, Blackburn CC. 2006. Identification of a tandem duplicated array in the Rhox α locus on mouse chromosome X. Mamm Genome 17:178–187 [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Zhang J. 2006. Remarkable expansions of an X-linked reproductive homeobox gene cluster in rodent evolution. Genomics 88:34–43 [DOI] [PubMed] [Google Scholar]
- 24. MacLean JA, 2nd, Wilkinson MF. 2010. The Rhox genes. Reproduction 140:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rao MK, Wayne CM, Wilkinson MF. 2002. Pem homeobox gene regulatory sequences that direct androgen-dependent developmentally regulated gene expression in different subregions of the epididymis. J Biol Chem 277:48771–48778 [DOI] [PubMed] [Google Scholar]
- 26. Lindsey JS, Wilkinson MF. 1996. An androgen-regulated homeobox gene expressed in rat testis and epididymis. Biol Reprod 55:975–983 [DOI] [PubMed] [Google Scholar]
- 27. Cosentino MJ, Cockett AT. 1986. Structure and function of the epididymis. Urol Res 14:229–240 [DOI] [PubMed] [Google Scholar]
- 28. Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I. 2011. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology 152:689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Hara L, Welsh M, Saunders PT, Smith LB. 2011. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology 152:718–729 [DOI] [PubMed] [Google Scholar]
- 30. Ezer NRB, ed. 2002. Androgenic regulation of the structure and functions of the epididymis. New York: Kluwer Academic/Plenum Publishers [Google Scholar]
- 31. Walsh CP, Bestor TH. 1999. Cytosine methylation and mammalian development. Genes Dev 13:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maiti S, Doskow J, Li S, Nhim RP, Lindsey JS, Wilkinson MF. 1996. The Pem homeobox gene. Androgen-dependent and -independent promoters and tissue-specific alternative RNA splicing. J Biol Chem 271:17536–17546 [DOI] [PubMed] [Google Scholar]
- 33. Araki Y, Suzuki K, Matusik RJ, Obinata M, Orgebin-Crist MC. 2002. Immortalized epididymal cell lines from transgenic mice overexpressing temperature-sensitive simian virus 40 large T-antigen gene. J Androl 23:854–869 [PubMed] [Google Scholar]
- 34. Bhardwaj A, Rao MK, Kaur R, Buttigieg MR, Wilkinson MF. 2008. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol Cell Biol 28:2138–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barbulescu K, Geserick C, Schüttke I, Schleuning WD, Haendler B. 2001. New androgen response elements in the murine pem promoter mediate selective transactivation. Mol Endocrinol 15:1803–1816 [DOI] [PubMed] [Google Scholar]
- 36. Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F. 2007. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci USA 104:4961–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. 2008. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol 22:781–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ko LJ, Engel JD. 1993. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol 13:4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merika M, Orkin SH. 1993. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol 13:3999–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang Y, Tarzami S, Burch JB, Evans T. 1998. Common role for each of the cGATA-4/5/6 genes in the regulation of cardiac morphogenesis. Dev Genet 22:263–277 [DOI] [PubMed] [Google Scholar]
- 41. Ballestar E, Esteller M. 2002. The impact of chromatin in human cancer: linking DNA methylation to gene silencing. Carcinogenesis 23:1103–1109 [DOI] [PubMed] [Google Scholar]
- 42. Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D. 2007. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39:457–466 [DOI] [PubMed] [Google Scholar]
- 43. Lindsey JS, Wilkinson MF. 1996. Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev Biol 179:471–484 [DOI] [PubMed] [Google Scholar]
- 44. Orgebin-Crist MC. 1969. Studies on the function of the epididymis. Biol Reprod 1(Suppl 1):155–175 [DOI] [PubMed] [Google Scholar]
- 45. Robaire B, Hermo L. 1988. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. New York: Raven Press [Google Scholar]
- 46. Rao MK, Wayne CM, Meistrich ML, Wilkinson MF. 2003. Pem homeobox gene promoter sequences that direct transcription in a Sertoli cell-specific, stage-specific, and androgen-dependent manner in the testis in vivo. Mol Endocrinol 17:223–233 [DOI] [PubMed] [Google Scholar]
- 47. Hu Z, Dandekar D, O'Shaughnessy PJ, De Gendt K, Verhoeven G, Wilkinson MF. 2010. Androgen-induced Rhox homeobox genes modulate the expression of AR-regulated genes. Mol Endocrinol 24:60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benoit J. 1926. Recherches anatomiques, cytologiques et histophysiologiques sur les voies excretices du testicle chez les mammifères. Archs Anat Histol Embryol 5:173–412 [Google Scholar]
- 49. Hamzeh M, Robaire B. 2010. Identification of early response genes and pathway activated by androgens in the initial segment and caput regions of the regressed rat epididymis. Endocrinology 151:4504–4514 [DOI] [PubMed] [Google Scholar]
- 50. Costello JF, Vertino PM. 2002. Methylation matters: a new spin on maspin. Nat Genet 31:123–124 [DOI] [PubMed] [Google Scholar]
- 51. Wayne CM, Sutton K, Wilkinson MF. 2002. Expression of the pem homeobox gene in Sertoli cells increases the frequency of adjacent germ cells with deoxyribonucleic acid strand breaks. Endocrinology 143:4875–4885 [DOI] [PubMed] [Google Scholar]
- 52. Rasmussen R. 2001. Rapid cycle real-time PCR, methods and applications. In: Meuer S, Wittwer C, Nakagawara K, eds. Quantification on the LightCycler. Heidelberg: Springer Press; 21–34 [Google Scholar]
- 53. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. 1996. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93:9821–9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MacLean JA, 2nd, Hayashi K, Turner T, Wilkinson MF. 14 March 2012. The Rhox5 homeobox gene regulates the region-specific expression of its paralogs in the epididymis. Biol Reprod 10.1095/biolreprod.112.099184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.