Abstract
Purpose
Connective tissue growth factor (CTGF) is a profibrotic factor that induces extracellular matrix (ECM) production and angiogenesis, two processes involved in diabetic retinopathy (DR). In this study, we examined whether insulin therapy or a CTGF-specific small interfering RNA (siRNA) administered to diabetic rats decreased the levels of CTGF and of selected putative downstream genes in the retina.
Methods
Rats with streptozotocin-induced diabetes were used. Animals received either no treatment for 12 weeks or were administered constant insulin therapy. MRNA and protein levels of CTGF and select ECM genes were determined using real-time PCR and western blotting of the retina. Localization of CTGF in the retina was visualized using immunohistochemistry. A group of diabetic rats received intravitreal injection of CTGF siRNA, and the retinas were examined three days later.
Results
CTGF mRNA and protein significantly increased in the retinas of diabetic rats. Immunohistochemistry indicated that retinal Müller cells of diabetic rats expressed CTGF. Hyperglycemia upregulated mRNA levels of fibronectin, laminin β1, collagen IVα3, and vascular endothelial growth factor (VEGF), and this increase was prevented by insulin therapy. Treatment of diabetic rats with CTGF siRNA decreased laminin β1, collagen IVα3 mRNA, and CTGF mRNA and protein but did not affect fibronectin or vascular endothelial growth factor mRNA levels.
Conclusions
These results indicate that CTGF and ECM genes can be regulated using insulin. Importantly, these results also suggest that CTGF regulates changes in ECM molecules in DR.
Introduction
Diabetic retinopathy (DR) is the leading cause of visual impairment and blindness among adults of working age in the United States [1]. DR can be divided into two stages. The first stage is non-proliferative DR, characterized by retinal edema, microaneurysms, venous bleeding, and soft exudates. The second stage, proliferative DR, is characterized by angiogenesis, retinal detachment, blindness, and an increased number of blood vessels with altered vascular permeability. DR occurs because of altered blood flow, pericyte loss, tissue hypoxia, and basement membrane thickening provoked by increased production of collagen IV, laminin, and fibronectin [2-4]. These changes were detected after 12 and 17 weeks following the appearance of diabetes, respectively [5,6]. In addition, there is also dysregulation of remodeling proteins such as matrix metalloproteinease-2, matrix metalloproteinease-9 (MMP-9), plasminogen activator inhibitor-1, tissue inhibitor of metalloproteinease-1, and other proteins [7-9].
Connective tissue growth factor (CTGF) is a profibrotic factor that induces extracellular matrix (ECM) production and angiogenesis [10], two processes involved in the development of DR. CTGF is one of the six members of the CCN family of proteins. The CCN acronym is derived from the names of the first three members of the family of proteins: Cyr61 (cysteine-rich protein 61), CTGF, and NOV1 (nephroblastoma overexpressed gene-1). The CCN family of proteins is involved in a wide range of functional pathways such as cell adhesion, cell survival, angiogenesis, tumorigenesis, and wound healing [11]. CTGF is upregulated in human and rodent models of DR [12,13] and is induced by glucose [5,13] and advanced glycation end-products [5]. In addition, CTGF is upregulated by vascular endothelial growth factor (VEGF) [14,15], which is increased in patients with diabetes and is a critical regulator of vascular permeability and angiogenesis [16].
The exact role of CTGF in the progression of DR has yet to be determined. Although CTGF knockout is embryonic lethal [17], CTGF heterozygote mice have a 50% decrease in CTGF levels in plasma and urine and show decreased retinal basal lamina thickening in diabetes [6]. In addition, CTGF is responsible for the development of fibrosis, not angiogenesis, which results in scarring of the retina and blindness [18]. Studies of the kidney strengthened the possibility that CTGF mediates the alterations of ECM during hyperglycemia [19].
In this study, we sought to determine the role of CTGF in non-proliferative DR. First, we tested whether the increase in CTGF levels with hyperglycemia could be attenuated through insulin therapy and whether this treatment affected the level of expression of key ECM molecules. Since glycemic levels fluctuate during insulin therapy, we also tested whether a specific inhibition of CTGF using siRNA affects the levels of selected ECM molecules that increase in the diabetic retina.
Methods
Diabetic animal model
Male Sprague Dawley Rats (Charles River, Troy, NY), weighing approximately 200 g, received a single (IP) injection of 80 mg/kg streptozotocin (STZ; Sigma, St. Louis, MO) dissolved in 0.1 M citrate buffer (pH 4.5) [20]. Control non-diabetic animals were injected with an equal volume of citrate buffer. Fasting blood glucose (FBG) levels were measured using a PrecisionXtra blood glucose monitor (Abbot, Alameda, CA). Animals with FBG greater than 350 mg/dl were considered diabetic. The first day of recorded hyperglycemia was considered day 1 of the experiment. Animals were euthanized with Euthasol (120 mg/kg; Vibrac Corp., Fort Worth, TX) and sacrificed after 8 and 12 weeks of hyperglycemia. Eyes were enucleated, and the retina dissected in nuclease free ice-cold PBS (137 mM sodium chloride, 2.7 mM potassium chloride, 8 mM sodium phosphate dibasic, 2 mM potassium phosphate monobasic, pH 7.4). The animal procedures were in accordance with the institutional Animal Care and Use Committee and with the standards established by the New York State Department of Health, the USA Public Health Service, the USA Department of Agriculture, and the Association for Assessment and Accreditation of Laboratory Animal Care.
