Abstract
Foxp3+ regulatory T cells (Tregs) maintain self-tolerance and adoptive therapy, and using Foxp3+ Tregs has been proposed as treatment for autoimmune diseases. The clinical use of Tregs will require large numbers of cells and methods for in vitro expansion of Tregs are being developed. Foxp3+ Tregs can be divided into 2 subpopulations based on expression of the transcription factor, Helios. Foxp3+Helios+ Tregs (70%) are thymic-derived, whereas Foxp3+Helios− Tregs (30%) are induced in the periphery. Foxp3+Helios+ Tregs differ from Foxp3+Helios− Tregs in terms of epigenetic changes at the Foxp3 locus, their capacity to produce effector cytokines, and their stability of Foxp3 expression on days to weeks of expansion in vitro. Addition of a 25 mer DNA oligonucleotide of random composition for a short period during the expansion of Foxp3+ Tregs in vitro results in prolonged stabilization of the Foxp3+Helios+ subpopulation and yields an optimal population for use in cellular biotherapy.
Introduction
Foxp3+ regulatory T cells (Tregs) are a unique subset of CD4+ T cells, which are responsible for self-tolerance and the prevention of autoimmune disease.1 Adoptive Treg infusion has been suggested as a potential therapy for the prevention of graft-versus-host disease (GVHD) after stem cell transplantation, organ allograft rejection, and for the treatment of autoimmune diseases such as type I diabetes and multiple sclerosis.2,3 Adoptive transfer of Foxp3+ Tregs in mouse models has been shown to prevent acute and chronic GVHD without negative effects on the graft versus leukemia response.4 More recently, several groups have reported that cotransfer of expanded Tregs from umbilical cord samples5 or from peripheral blood appears to be both safe,6 and in one study, remarkably effective in preventing acute GVHD after stem-cell transplantation.7
Although considerable enthusiasm has been generated for adoptive Treg therapy, several major issues remain to be resolved. First, most clinical applications of Treg therapy will require large numbers of cells and optimal methods for Treg expansion are now being explored. Expansion of highly purified populations of human Tregs also frequently results in loss of Foxp3 expression during the expansion process. Secondly, in contrast to studies in the mouse, Foxp3 expression can be readily induced during in vitro stimulation of conventional human T cells.8 T cells induced in vitro to express Foxp3 frequently lack a Treg phenotype, continue to make effector cytokines, and lack in vitro suppressive function.8 Thus, expression of Foxp3 cannot be considered a completely reliable marker for functional human Tregs.
Several approaches have been used to address these problems. Combined use of several surface markers (CD127lo and CD25hi) has facilitated isolation of more highly enriched populations of Foxp3+ T cells with less contamination by CD25int activated T cells.9 Addition of inhibitors of the mammalian target of rapamycin (mTOR) pathway, such as rapamycin, block the expansion of contaminating conventional T cells and favor the expansion of Tregs, but purity greater than 60% is rarely achieved after several rounds of expansion depending on the starting population.10 CD4+CD25+CD45RA+Foxp3+ T cells, although a minor subpopulation (5%-30%) of the Foxp3+ pool in adults, appear to have a greater propensity to expand in culture and have enhanced stability of Foxp3 expression compared with CD4+CD25+CD45RO+Foxp3+ T cells.11
Foxp3+ Tregs can be divided into 2 potentially distinct subpopulations. One population is generated in the thymus and has been termed natural (n) Tregs. A second population is generated extrathymically in peripheral sites and has been termed induced (i) Tregs or adaptive Treg. We have recently demonstrated12 that the transcription factor, Helios, a member of the Ikaros gene superfamily, is expressed in 70% of both mouse and human Foxp3+ T cells. Foxp3+Helios− T cells are primarily iTregs as Foxp3+ T cells induced in vitro are Helios−, and Foxp3+ T cells induced in vivo in response to oral antigen administration, antigen administered intravenously, or T cells activated in response to lymphopenia are almost exclusively Helios−.
Here, we further characterize the Foxp3+Helios+ and Foxp3+Helios− Treg subpopulations in terms of epigenetic changes at the Foxp3 locus, their capacity to produce effector cytokines, and stability of Foxp3 expression during days to weeks of expansion in vitro. We demonstrate that addition of a 25 mer DNA oligonucleotide of random composition for a short period during the initial expansion of Foxp3+ Tregs results in prolonged stabilization of the Foxp3+Helios+ subpopulation. Taken together, our results suggest that Foxp3+Helios+ Tregs represent the optimal subpopulation for cellular biotherapy in humans and that addition of DNA oligonucleotides during expansion cultures will facilitate expansion of this potent and more stable subpopulation.
Methods
Cytokines and antibodies
Recombinant human IL-2 and recombinant mouse transforming growth factor (TGF) β were purchased from Peprotech. Viability fluorescence dye was purchased from eBioscience. Anti–human CD28 antibody (clone 28.2) was also purchased from eBioscience, and anti–human CD3ϵ antibody (clone 64.1) was grown and purified in house. Anti-CD3/anti-CD28 coated stimulating and expander beads were purchased from Invitrogen.