Cell cultures
Rat-2 fibroblasts (ATCC, Manassas, VA) were maintained in vitro as previously described [21]. Briefly, cells were maintained in Dulbeco’s Modified Eagle Medium (DMEM, Sigma) supplemented with sodium bicarbonate (1.5 g/l; Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (FBS, Invitrogen). Rat-2 cells were seeded at a density of 5.8×105 cells in a T-25 tissue culture flask (Falcon; BD, San Jose, CA) and allowed to adhere overnight. Transfection agent (siPORT-Amine; Ambion, Austin, TX) was diluted (1:40) in Opti-MEM (Invitrogen) and incubated at room temperature for 10 min. Three CTGF siRNAs (Ambion) were individually tested for their effect on CTGF mRNA and protein. Sequences for these siRNAs are shown in Table 1. Each siRNA (50 mM), diluted in 900 µl of Opti-MEM, was added to the transfection mix and incubated at room temperature for 10 min. The siRNA/transfection agent complexes were added to suspended cells (1.8×106), and the mixture was placed in a T-75 flask in medium containing 10% FBS. siRNA transfected cells were incubated at 37 °C with CO2 for 24 h. Then the cells were processed and treated with transforming growth factor (TGF)-β1 (R&D Systems, Minneapolis, MN) as previously described [21]. In brief, cells were incubated with 0.5% FBS DMEM medium containing TGF-β1 (0.1 ng/ml) for 4 or 6 h before harvesting. After TGF-β1 treatment, cells were trypsinized with 0.25% trypsin-EDTA (Trypsin-EDTA; Invitrogen) and pelleted by centrifugation at 200 g for 5 min (International Equipment Company, Chattanooga, TN). Cell pellets were washed in RNase-free ice-cold PBS and centrifuged at 1,300× g (MTX-150; Tomy Tech, Menlo, CA) for 10 min at 4 °C and then used for total RNA isolation or protein extraction.
Table 1. Sequences for siRNAs.
| Name of siRNA | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|
| Scrambled siRNA |
Sequence undisclosed by company |
|
|
CTGF siRNA I * |
GGAUUACAGUAGCACAUUATT |
UAAUGUGCUACUGUAAUCCTT |
|
CTGF siRNA II |
GGGACACGAACUCAUUUAGTT |
CUAAAUGAGUUCGUGUCCCTT |
|
CTGF siRNA III |
CGAACUCAUUUAGACUAUATT |
UAUAGUCUAAAUGAGUUCGTG |
| Scrambled siRNA* | UAAGGCUAUGAAGAGAUACUU | PGUAUCUCUUCAUAGCCUUAUU |
*Denotes siRNA used for intravitreal injections.
Insulin therapy
A subset of diabetic animals (FBG≥350 mg/dl) was implanted with subcutaneous insulin pellets (LinShin Canada Inc., Toronto, Canada) for 12 weeks, on the first day of hyperglycemia according to the company’s recommendations. FBG was measured 1 week after insulin pellet implantation (day 1 of the experiment for the group treated with insulin), and weekly thereafter. Animals with poor glycemic control received two additional insulin pellets.
Intravitreal small interfering ribonucleic acid injections
After 12 weeks of hyperglycemia, a subset of rats received a single intravitreal injection of siRNA. Animals were anesthetized with vaporized isoflurane. Eyes were visualized with a dissecting microscope, and the sclera was pierced behind the ora serrata using a borosilicate glass capillary (World Precision Instruments, Sarasota, FL) that was heat pulled to obtain a thin diameter. siRNAs (Table 1) were modified for in vivo studies by Dharmacon (Lafayette, CO), using undisclosed techniques due to proprietary considerations. siRNA was diluted in sterilized, RNase/DNase-free, physiologic PBS for a final concentration of 2.5 µg/µl. siRNA was injected using a sterilized Nanofill syringe attached to a 33G beveled needle (World Precision Instruments) inserted in the opening produced by the capillary needle. One eye was injected with 3 µl (7.5 µg) of CTGF siRNA and the contralateral eye with scrambled siRNA (7.5 µg). Animals were sacrificed at 3 and 10 days post siRNA injection.
To track and visualize siRNA inside the layers of the retina, a Label IT siRNA Tracker Kit (Mirus, Madison, WI) was used to label the scrambled siRNA with Cy3. Briefly, about 10 mg of siRNA (Silencer® Negative Control #2 siRNA; Ambion) was incubated with 5 μl labeling buffer A (Mirus) and 10 μl labeling reagent (Mirus) at 37 °C for 1 h, unreacted Label IT siRNA Tracker Reagent was removed from the labeled siRNA with ethanol precipitation, and labeled siRNA was spun, washed with ethanol 70%, and resuspended in PBS. Purified, labeled siRNA was quantified with spectrophotometry. Five μg (in 2 μl PBS) of labeled siRNA was injected in one eye. The contralateral eye was injected with the same volume of unlabeled siRNA. Eyes were enucleated 3 days later, placed in mounting medium, snap frozen in liquid nitrogen, and cryosectioned, and cells were visualized by staining the nuclei with To-Pro Blue (Invitrogen).
Immunohistochemistry
Animals were euthanized, and the eyes were immersed in embedding matrix (ThermoFisher, Waltham, MA) and snap frozen in liquid nitrogen. Then 20 µm sections were obtained. The plane of section was parallel to the optic nerve. Cryosections were fixed in ice-cold acetone (10 min), and post-fixed in Zamboni’s fixative for 2 min. Slides were washed in 0.1 M PBS, and sequentially incubated in PBS containing 0.3% hydrogen peroxide for 20 min, in blocking buffer (PBS containing 3% horse serum and 0.01% saponin) for 30 min, and incubated with goat anti-human CTGF antibody (diluted 1:100 in 0.01 M PBS; R&D) at 4 °C overnight. The specificity of this antibody has been previously described [22-26]. The next day, slides were incubated for 1 h with Alexa Fluor 488 conjugated with antigoat immunoglobulin G (diluted 1:200 in 0.01 M PBS; Invitrogen), washed in PBS, fixed in 4% paraformaldehyde for 5 min, and incubated overnight with mouse anti-vimentin antibody (1:500 in 0.01 M PBS). The following day, slides were incubated for 1 h with Alexa-Fluor 594 (1:200 in 0.01 M PBS; Invitrogen) and coverslipped using Vectashield mounting medium (Vector, Burlingame, CA).
Confocal microscopy
Confocal images were obtained using a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA) connected to a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY). Images of 1240×1240 pixels were processed using Photoshop (Adobe, San Jose, CA). All images were captured using similar settings on the confocal microscope.