Isolation, stimulation, and expansion of human regulatory T cells
Human peripheral blood mononuclear cells (PBMC)s were prepared from 20- to 80-year-old healthy male donors by the Department of Transfusion Medicine at the National Institutes of Health. The acquisition of blood products was approved according to the International Review Board, and in accordance with the Declaration of Helsinki. CD4+CD25hiCD127− Tregs were isolated by cell sorting on a FACS Aria. Sorted Tregs (0.2 × 106) were resuspended in complete RPMI-1640 supplemented with 10% heat-inactivated FCS, and stimulated in plates coated with anti-CD3ϵ (10 μg/mL) and anti-CD28 (2 μg/mL) in the presence of IL-2 (150 U/mL) for 5 to 6 days. Stimulated cells were washed in RPMI 1640-10 media twice and expanded in complete RPMI 1640-10 with recombinant IL-2 (150 U/mL) for 5 to 10 days in the presence of Treg-expander magnetic beads (Cell:Bead = 4:1). To evaluate the purity of sorted Tregs, cells were stained with anti-Foxp3 and anti-Helios (Biolegend) in Foxp3-staining buffer (eBioscience). All flow cytometry was performed on a FACS LSRII (Becton Dickinson) and analyzed with FlowJo Version 9.4.10 software (TreeStar).
Measurement of cytokine production
Stimulated or rested cells were restimulated with PMA (12-O-Tetradecanoylphorbol-13-acetate, 50 ng/mL) and Ionomycin (1 μg/mL) for 4 hours in the presence of Golgistop (0.75 μL/mL) at 37°C. The cells were fixed, permeabilized, and stained for Foxp3 and Helios expression, as well as IL-2-FITC, IFNγ-PECy7, and IL17A-APC. All the antibodies were purchased from eBioscience.
DNA methylation analysis of the TSDR in freshly isolated and fixed Tregs
Freshly isolated or in vitro expanded Tregs were harvested, and washed twice in PBS. Extraction of genomic DNA was performed with DNeasy Blood&Tissue Kit (QIAGEN). Fixed, stained cells were FACS sorted, washed in PBS twice, and genomic DNA was extracted with QIAamp DNA FFPE tissue Kit (QIAGEN). Bisulfite conversion, pyrosequencing, and data analysis were done by EpigenDx. Eleven cytosine guanine dinucleotides (CpGs) of the human Treg specific demethylation region (TSDR) were analyzed (−2376 to −2263 from transcriptional start site (TSS) ENST00000376207). The percentage methylation of each sample indicates the mean value of all 11 CpGs.
Cell labeling with CFSE
Freshly isolated Tregs or 5 day-stimulated Tregs and effector T cells (Teffs) were washed 3 times in PBS, and labeled with 1μM of carboxyfluorescein diacetate succinimidyl ester (CFSE) or 5μM of cell proliferation dye at 37°C for 10 minutes. The cells were then washed in 10% serum containing media 4 times to remove unlabeled excess CFSE and cell proliferation dye.
In vitro suppression assay
The protocol was previously described.12 Briefly, responder CD4+ T cells and γ-irradiated PBMCs were prepared from the same donor of Tregs and were frozen at −80°C. Expanded Tregs (5 × 104) were cocultured with thawed responder CD4+ T cells at different ratios indicated in the presence of thawed γ-irradiated PBMCs (5 × 104) and soluble anti-CD3 (OKT3, 2 μg/mL). Cell proliferation was assayed by 3H-TdR incorporation during the last 8 hours.
Oligonucleotides
ssRNA40 (TLR8 agonist), ssRNA41 (control oligoribonucleotide), CL264 (TLR7 agonist), CpG oligodeoxynucleotide (ODN; ODN2395), and Toll-like receptor(TLR) 9 antagoinst (ODN TTAGGG) were purchased from Invivogen. The other ODNs were custom synthesized by IDT.
Quantitative PCR of endosomal TLRs
Conventional and Tregs were prepared by FACS-sorting. TaqMan primers sets of endosomal TLRs were purchased from Applied Biosystems.
Statistical snalysis
The paired Student t test was used for comparison of group values.
Results
CD4+Foxp3+ Helios+ Tregs are stable, do not produce inflammatory cytokines, and possess a demethylated TSDR
We initially evaluated the possibility that the instability might be related to the different properties of the Helios+ and Helios− subpopulations. One epigenetic marker that has been used to distinguish nTregs from iTregs is the methylation status of the Treg specific demethylation region (TSDR) of the Foxp3 gene.13 Demethylation of the TSDR region correlates with the thymic origin of Foxp3+ T cells and stable expression of Foxp3, whereas T cells induced to express Foxp3 in vitro have a fully methylated TSDR region and lose expression of Foxp3 in vivo or in vitro. To determine whether a correlation exists between Helios expression and TSDR methylation status, we isolated 4 different CD4+ subsets (Foxp3+Helios+, Foxp3+Helios−, Foxp3−Helios+, and Foxp3−Helios−) by FACS sorting fixed, freshly isolated human CD4+ T cells (Figure 1A), and analyzed the methylation of the TSDR in each subpopulation. As expected, Foxp3− T cells expressed a fully methylated TSDR regardless of Helios expression (Figure 1A). In contrast, Foxp3+Helios+ cells were fully demethylated in all donors, whereas the TSDR region of the Fox3+Helios− subset was ∼ 45% methylated. One interpretation of these results is that the Foxp3+Helios− subpopulation is composed of 2 subpopulations, one of which expresses a fully methylated TSDR that can potentially lose Foxp3 expression on in vitro expansion, whereas the second probably represents a population with greater stability of Foxp3 expression.
Figure 1.
Foxp3+Helios+ Tregs have uniformly demethylated TSDR and do not produce inflammatory cytokines. (A) CD4+CD25hi cells were isolated from the buffy coat of a male donors (n = 7), fixed-permeabilized, and stained for intracellular expression of Foxp3 and Helios. Stained cells were sorted into the 4 different fractions. Upper plot shows the staining and gating condition before sorting. For DNA methylation analysis of the TSDR, bisulfite modification of genomic DNA was performed after extraction from the sorted fractions. Methylation of CpG was read and analyzed by the pyrosequencing method. Results from 7 donors are shown in the lower plot. (B) CD4+CD25+ cells were sorted from the buffy coat and were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies for 6 days. The cells were then expanded for an additional 5 days with anti-CD3/anti-CD28 antibody-coated magnetic beads (CD3/CD28-beads). Intracellular staining for IFNγ (n = 4), IL17A (n = 5), and IL2 (n = 4) was performed after restimulation with PMA and ionomycin. Top dot plot is an example to show the gating of each subset separated by Foxp3 and Helios expression in the expanded population.
As another approach, we measured effector cytokine production by the expanded cells. We previously demonstrated that a low percentage (1%-5%) of freshly explanted Foxp3+ T cells produced effector cytokines (IL-2, IFNγ) and that most cytokine-producing cells were in the Foxp3+Helios− subpopulation.14 We sorted CD4+CD25+ T cells from normal donors and stimulated the cells for a total of 11 days with plate-bound anti-CD3 and anti-CD28 in the presence of IL-2. Although ∼ 90% (data not shown) of the starting population was Foxp3+, after 12 days of expansion only ∼ 50% of the cells remained Foxp3+ (Figure 1B). On restimulation in vitro with PMA/ionomyin, 5%-20% of the expanded Foxp3+Helios− subpopulation produced IL-2, IL-17A, or IFNγ, whereas the frequency of cytokine producers remained low in the Foxp3+Helios+ subpopulation (Figure 1B). These experiments suggest that one of the major factors that control the maintenance of Foxp3 expressing Tregs on expansion in vitro is the heterogeneity of the starting population. The Foxp3+Helios− subpopulation appears to contain a high percentage of Tregs with methylated TSDRs that have the potential for loss of expression of Foxp3. This same subpopulation also contains a high percentage of effector cytokine producing cells that would not be ideal for cellular immunotherapy.
The addition of CpG ODN results in enhanced maintenance of Foxp3+Helios+ cells during in vitro expansion
One goal of this study was to identify a cytokine or small molecule that would facilitate the preferential expansion of the Foxp3+Helios+ subpopulation of Tregs. We initially selected 2 potential candidates, TGFβ and the TLR9 agonist CpG ODN. TGFβ can induce Foxp3 expression in human T cells and some studies have suggested that it can potentiate maintenance of Foxp3 expression. Some studies have claimed that engagement of certain TLRs reverses Treg-mediated suppression by reducing Foxp3 expression,14 but others have claimed that it leads to greater viability of Foxp3+ Tregs without loss of Foxp3 expression.15 We could readily demonstrate that human Tregs expressed TLR9, but in contrast to other studies,16 we could not detect TLR8 or TLR7 expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). When sorted CD4+CD25hi CD4+ T cells were expanded in the presence of TGFβ and the TLR9 agonist for 5 days, the frequency of the Foxp3+Helios+ positive cells was higher than cells expanded in the absence of TGFβ and the ODN (data not shown). Interestingly, when the 5-day expanded cells were washed, and restimulated for an additional 7 days in the absence of TGFβ and CpG ODN, the cells initially exposed to TGFβ/ODN maintained a high frequency of Foxp3+Helios+ cells, whereas the cells initially expanded in the absence of TGFβ/ODN had a markedly decreased percentage of the Foxp3+Helios+ cells. The CpG ODN was the major factor involved in stabilization of the Foxp3+Helios+ population, as cells expanded in the presence of TGFβ alone had only a modest increase in Foxp3+Helios+ cells, whereas cells expanded with the ODN alone resembled the population treated with the combination of TGFβ/ODN (Figures 2A-B).
Figure 2.