Quantitative real-time polymerase chain reaction
Total RNA was extracted using the RNAeasy Kit (Qiagen, Germantown, MD) as described in Winkler et al. [21]. Briefly, concentration and purity of RNA were determined by spectrophotometry. RNA integrity was verified by electrophoresis in 1.2% denaturing formaldehyde gel (1.2 g agarose, 10 ml 10× FA gel buffer [200 mM 3-[N-morpholino] propanesulfonic acid; MOPS], 50 mM sodium acetate, 10 mM EDTA, pH 7.0) in 100 ml RNase-free water). RNA preparations were treated with recombinant DNase-1 (DNA-free kit; Ambion). One microgram of total RNA was transcribed using SuperScript III reverse transcriptase and oligo(dT)18 primer (Invitrogen). Real-time PCR was performed using RT2 SYBR Green Mix (SA Biosciences, Frederick, MD) and performed on a StepOnePlus cycler (Applied Biosystems, Carlsbad, CA). The cycling conditions were 95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. mRNA levels were normalized to an appropriate housekeeping gene, TATA-binding protein (TBP) [27] or acidic ribosomal protein P0 (ARPP0) [28]. Primer sequences for laminin β1, fibronectin 1, and collagen IVα3 were taken from a previous publication [5]. Details of the primers are shown in Table 2. Data was analyzed using the 2-∆∆C(T) method [29]. Each sample was run in triplicate, and each real-time PCR was repeated three times.
Table 2. Primer Sequences.
| Gene name | 5′ Primer | 3′ Primer | Catalog number/reference |
|---|---|---|---|
| Connective tissue growth factor (CTGF) |
Purchased from SA Biosciences |
PPR46426A |
|
| Laminin β1 (Lam) |
GCGTAAAGCTGCCCAGAACTCTG |
TCCTCCTGGCATCTGCTGACTC |
[5] |
| Fibronectin (FN) |
CAGCCTACGGATGACTCATGC |
CAGATAACCGCTCCCATTCCT |
[5] |
| Collagen 4α3 (Col 4a) |
CCCTTGAGCCCTACGTTAGCA |
CCTCAGAGCCTCCACTTGTAAACA |
[5] |
| Acidic ribosomal phosphoprotein P0 (ARPP0) |
GTCCAACTACTTCCTCAAGATCATCCA |
ACATGCGGATCTGCTGCAT |
[28] |
| TATA binding protein (TBP) | Purchased from SA Biosciences | PPR47412A | |
Western blot analysis
Retinas were homogenized for 5 min in ice-cold lysis buffer (50 mM Tris HCl, pH 7.4, 5 mM EDTA, and 0.02% sodium azide) containing a cocktail of protease inhibitors (Sigma). After homogenization, samples were centrifuged at 28,000× g for 10 min at 4 °C. Protein concentration in cleared lysates was measured using bicinchoninic acid colorimetric assay (BCA kit; Pierce, Rockford, IL). Samples were fractionated on a 15% Tris-HCl sodium dodecyl sulfate–PAGE (SDS–PAGE) Ready Gel (Bio-Rad) for CTGF and VEGF and a 5% Tris-HCl SDS–PAGE Ready Gel (Bio-Rad) for laminin; 30 µg of total protein was loaded into each lane. After electrophoresis, protein samples were electroblotted onto nitrocellulose membrane (Amersham, Pittsburgh, PA), washed in Tris-buffered saline, and incubated overnight at 4 °C with goat anti-CTGF antibody (1:1,000; Santa Cruz, Santa Cruz, CA). Membranes were incubated with horseradish peroxidase–conjugated Donkey anti-goat secondary antibody (1:10,000; Santa Cruz), and bands were visualized using SuperSignal West Pico chemiluminescent detection reagents (Thermo Scientific) and Hyblot-CL Autoradiography Film (Denville, Metuchen, NJ). Membranes were subsequently stripped with Restore Western Blot Stripping (Thermo Scientific), incubated with antibodies to VEGF (1:1,000; Santa Cruz) and to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1,000; Santa Cruz) for loading control, and processed for immunodetection as described as above. GAPDH was chosen because changes in protein levels have been reported in vivo only after 12 months of hyperglycemia [30]. Different membranes were incubated with anti-laminin sera, a pan-specific antibody that recognizes multiple laminin isoforms (1:1,000; Novus, Littleton, CO). For loading controls, membranes were stained with Ponceau S. Densitometric analysis of the luminescent signal was performed at non-saturating exposures. Bands were scanned with Epson Perfection 2400 and analyzed using Image J software (NIH, Bethesda, MD). Densitometry was performed using proteins that were run on the same gel.
Statistical analysis
All values are shown as mean±SD. For comparison between two groups, the unpaired Student t test (two tail) was used. p≤0.05 was considered significant.
Results
Connective tissue growth factor messenger ribonucleic acid and protein increased because of hyperglycemia
To ascertain whether CTGF gene expression increased during hyperglycemia, the level of CTGF mRNA and protein in the retina of diabetic and non-diabetic control rats was compared. At 8 and 12 weeks of hyperglycemia, CTGF mRNA was sixfold (p<0.05) and sevenfold (p<0.001) higher than in controls, respectively (Figure 1A and Figure 2A). Similarly, CTGF protein increased 2.5 fold (p<0.05) at 8 weeks and 5.7 fold (p<0.05) at 12 weeks of hyperglycemia (Figure 1B,C and Figure 2B,C). These findings are in agreement with previous reports by others [5,12,13]. To ascertain the identity of the cells expressing CTGF, sections of retina were processed for simultaneous visualization of CTGF and vimentin, a marker of Müller cells [31]. This analysis indicated that in the retina of diabetic rats, CTGF is expressed by vimentin-positive Müller cells (Figure 3).
Figure 1.
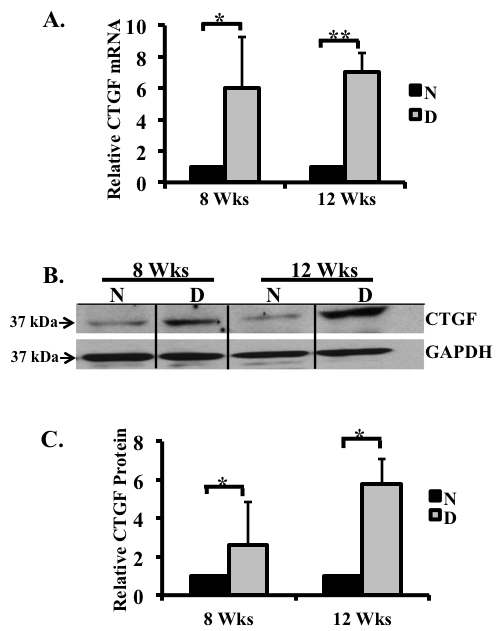
Connective tissue growth factor (CTGF) expression increased in retina of 8 and 12 week diabetic rats. CTGF mRNA expression in the retinas of non-diabetic and diabetic rats after 8 and 12 weeks of hyperglycemia were analyzed using real-time PCR and normalized to the housekeeping gene acidic ribosomal phosphoprotein P0 (ARPP0). A: CTGF mRNA levels increased six- and sevenfold at both time points. B: A representative western blot illustrating CTGF and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein expression in the retinas of diabetic rats after 8 and 12 weeks of hyperglycemia. Note the increased CTGF protein levels compared to the non-diabetic controls. C: Densitometric analysis of three separate immunoblots indicates a 2.5 and 5.7 fold increase in the CTGF protein after 8 and 12 weeks of hyperglycemia, respectively. (*p<0.05, **p<0.001 diabetic versus non-diabetic) n=non-diabetic; D=diabetic. Proteins ran on a 4%–15% gradient gel. n=3/age.
Figure 2.
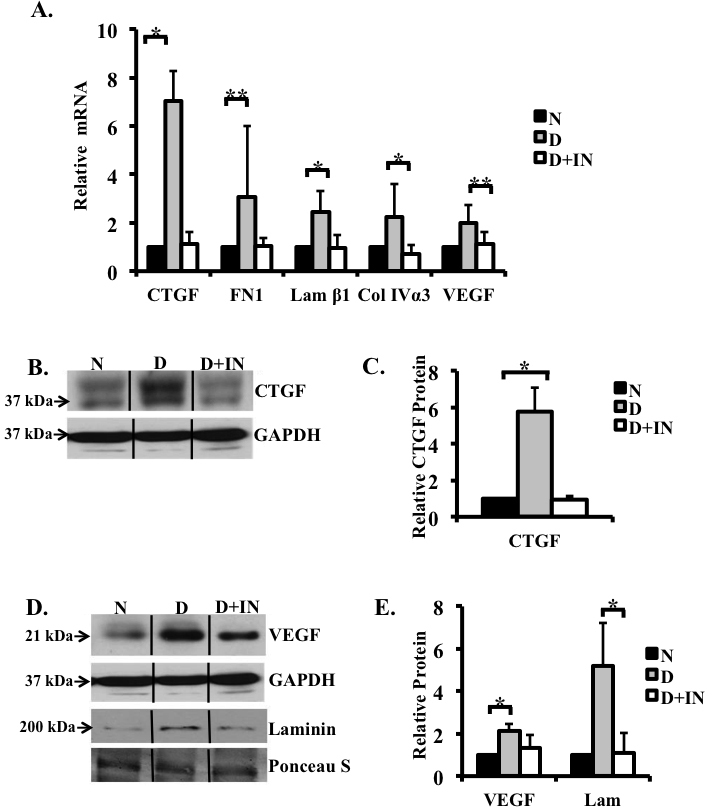
Insulin therapy in diabetic rats abolished hyperglycemia-induced increases in levels of selected genes and proteins. mRNA expression in the retinas of non-diabetic, diabetic, and diabetic rats treated with insulin were analyzed using real-time PCR and normalized to the housekeeping gene acidic ribosomal phosphoprotein P0 (ARPP0). A: Real-time PCR analysis of mRNA extracted from the retina. Note the increase in the levels of connective tissue growth factor (CTGF; sevenfold), fibronectin (threefold), laminin β1 (2.5-fold), collagen IVα3 (2.3 fold), and vascular endothelial growth factor (VEGF; twofold) mRNAs in the rat retinas after 12 weeks of hyperglycemia and that this increase was prevented with insulin therapy. B: A representative immunoblot showing CTGF expression in the retinas of normoglycemic control, diabetic, and insulin-treated rats. Proteins were ran on a 15% SDS–PAGE gel, allowing for better protein separation, hence the double band for CTGF. C: Densitometric analysis of three different western blots indicated that the level of CTGF increased following 12 weeks of hyperglycemia, when compared to the non-diabetic controls. In contrast, CTGF expression in the retinas of diabetic rats treated with insulin for 12 weeks remained near the control levels. D: A representative western blot for VEGF and laminin in retinal extracts shows an increase in retinal VEGF and laminin in the hyperglycemic state, and this increase was inhibited in the insulin-treated hyperglycemic rats. E: Densitometric analysis of three different western blots revealed a 2.1-fold increase in VEGF and a 5.2-fold increase laminin. VEGF and laminin levels were similar in the control and insulin-treated diabetic animals. GAPDH and Ponceau S staining were used as a loading control. n=non-diabetic; D=diabetic; D+IN=insulin treated diabetic (*p<0.05, **p<0.001). n=3/group.
Figure 3.

Connective tissue growth factor (CTGF) was detected in vimentin-positive Müller cells in the retina of diabetic rats. A: Müller cells labeled with filament protein vimentin throughout the diabetic retina. B: CTGF staining in the 12-week diabetic retina is also seen throughout the retina. C: Merge photomicrograph for vimentin and CTGF shows that CTGF colocalizes with vimentin in the diabetic retina. Bar=60 um. D: High magnification of the area indicated with a dotted rectangle in C. Bar=5 um. V=Vitreous, ONL=Outer Nuclear Layer, S=Sclera.
In addition to an increase in CTGF, hyperglycemia affected the level of expression of VEGF and of possible downstream ECM genes in the retina. Comparison of the retina of 12-week diabetic and normoglycemic rats revealed a significant increase in fibronectin (threefold, p<0.001), laminin β1 (2.5 fold, p<0.05), collagen IVα3 (2.3 fold, p<0.05), and VEGF (twofold, p<0.001) mRNAs (Figure 2A). Protein levels of VEGF and laminin also increased significantly (2.1 fold and 5.2 fold, respectively [p<0.05]) in the retina after 12 weeks of hyperglycemia (Figure 2D,E). These results confirm previous observations obtained using a similar model [4,32].