Addition of CpG ODN increases the frequency of Foxp3+Helios+ cells during in vitro expansion. (A) FACS-sorted CD4+CD25+ cells (left plot, square gate) were stimulated with plate-bound anti-CD3 and anti-CD28 in the presence of TGFβ (5 ng/mL) and CpG ODN (ODN2395, 2.5μM) for 5 days. The cells were then washed and incubated for 2 additional days in IL-2 containing media. Foxp3 and Helios staining was performed on gated viable CD4+ cells. (B) Summary of CpG ODN mediated enhancement of Foxp3+Helios+ cells during expansion. Culture conditions for stimulation and expansion are same as in panel A. Donors (n = 15) ranged in age from 19 to 75 and were all male. (C) The CpG ODN enhances the frequency of Foxp3+Helios+ cells in both freshly isolated and preactivated T cells. Cells were isolated and stimulated for 5 days as in panel A. The CpG ODN was added at the time points indicated in the figure.
To determine the longevity of the effects of the ODN on maintenance of the Foxp3+Helios+ cells, sorted CD4+CD25hi cells were expanded in the presence or absence of the ODN for 5 days, washed, and restimulated for an additional 6 or 19 days. Marked enhancement of the frequency of the Foxp3+Helios+ cells was observed after 12 days in culture and the enhancement was still prominent after a total of 25 days in culture, even though some decrease in the frequency of the Foxp3+Helios+ cells was observed in the cells initially exposed to the ODN (supplemental Figure 2). It should also be noted that continuous presence of the ODN for the entire 12 days of expansion resulted in a modest increase (75.3% vs 63.7%) in the frequency of Foxp3+Helios+ cells compared with cells that had just been treated for the initial 5 days (Figure 2C). Interestingly, when cells that had been previously expanded for 5 days in the absence of the ODN, were restimulated for 7 additional days in the presence of the ODN, stabilization (38.1% vs 22.2%) of the frequency of Foxp3+Helios+ cells was observed (Figure 2C). Thus, the ODN can also exert a stabilizing effect on Foxp3+Helios+ cells that had been previously stimulated in the absence of the ODN.
Foxp3+Helios+ cells expanded with CpG ODN are functional Tregs
We next compared the TSDR methylation status of Foxp3+ cells expanded in the presence or absence of the ODN for 19 to 21 days. CpG ODN treatment resulted in a high frequency of Foxp3+Helios+ cells (68% vs 19%). The TSDR of the entire population of cells expanded in the presence of the CpG ODN was very highly (79%) demethylated, whereas the TSDR of the cells expanded in the absence of the ODN was only < 25% demethylated (Figure 3A).
Figure 3.
The Foxp3+Helios+ T cells expanded in the presence of the CpG ODN are functional Tregs. (A) The TSDR of CD4+CD25+ T cells expanded in the presence of the CpG ODN is fully demethylated. FACS-sorted CD4+CD25+ cells were stimulated with plate-bound anti-CD3 and anti-CD28 in the presence of CpG ODN (2.5μM) for 5 days. The cells were then expanded 14 to 16 days in IL-2 containing media without CpG ODN and methylation analysis was performed as in Figure 1A. (B) Two-week expanded, CpG ODN-treated Tregs were treated as in Figure 1B with PMA/ionomycin and analyzed for intracellular cytokine expression. (C) The population of cells expanded in the presence of the CpG ODN exhibits greater suppressive activity than cells expanded in the absence of the ODN. Tregs were expanded as in panel A and cultured for 3 days at different ratios with freshly isolated CD4+CD25− cells (5 × 104 cells/well) in the presence of γ-irradiated PBMCs (5 × 104) and soluble anti-CD3 (2 μg/mL). 3H-TdR incorporation was measured during the last 18 hours of culture.
The cell populations expanded for 2 weeks in the presence of the ODN were restimulated for 4 hours with PMA/ionomycin and cytokine production on gated Foxp3+Helios+ and Foxp3+Helios− populations was measured. Foxp3+Helios+ Tregs expanded in the presence of the ODN produced low to undetectable levels of IL-2, IFNγ, or IL-17, whereas Foxp3+Helios− cells produced significant levels of all 3 cytokines (Figure 3B). Lastly, the population of Foxp3+ T cells that had been expanded in the presence of the ODN, which contained a high percentage of Foxp3+Helios+ T cells, exhibited greater suppressive activity in a standard in vitro suppression assay (Figure 3C) than cells that had been expanded in the absence of the ODN. Taken together, these studies demonstrate that the Foxp3+Helios+ cells that had been expanded in the presence of the ODN retain all the properties of fully functional Tregs.