Insulin therapy regulates the levels of connective tissue growth factor, vascular endothelial growth factor, and extracellular matrix molecules
To ascertain whether insulin therapy affects the level of expression of CTGF, VEGF, and possible downstream ECM molecules in the retina, a subset of rats with STZ-induced diabetes received insulin therapy at 7 days post-STZ for 12 weeks. Insulin therapy normalized blood glucose levels and reduced weight loss in diabetic rats (Table 3). The levels of CTGF, fibronectin, laminin β1, collagen IVα3, and VEGF mRNAs in the retinas of diabetic rats receiving insulin therapy were similar to that of controls (Figure 2A). Similarly, CTGF, VEGF, and laminin proteins were similar in insulin-treated STZ and non-diabetic control (Figure 2B-E). To confirm the effect of insulin on CTGF, the levels of CTGF in the retinas of the control, diabetic, and insulin-treated diabetic rats were compared with immunohistochemistry. The level of CTGF immunostaining was undetectable in control retinas (Figure 4A), increased in diabetic retinas (Figure 4B), and significantly reduced in the retinas of diabetic rats treated with insulin (Figure 4C). In addition, we confirmed previous reports indicating that insulin treatment normalized fibronectin levels in the retinas of diabetic rats (Figure 2A) [33]. These findings indicate that insulin prevented the glucose-dependent increase in the expression of these selected cytokines and ECM genes.
Table 3. Average blood glucose and weight of control, diabetic and insulin treated animals.
| Parameter | Control (non-diabetic) n=6 | Diabetic (untreated) n=12 | Insulin treated diabetic n=8 |
|---|---|---|---|
| Blood glucose (mg/dl) |
117±13.8 |
405±68 |
135±89 |
| Weight (g) | 729±52 | 335±71 | 524±60 |
Figure 4.
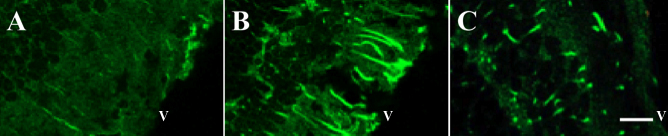
Hyperglycemia induces connective tissue growth factor (CTGF) expression in Müller cells. A representative photomicrograph illustrates retinas processed for visualization of CTGF. The CTGF level is low in the retinas of the control rats (A) and increased in the retinas of the diabetic rats after 12 weeks of hyperglycemia (B). Insulin therapy for 12 weeks decreased the staining intensity of CTGF (C). All images were captured at the same magnification. V=vitreous side of the retina. Bar=30 um. n=3/group.
Effect of connective tissue growth factor small interfering ribonucleic acid on the expression of connective tissue growth factor and possible downstream targets
To further analyze the contribution of CTGF in regulating ECM molecules, we developed a siRNA-approach to inhibit CTGF. We tested if inhibition of CTGF by siRNA affected the expression of laminin β1, fibronectin 1, collagen IVα3, and VEGF. In addition, we also tested the effect of the treatment on glial fibrillary acidic protein (GFAP), an intermediate filament protein that increases in the retina during DR [34,35],
We first used an in vitro system to determine the specificity of three siRNAs in downregulating CTGF levels in a rat cell line. Since we previously showed that TGF-β induces a twofold increase in CTGF mRNA and a significant increase in the CTGF protein in Rat-2 fibroblasts [21], we tested whether the induction of CTGF was inhibited by any of the three CTGF siRNAs (siRNA I–III) alone or in combination. Previous studies showed that CTGF protein levels in Rat-2 cells were undetectable in the absence of TGF-β [21]. Real-time PCR showed that siRNA I and siRNA III decreased TGF-β induced CTGF mRNA by 75% and 86% (p<0.05), respectively (Figure 5A). In contrast, siRNA II had no effect on reducing CTGF mRNA (data not shown). Western blot analysis using siRNA I and siRNA III revealed that CTGF protein decreased by 49% and 46%, respectively (Figure 5B,C). The combination of siRNA I and III did not result in further inhibition of CTGF (data not shown). siRNA I was chosen for the in vivo experiments because it gave the greatest decrease in CTGF protein.
Figure 5.
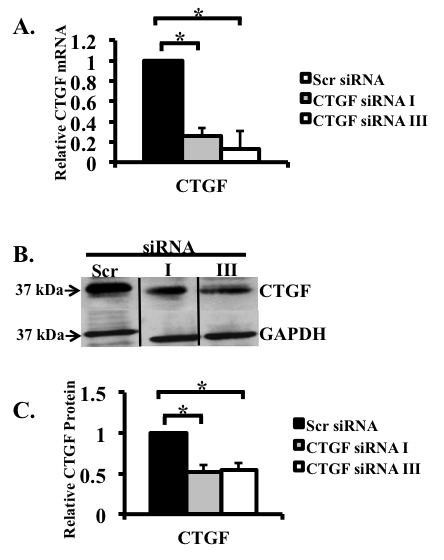
siRNA decreased transforming growth factor-β1 (TGF-β)-induced connective tissue growth factor (CTGF) in Rat-2 fibroblasts. CTGF mRNA expression in Rat-2 cells treated with TGF-β and siRNA were analyzed using real-time PCR and normalized to the housekeeping gene acidic ribosomal phosphoprotein P0 (ARPP0). A: The effect of two separate CTGF siRNAs (I and III) and a control scrambled siRNA on TGF β-induced CTGF expression in Rat-2 fibroblasts was tested. Real-time PCR showed that siRNA I and siRNA III decreased CTGF mRNA by 75% and 86%, respectively. B: Arepresentative western blot documenting that TGF-β induced CTGF protein decreased by siRNA I and III. Proteins were run on the same gel, but not in adjacent lanes. C: Densitometric analysis summarizing the results of three separate western blots shows that siRNA I and siRNA III decreased CTGF protein by 49% and 46%, respectively. (*p<0.05). n=3/group.
Then we sought to determine whether the siRNA reached all layers of the retina. Animals were given a single intravitreal injection of Cy3-labeled scrambled siRNA, and the eyes were harvested 3 days after the injection, sectioned, and viewed using confocal microscopy. Labeled siRNA was distributed throughout the retina from the ganglion cell to the photoreceptor layers (Figure 6A,B,D). The contralateral eyes were injected with unlabeled siRNA to test whether the light emissions observed in the eyes injected with labeled siRNA was due to autofluorescence produced by the siRNA. Retinas of rats that received unlabeled siRNA did not show any detectable fluorescence (Figure 6C). The distribution of labeled siRNA throughout the retina confirms previous reports by Shen et al. [36].
Figure 6.
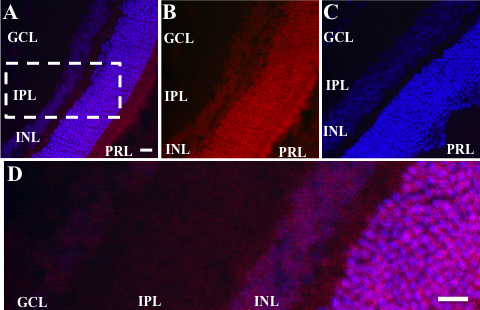
Intravitreal injection of labeled siRNA penetrates the retina. A: Confocal microscopy revealed that the scrambled siRNA covalently bound to Cy3 (Red) was distributed throughout the layers of the retina when injected into the eyes of control rats. The label was distributed throughout the retina from the ganglion cell layer (GCL) to the photoreceptor layer (PRL). Nuclei were stained with To-Pro Blue. B: A photomicrograph illustrates panel A with To-Pro Blue removed. Note the distribution of Cy3 labeled siRNA throughout the layers of the retina. C: In contrast, the retinas of rats injected with unlabeled scrambled siRNA did not contain labeled particles, indicating that the label was not due to autofluorescence or artifacts from the injection. Bar=30 um. D: Illustrates higher magnification of inset depicted with dotted rectangle in A. Bar=15 um. IPL=inner plexiform layer, INL=inner nuclear layer. n=3/group.
To determine whether the siRNA treatment had a beneficial effect on the retinas of diabetic rats, 12-week hyperglycemic rats were given a single intravitreal injection of CTGF siRNA in one eye (the experimental retina), while the contralateral eye was injected with scrambled siRNA (the control retina). Three days later, the retinas were dissected and processed for mRNA and protein analysis. Administering CTGF siRNA induced a decrease in CTGF (33%, p<0.05), collagen IVα3 (71%, p<0.05), and laminin β1 (63%, p<0.05) mRNA levels (Figure 7A). Levels of GFAP mRNA decreased by 44% (p<0.05) in the CTGF siRNA–treated retina (Figure 7A). Importantly, the level of CTGF protein was 54% lower (p<0.05) in the experimental retinas compared to the control retinas (Figure 7B-C). In contrast, administering CTGF siRNA did not affect VEGF or fibronectin levels, which remained similar in the experimental and control retinas (Figure 7A). The effect of the siRNA treatment decreased with time since the CTGF mRNA levels were similar in the experimental and control retinas 10 days after CTGF siRNA was injected (Figure 7D).
Figure 7.
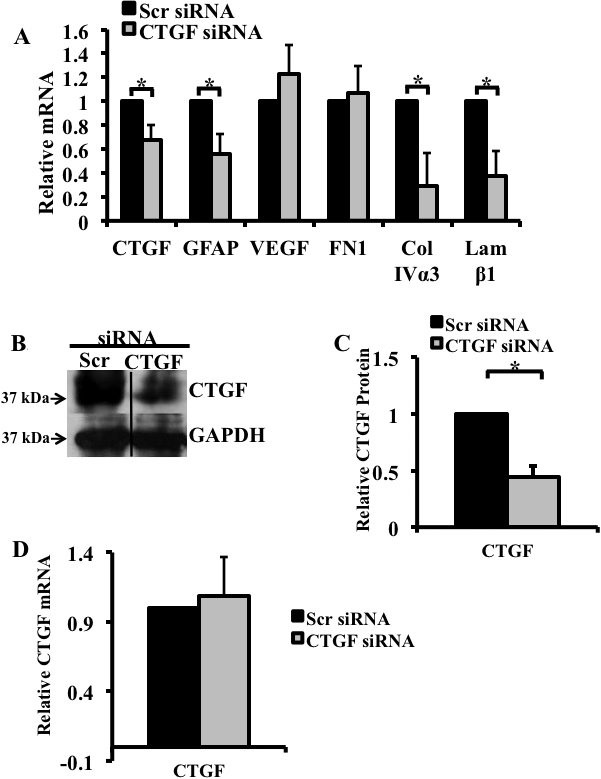
siRNA treatment significantly decreased hyperglycemia-induced increase in connective tissue growth factor (CTGF) mRNA and protein, glial fibrillary acidic protein (GFAP), collagen IVα3 and laminin-β1 gene expression. mRNA expression for CTGF and selected genes in the retinas of the diabetic rats after 12 weeks of hyperglycemia were analyzed using real-time PCR and normalized to the TATA-binding protein (TBP). A: Real-time PCR revealed that 3 days post intravitreal injection, CTGF siRNA induced a decrease in CTGF (33%), GFAP (44%), collagen IVα3 (71%), and laminin β1 (63%) mRNAs. In contrast, CTGF siRNA did not affect the level of fibronectin or vascular endothelial growth factor (VEGF). B: Immunoblot analysis of CTGF levels in the retinas following a single intravitreal injection of CTGF siRNA or scrambled siRNA into left and right eye, respectively. The concentration of the CTGF protein is lower in eyes injected with CTGF siRNA. C: Densitometric analysis of three independent experiments revealed a 54% decrease in CTGF protein in retinas injected with CTGF siRNA compared to retinas injected with a scrambled (non-specific) siRNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. D: Real-time PCR showed that CTGF siRNA had no effect on CTGF expression 10 days after injection. (*p<0.05). n=5/group.
Discussion
Although insulin therapy aids in preventing DR, since insulin stabilizes endothelial barrier function [37] and decreases microvascular leakage [38], the role of insulin in regulating ECM molecules is not well defined. In this study, our first goal was to determine whether CTGF, a profibrotic molecule induced by high glucose [5,13], was downregulated with insulin therapy and whether this decrease also affected extracellular matrix components proposed to be controlled by CTGF [19]. We found that the elevated levels of CTGF mRNA and protein induced by hyperglycemia were prevented by exogenous insulin treatment. Moreover, insulin therapy also inhibited the upregulation of several components of the extracellular matrix such as laminin β1, collagen IVα3, and fibronectin, and of GFAP and VEGF.