CpG ODN stabilizes Foxp3 and Helios expression in all Treg subsets
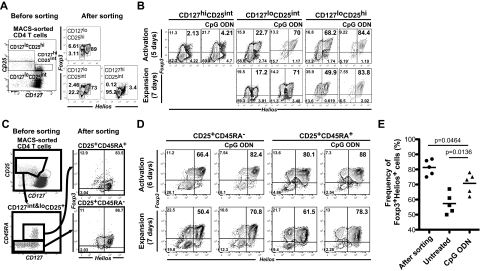
The coexpression of CD25 and low levels of CD127 has been used to distinguish CD25+Foxp3+ from CD25+Foxp3− Teff cells.10 To determine the relationship between Foxp3/Helios expression and the level of expression of CD127, we sorted freshly isolated CD4+ T cells into 3 subsets based on expression of CD25 and CD127 and analyzed the relative expression of Foxp3 and Helios (Figure 4A). Most CD127hiCD25int cells did not express either Foxp3 or Helios. Both CD127low CD25hi and CD127lowCD25int contained a high percentage of Foxp3+Helios+ T cells. We sorted these 3 subpopulations and expanded them in the presence or absence of the CpG ODN (Figure 4B). The ODN had minimal effects on the composition of the CD127hiCD25int cells except for a slight increase in the Foxp3+Helios− subset (11.3% to 21.7%, Figure 4B left 2 panels). Addition of the ODN to the expansion of the CD127lowCD25hi cells enhanced the recovery of Foxp3+Helios+ cells (68.2% vs 89%) on day 5 and this enhancement was maintained for an additional 7 days of culture in the absence of the ODN. The most interesting results were observed with the CD127lowCD25int cells which rapidly lost Foxp3+Helios+ cells (73% to 22.7%) during the initial 5 days of expansion, but retained Foxp3+Helios+ cells when expanded in the presence of the ODN (73% to 71%) and maintained this frequency of Foxp3+Helios+ cells after a further 7 days expansion in the absence of the ODN. These results suggest that the subpopulation of Foxp3+Helios+ cells that express lower levels of CD25 are much less stable than those that express higher levels of CD25, but that expansion in the presence of the ODN exerts a major effect on Foxp3/Helios stabilization in this subpopulation.
Figure 4.
An increased frequency of Foxp3+Helios+ T cells is seen in the presence of the CpG ODN during expansion of distinct populations of Tregs. (A) Sorting strategy used for isolation of CD127hiCD25+, CD127loCD25int, and CD127lo25hi T cells. Expression of Foxp3 and Helios was analyzed in the different populations after sorting. (B) CpG ODN-mediated enhancement of the frequency of Foxp3+Helios+ cells in the sorted populations. (C) Sorting strategy for the isolation of CD25+CD45RA+ and CD25+CD45RA− Treg subpopulations. Expression of Foxp3 and Helios in the cells was performed after sorting. (D) CpG ODN-mediated enhancement of the frequency of Foxp3+Helios+ cells in the sorted CD25+CD45RA+ and CD25+CD45RA− Treg subpopulations after activation and subsequent expansion. (E) Enhancement of Foxp3+Helios+ cells in 2 week-expanded CD25+CD45RA+ Tregs (n = 5).
In addition to the use of the levels of CD127 expression to isolate Foxp3+ Treg cells, coexpression of CD25 and CD45RA has defined a subpopulation of Foxp3+ T cells that are regarded as naive,11 exhibit more stable Foxp3 expression on expansion in vitro,17 and express fully demethylated TSDRs.18 We sorted CD45RA+ and CD45RA− cells from CD4+CD25+CD127low peripheral CD4+ T cells and found that Helios expression was the same in both RA+ and RA− populations (Figure 4C). The groups were then stimulated in the presence or absence of CpG ODN for 6 days, washed, and expanded in the absence of the ODN for an additional 7 days (Figures 4D-E). After 6 days of stimulation without ODN, 94% of CD25+CD45RA+ cells remained Foxp3+, whereas CD25+CD45RA− subpopulation began to lose Foxp3 expression (98% to 77.6%). The frequency of Foxp3+Helios+ cells was also somewhat higher in the CD25+CD45RA+ subpopulation (81%) than in CD25+CD45RA− group (66%). Both cell populations had decreased frequencies of Foxp3+Helios+ T cells after an additional 7 days of expansion in the absence of the ODN, although the total frequency of Foxp3+ cells remained stable. Addition of the ODN to both groups resulted in enhanced frequencies of Foxp3+Helios+ cells during the 7 days of expansion in the absence of the ODN. Thus, Foxp3+CD45RA+ cells appear to exhibit more stable expression of Foxp3 on expansion in vitro, but the frequency of Foxp3+Helios+ cells does diminish and can be rescued in both the CD45RA+ and CD45RA− populations by stimulation in the presence of the ODN.
CpG ODN directly stabilize Helios-expressing Foxp3+ cells and do not inhibit the proliferation of Foxp+Helios− or Foxp3−Helios− cells
Our studies thus far have demonstrated that expansion of Foxp3+ Tregs in the presence of the CpG ODN results in an enhanced frequency of Foxp3+Helios+ T cells. However, we have not directly demonstrated that this enhancement is the result of a direct effect of the ODN on existing Foxp3+Helios+ cells or a suppressive effect on the expansion of Foxp3+Helios− or Foxp3−Helios− cells that are also present in the starting populations. To address this issue, we prepared a starting population that contained 50% Foxp3+Helios+ cells rather than the higher percentages used in the other studies (Figure 5A). Under these conditions we could directly observe the differential effects of the ODN on Helios+ and Helios− cells. We labeled the mixture with CFSE and stimulated the cells for 6 days in the presence or absence of the ODN. Stabilization of the Foxp3+Helios+ cells was seen after expansion in the presence of the ODN (Figure 5A, 30% vs 11.1%). Addition of the CpG ODN slightly suppressed the proliferation of all 3 subsets (Foxp3+Helios+, Foxp3+Helios−, and Foxp3−Helios−; Figure 5A, right histograms). As there was no significant difference in proliferation between the Foxp3+ and the Foxp3− populations in the presence of absence of the ODN (Figure 5B), the enhanced frequency of the Foxp3+Helios+ subset in the ODN treated group is consistent with a direct stabilizing effect of the ODN on this subpopulation.