Importantly, we determined that Müller cells in the diabetic retina immunostained with a specific antibody to CTGF. Although CTGF is a secreted protein, it is not surprising that it is localized within the cytoplasm of Müller cells. Other secreted proteins, such as hormones, are also localized to the cytoplasm of the cells that synthesize them. In contrast to our findings, previous immunohistochemical studies reported the expression of CTGF by cells of the ganglion cell and inner plexiform layers in rodents [5,15] and in microglial cells and pericytes in humans [12]. The discrepancy between our results and that of others could be due to the different histological techniques employed in the analysis and/or to the specificity of the antibodies used. In situ hybridization studies reported the expression of CTGF mRNA by structures located in the ganglion cell layer [13] and proposed that it was expressed by the end feet of Müller cells [13]. Although in situ hybridization identifies the site of mRNA expression, identifying the cell type that is labeled is often difficult. For that reason, we used immunohistochemistry to visualize CTGF. Our results are in agreement with the cell type proposed by that group to synthesize CTGF. The antibody did not stain CTGF bound to the cell membrane of cells that respond to the action of this cytokine. Although a specific CTGF receptor has not yet been identified, CTGF appears to perform many of its functions through integrins, heparin sulfate–containing proteoglycans, and the low-density lipoprotein receptor–related protein [39]. Although CTGF probably binds to any or all of these receptors, its levels at the cell membrane are probably below the sensitivity of the techniques used.
Müller cells become activated by high glucose levels and upregulate the expression of GFAP, and of VEGF and other angiogenic cytokines [35,40]. The localization of CTGF to Müller cells has important clinical applications since these cells participate in maintaining the homeostasis of the retinal extracellular milieu and protect neurons via a release of neurotrophic factors; disturbance of these cells could lead to retinal dysfunction [40].
Although insulin therapy downregulated the level of expression of CTGF and ECM molecules, oscillations in blood glucose occur even in the presence of tight glycemic control [41], and these changes could affect retinal function even in well controlled patients [42]. To circumvent possible harmful effects of blood glucose oscillations, we sought to develop a siRNA approach for short-term inhibition of CTGF. Our goal was to determine whether a reduction in CTGF expression in diabetic rats affected the activity of genes regulating the synthesis of selected ECM molecules. We found that the siRNA treatment specifically decreased CTGF expression in vitro and in vivo. In vivo, siRNA molecules labeled with Cy3 were found in all layers of the retina. This observation suggests that the injected molecule reaches all retinal cells but affects only the level of CTGF expression by Müller cells. Since other retinal cell types do not express CTGF, their gene expression is not likely to be affected due to the exquisite specificity of the siRNA approach.
We demonstrated that siRNA treatment decreased CTGF mRNA and protein. This effect is specific, because the treatment downregulated the 36–38 kDa form of CTGF, the primary form of the cytokine [43]. In contrast to our finding, a recent study using a similar approach reported a decrease in a 55 kDa protein probed with a CTGF antibody and no change in the 36–38 kDa forms [44]. The identity of the 55 kDa protein is unknown. Moreover, the specificity of the siRNA treatment in Yang et al.’s report is unclear since the synthetic oligonucleotides used in that report also decreased VEGF [44]. In contrast, in our study the CTGF siRNA did not affect VEGF mRNA levels, supporting multiple reports indicating that VEGF is upstream of CTGF [14,15,45].
Our results reveal that the CTGF-specific siRNA resulted in a decrease in laminin β1 and collagen IVα3 mRNA levels, indicating that these two genes are CTGF targets. In contrast, inhibition of CTGF did not change fibronectin mRNA levels. This result was unexpected because studies in other tissues reported that CTGF regulates fibronectin expression [19,46]. This discrepancy could be due to differences in the half-life of fibronectin mRNA in different cell types. We also found that CTGF siRNA reduced GFAP expression, indicating that CTGF regulates the level of expression not only of extracellular molecules but also genes coding for intracellular proteins. We did not examine the protein levels of ECM molecules following the siRNA treatment. The short duration of action of the siRNA and the relatively long half-life of extracellular matrix proteins [47] suggest that, in our studies, the siRNA treatment did not affect protein levels.
Although the siRNA experiments support a role for CTGF in regulating ECM molecules, the effect of the injected siRNA in diabetic rats lasted for only 3 days since the experimental and contralateral control retinas expressed similar levels of CTGF mRNA 10 days after the injection. Other studies also reported a short duration of siRNA treatments [36,48]. Therefore, although our findings support the use of an siRNA approach to complement insulin therapy, future studies should seek to circumvent its limitations. One limitation of siRNA treatment is the ability of siRNA to nonspecifically bind to toll-like receptor 3, resulting in an off-target antiangiogenic response [49]. In addition, the approach requires the development of chemically modified siRNA molecules that will stabilize and extend the duration of the treatment when combined with appropriate transfection reagents. In conclusion, our results clearly demonstrate that CTGF regulates the level of expression of ECM molecules as well as GFAP, a specific glial cell filament.
Acknowledgments
There are no potential conflicts of interest relevant to this article. Parts of this study were presented in abstract form at the American Diabetes Association scientific sessions 2010, Orlando, Florida, June 25–29. The authors wish to thank Dr. John Danias (SUNY Downstate Medical Center, Brooklyn, NY) for critically reading the manuscript. This work was supported by a pilot project grant from the Juvenile Diabetes Research Foundation International.