Figure 5.
CpG ODN acts directly on Foxp+Helios+ cells. The enhancement of the frequency of Foxp3+Helios+ Tregs by CpG ODN is not secondary to differential effects of the ODN on the proliferation of Foxp3+Helios+, Foxp3+Helios−, or Foxp3−Helios− subpopulations. A mixture containing 50% CD4+CD25+ and 50% CD4+CD25− T cells was created after sorting. After labeling with CFSE, the mixture was stimulated for 5 days with plate-bound anti-CD3/CD28 in the presence or absence of the CpG ODN (2.5μM). (A) The extent of CFSE dilution was analyzed by gating on the 3 subsets (Foxp3+Helios+, Foxp3+Helios−, and Foxp3−Helios−). (B) The proliferation of the Foxp3+ and Foxp3− T cells is identical in the presence and absence of the ODN. (C) The optimal size ODN do not inhibit Treg expansion. CD4+CD127loCD25+ cells (1 × 105) were isolated by FACS sorting, stimulated for 5 days in the presence of different length ODNs (10 mer, 10-bp long ODN; 25 mer, 25-bp long ODN; 50 mer, 50-bp long ODN; 100 mer, 100-bp long ODN). Stimulated cells were washed and expanded for an additional 7 days in the absence of the ODN. Cell yield and percent Foxp3+ and Helios+ cells were quantitated by FACS. The graph indicates the absolute number (mean ± SD) of Foxp3+Helios+ cells (open box) and non-Foxp3+Helios+ cells (closed box) recovered on day 12. Data shown is the 1 of 4 independent experiments from different donors.
As all of the studies above (Figures 1–4) used 22-24 mer ODNs, it was of interest to determine the optimal size of the ODN on stabilization of Foxp3+Helios+ cells by comparing the activity of ODNs ranging from 10 mer to 100 mer (Figure 5C). The 10 mer had no activity compared with the untreated cells, whereas the 50 mer and 100 mer increased the frequency of the Foxp3+Helios+ cells after 5 days of culture, but were also moderately toxic as their use resulted in a decreased cell yield. Optimal results were obtained with the 25 mer ODN including stabilization of the frequency of Foxp3+Helios+ cells and an increase in the absolute number of recovered Foxp3+Helios+ cells after 12 days of expansion. Although the ODN do exert a slightly negative effect on cell proliferation as measured by CFSE dilution (Figure 5A), the presence of the ODN is only required during the initial 5 days of culture. After removal of the ODN, the treated cells expand at a rate slightly greater than the controls resulting in a higher yield of cells on day 12 (Figure 5C).
ODN stabilizes Helios-expressing Foxp3+ cells via a cytosomal universal DNA sensor, not via an endosomal TLR
We have thus far assumed that the CpG ODN used in our studies was mediating its effects on stabilization of Foxp3+Helios+ T cells by acting via TLR9 which is expressed in Foxp3+ human Tregs (supplemental Figure 1). Surprisingly, expansion of Tregs in the presence of both the TLR9 agonist and a TLR9 antagonist (ODN TTAGGG) resulted in stabilization of the Foxp3+Helios+ subset (Figure 6A). When both the CpG ODN and the TLR9 antagonist were simultaneously added to the expansion cultures, reversal of the stabilization of the Foxp3+Helios+ subset was not observed (data are not shown). Although we could not detect TLR8 or TLR7 expression in Tregs by RT-PCR, we compared the effects of a TLR8 agonist (ssRNA40) and a TLR7 agonist (CL264) with the TLR9 agonist. Neither the TLR8 nor the TLR7 agonists had any effect on the stabilization of Foxp3+Helios+ T cells, whereas the CpG ODN enhanced the frequency of this subset as noted in our other studies (Figure 6B). Thus, it is unlikely that that CpG ODN–mediated stabilization of Foxp3+Helios+ cells is mediated by TLR signaling pathway and that another nucleotide-recognizing sensor is involved in this stabilization.
Figure 6.
Random ODNs also stabilize Foxp3+Helios+ T cells. (A) Both a TLR9 agonist and a TLR9 antagonist stabilize Helios+ Foxp3+ Tregs. Cells were isolated as in Figure 5A and stimulated in the presence of 2.5μM CpG ODN or ODN TTAGGG for the indicated time. (B) TLR7 and TLR8 agonists do not stabilize Helios+Foxp3+ Tregs. Cells were isolated as in panel A and stimulated in the presence of CpG ODN, 10μM of ssDNA40 (CL264, TLR8 agonist), ssDNA40 (TLR7 agonist), or ssRNA41 (negative control for ssDNA40). (C) Random sequence ODNs stabilize Helios+Foxp3+ Tregs. Left panel, CD4+CD127loCD25+ cells were sorted and stimulated for 5 days in the presence of 2.5μM of ODN TTAGGG or ODN NNNGGG. The cells were then expanded with anti-CD3/CD28 beads in the absence of the ODN and stained for Foxp3 and Helios expression 8 days later. Right panel, same conditions as in left panel except that different ODNs were added (4X indicates 4-times repeat [24 mers] of 6 bp nucleotides). (D) Stabilization of Foxp3 and Helios expression by ODN requires physical stability and proper size (> 10 mer) of the ODN. CD4+CD127loCD25hi cells were stimulated and expanded as same as panel (C) in the presence of phosphorothioate backboned 10 mer of ODN (ODNps10), phosphodiester backboned 25 mer of ODN (ODNpe25), and phosphothioate backboned 25 mer of ODN (ODNps25) at a concentration of 2.5μM. (E) ODNs are localized in cytosol of Tregs. MACS-sorted CD4+ T cells were isolated from buffy coat, stimulated with plate-bound anti-CD3/CD28 for 18 hours in the presence of Biotin-5-ODN or unlabeled ODN, and then washed 3 times in FACS staining buffer. Extra and intracellular Biotin-5-ODN in stimulated cells was stained with AlexaFluor 488– or 546–conjugated strepavidins (Invitrogen). For the confocal microscopic analysis, fixed and stained cells were mounted with Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI;blue, Vector Laboratories). Images were taken using a Zeiss LSM 710 Laser Scanning Microscope (Carl Zeiss) equipped with a Plan-Apochromat 63×/1.4 oil-immersion objective at ambient temperature and processed using ZEN 2009 software (Carl Zeiss). The white bar represents 10 μm.
We next determined the optimal order or motif of the ODN sequence needed to stabilize Foxp3+Helios+ Treg. The TTA sequence from ODN TTAGGG was substituted with randomly selected nucleotides (NNN) and then tested for its effect on the stability of Tregs (Figure 6C). ODN NNNGGG behaved in identical fashion to ODN TTAGGG. Moreover, a completely randomized ODN (ODN NNNNNN) also efficiently stabilized Foxp3+Helios+ cells. As the ODN appeared to be sensed in a nonsequence-specific manner, we examined whether the composition of nucleotide base plays any role stabilization. Excluding A, T, G, or C from the random synthesized ODN did not diminish the stabilizing effect (data not shown). In contrast to phosphorothioate ODN (ODNps), phosphodiester ODN (ODNpe) failed to stabilize Foxp3+Helios+ Tregs even when the ODNpe was added every 24 hours during the 5-day expansion culture (Figure 6D).
To address cellular localization of the purported ODN sensor, we synthesized a 5′-biotin–conjugated ODN (Biotin-ODN). The biotin-labeled ODN was as active the ODNps in stabilizing expression of Foxp3+Helios+ T cells (data not shown). Purified CD4+ T cells were stimulated in the presence of Biotin-ODN for 18 hours, and then evaluated for intracellular and extracellular expression of the ODN using 2 different fluorescence-conjugated streptavidins (Figure 6E left). In some cells, biotin-ODN was detected on the extracellular membrane, but all of the cells possessed Biotin-ODN intracellularly. The staining pattern of Biotin-ODN was identical in Foxp3−Helios− and Foxp3+Helios+ subsets. On analysis by confocal microscopy, Biotin-ODN was not detected in the nucleus or associated with the nuclear membrane. All of the ODN was aggregated in granule-like organelles in the cytoplasm (Figure 6E). Time-course analysis of Biotin-ODN on the FACS indicated that the ODN rapidly disappeared after washing during the expansion phase in the absence of extracellular ODN (supplemental Figure 3).
Discussion
Most studies designed to optimize Treg expansion in vitro have focused on obtaining the greatest expansion of the Tregs of the highest purity in terms of Foxp3 expression. Although the highest degree of purity may be achieved with a starting population of Tregs purified by FACS, cell sorting, as yet, has not been used for preparation of Tregs under good manufacturing practice conditions, therefore the starting preparations have used Tregs purified based on high expression of CD25 and low expression of CD127 using magnetic bead separation techniques such as the CliniMACS.19,20 After 3 weeks of expansion in vitro, only 60%-70% of bead-purified Tregs retain expression of Foxp3.20 It has been difficult to distinguish whether this reduction is secondary to loss of Foxp3 by cells that were initially Foxp3+ or by overgrowth of Foxp3− T cells that contaminated the starting samples.