References
- 1.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA, JDRF Diabetic Retinopathy Center Group Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 2.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA. 1990;87:404–8. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spirin KS, Saghizadeh M, Lewin SL, Zardi L, Kenney MC, Ljubimov AV. Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr Eye Res. 1999;18:490–9. doi: 10.1076/ceyr.18.6.490.5267. [DOI] [PubMed] [Google Scholar]
- 4.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem. 1996;44:1469–79. doi: 10.1177/44.12.8985139. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JM, Kuiper EJ, Klaassen I, Canning P, Stitt AW, Van Bezu J, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia. 2007;50:1089–98. doi: 10.1007/s00125-007-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper EJ, van Zijderveld R, Roestenberg P, Lyons KM, Goldschmeding R, Klaassen I, Van Noorden CJ, Schlingemann RO. Connective tissue growth factor is necessary for retinal capillary basal lamina thickening in diabetic mice. J Histochem Cytochem. 2008;56:785–92. doi: 10.1369/jhc.2008.950980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575–96. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazionis L, Yau J, Rowley K, Itsiopoulos C, O'Dea K, Wong TY, Jenkins A. Plasminogen activator inhibitor-1 (PAI-1) activity and retinal vascular calibre in type 2 diabetes. Diabetes Res Clin Pract. 2010;87:192–9. doi: 10.1016/j.diabres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Kowluru RA. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Invest Ophthalmol Vis Sci. 2010;51:4320–6. doi: 10.1167/iovs.09-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–75. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 11.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–73. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol. 2004;88:1082–7. doi: 10.1136/bjo.2003.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tikellis C, Cooper ME, Twigg SM, Burns WC, Tolcos M. Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology. 2004;145:860–6. doi: 10.1210/en.2003-0967. [DOI] [PubMed] [Google Scholar]
- 14.Suzuma K, Naruse K, Suzuma I, Takahara N, Ueki K, Aiello LP, King GL. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem. 2000;275:40725–31. doi: 10.1074/jbc.M006509200. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper EJ, Hughes JM, Van Geest RJ, Vogels IM, Goldschmeding R, Van Noorden CJ, Schlingemann RO, Klaassen I. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest Ophthalmol Vis Sci. 2007;48:4267–76. doi: 10.1167/iovs.06-0804. [DOI] [PubMed] [Google Scholar]
- 16.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS ONE. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–68. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 20.Blades RA, Bryant KR, Whitehead SA. Feedback effects of steroids and gonadotrophin control in adult rats with streptozotocin-induced diabetes mellitus. Diabetologia. 1985;28:348–54. doi: 10.1007/BF00283142. [DOI] [PubMed] [Google Scholar]
- 21.Winkler JL, Kedees MH, Teitelman G. Adenoviral-mediated inhibition of connective tissue growth factor in Rat-2 cells. Biochem Biophys Res Commun. 2007;358:209–14. doi: 10.1016/j.bbrc.2007.04.109. [DOI] [PubMed] [Google Scholar]
- 22.Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol. 2006;146:362–70. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, Maga TK, Arnaud-Dabernat S, Palmieri D, Stetler-Stevenson WG, Lacombe ML, Meltzer PS, Steeg PS. Nm23–H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res. 2007;67:7238–46. doi: 10.1158/0008-5472.CAN-07-0962. [DOI] [PubMed] [Google Scholar]
- 24.Inkinen K, Wolff H, Lindroos P, Ahonen J. Connective tissue growth factor and its correlation to other growth factors in experimental granulation tissue. Connect Tissue Res. 2003;44:19–29. [PubMed] [Google Scholar]
- 25.Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ, Chau YP, Kuo ML. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Milliat F, François A, Isoir M, Deutsch E, Tamarat R, Tarlet G, Atfi A, Validire P, Bourhis J, Sabourin JC, Benderitter M. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol. 2006;169:1484–95. doi: 10.2353/ajpath.2006.060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasir I, Kedees MH, Ehrlich ME, Teitelman G. The role of pregnancy hormones in the regulation of Pdx-1 expression. Mol Cell Endocrinol. 2005;233:1–13. doi: 10.1016/j.mce.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–55. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Kanwar M, Kowluru RA. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–34. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna G, Lewis GP, Banna CD, Skalli O, Fisher SK. Expression profiles of nestin and synemin in reactive astrocytes and Muller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Mol Vis. 2010;16:2511–23. [PMC free article] [PubMed] [Google Scholar]
- 32.Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- 33.Cherian S, Roy S, Pinheiro A. Tight glycemic control regulates fibronectin expression and basement membrane thickening in retinal and glomerular capillaries of diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:943–9. doi: 10.1167/iovs.08-2377. [DOI] [PubMed] [Google Scholar]
- 34.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–20. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 35.Rungger-Brändle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–80. [PubMed] [Google Scholar]
- 36.Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, Hackett SF, Zinnen S, Kossen K, Fosnaugh K, Vargeese C, Gomez A, Bouhana K, Aitchison R, Pavco P, Campochiaro PA. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–34. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 37.Gündüz D, Thom J, Hussain I, Lopez D, Härtel FV, Erdogan A, Grebe M, Sedding D, Piper HM, Tillmanns H, Noll T, Aslam M. Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3-kinase/Akt-mediated Rac1 activation. Arterioscler Thromb Vasc Biol. 2010;30:1237–45. doi: 10.1161/ATVBAHA.110.203901. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki R, Whitt SP, Huxley VH. Permeability response of the rat mesenteric microvasculature to insulin. Clin Hemorheol Microcirc. 2006;34:259–63. [PMC free article] [PubMed] [Google Scholar]
- 39.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–44. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:2198–202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henricsson M, Nilsson A, Janzon L, Groop L. The effect of glycaemic control and the introduction of insulin therapy on retinopathy in non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:123–31. doi: 10.1002/(SICI)1096-9136(199702)14:2<123::AID-DIA306>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 43.Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor-(CTGF, CCN2)–a marker, mediator and therapeutic target for renal fibrosis. Nephron, Exp Nephrol. 2010;114:e83–92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Z. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol (Copenh) 2010;88:652–9. doi: 10.1111/j.1755-3768.2009.01641.x. [DOI] [PubMed] [Google Scholar]
- 45.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 46.Xiao L, Sun L, Liu FY, Peng YM, Duan SB. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-beta1 in cultured human peritoneal mesothelial cells. Ther Apher Dial. 2010;14:27–34. doi: 10.1111/j.1744-9987.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 47.Halfter W, Dong S, Dong A, Eller AW, Nischt R. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye (Lond) 2008;22:1207–13. doi: 10.1038/eye.2008.19. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–7. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]