We reported that expression of the transcription factor, Helios, is a marker of thymus-derived nTregs,12 whereas Foxp3+Helios− T cells are probably iTregs. We have characterized several properties of the Helios+ and Helios− Foxp3 subpopulations. First, Foxp3+Helios+ Tregs have completely demethylated TSDR regions of the Foxp3 locus, whereas the Foxp3+Helios− cell populations are only 50% demethylated. This result is most consistent with heterogeneity in the Helios− Treg population that is probably related to decreased stability of Foxp3 expression in vivo and in vitro.15 Secondly, a higher percentage of effector-cytokine producing Foxp3+ T cells were present in the Helios− subset and the frequency of effector-cytokine producing cells in this subset was increased (∼ 10-fold) after expansion in vitro. IFNγ-producing Foxp3+ Tregs have also been shown to have methylated TSDRs and to be Helios− in patients with type I diabetes.21 It is probable that the Helios+ Treg represents a much more stable population, which is less probable to convert to a Teff cell during the course of an inflammatory response in vivo.
The goal of this study was to develop conditions for the selective expansion of the Foxp3+Helios+ subset in vitro for potential use in cellular therapy studies. We initially examined the effects of TGFβ and an ODN TLR9 agonist on the expansion of Foxp3+ T cells in vitro. The ODN TLR9 agonist enhanced the frequency of the Foxp3+Helios+ T cells in the expanded population. Interestingly, the effects of the ODN were observed after 5 days of exposure, but were also maintained for as long as an additional 16 days after the cells were washed and further expanded in the absence of the ODN. The effects of the ODN were not mediated indirectly by inducing the death of the Foxp3+Helios− cells (data not shown) or by modifying the rates of proliferation of either Foxp3+Helios+ or Foxp3+Helios− subsets, but rather by a direct effect on the Foxp3+Helios+ population resulting in stabilization of Foxp3 expression. The ODN effects were also observed when we expanded CD4+CD25+CD45RA+ T cells, a Treg subpopulation that has been shown to be more stable on expansion in vitro18 and to have a greater proliferative capacity than the CD4+CD25+CD45RO+ subset.
Although the effects of the ODN were observed when culturing highly enriched cell sorter purified CD4+CD25hiCD127lowFoxp3+ T cells, the ODN stabilized Foxp3/Helios expression in Tregs purified by magnetic beads that contained a lower percentage of Foxp3+ T cells in the starting population and also stabilized Foxp3/Helios expression in sorted CD4+CD25int cells that contained as few as 70% Foxp3+ cells. The stabilizing effects of the ODN were very consistent using cells isolated from buffy coats from multiple donors over a broad age range (Figure 2B). Some degree of stabilization of Foxp3/Helios was even observed in cell populations that had first been expanded in the absence of the ODN and had lost considerable expression of Foxp3/Helios. These results strongly suggest that the ODN may potentiate the stabilization of Foxp3/Helios in Tregs selected by less stringent techniques than cell sorting and allow a greater yield of Tregs by starting with a larger population of cells. Several other approaches have been used to stabilize Foxp3 expression in Treg expansion cultures including rapamycin,10 retinoic acid22 or immunosuppressive cytokines (IL-10, TGFβ). Although some of these studies appear promising, particularly when using flow sort-purified Tregs, bead-purified Tregs expanded for multiple rounds even in the presence of rapamycin had reduced percentages of Foxp3+ T cells.22 In preliminary studies, we have compared the effects of rapamycin with the ODN in the stabilization of Foxp3 and Helios expression. Although stimulation in the presence of rapamycin resulted in enhancement of the total percentage of Foxp3+ cells, only stimulation in the presence of the ODN led to stabilization of the Foxp3+Helios+ population (supplemental Figure 4).
We initiated these studies with an ODN that was well characterized as a TLR9 agonist, but a TLR antagonist was as effective, if not more effective, at stabilizing Foxp3/Helios expression. Moreover, any random sequence ODN was also capable of stabilizing Foxp3/Helios expression and the absence of any single nucleotide (adenine, guanine, cytidine, thymine) did not diminish the stabilizing activity. The optimal size of the ODN was a 25 mer, as a 10 mer was ineffective and longer ODNs appeared to have negative effects on the viability of Tregs in the expansion cultures. We could not detect the intracellular presence of an ODN with a phosphodiester backbone or a short-length ODN with a phosphothioate backbone (supplemental Figure 5) after 18 hours of incubation. A poly RNA ODN also failed to stabilize Foxp3 expression. Thus, it does not appear that the ODNs function through any known TLR.
At present, we have very little information on the cellular target of ODN action. By immunostaining using a Biotin-labeled ODN and confocal microscopy, we could demonstrate that the ODN appeared to be localized to cytoplasmic vesicles rather than the nucleus, which is consistent with earlier studies demonstrating that phosphorothioate-backboned ODN is taken up by cells via receptor-mediated endocytosis and then localized in endosomes and lysosomes.23 However, it is also possible that an ODN binding partner is located on the cell surface and that T-cell activation induces the cellular uptake of such a complex. It should also be noted that the optimal concentration of the ODN used in these studies (2.5μM) is approximately 10-fold higher that that of TLR agonists used to modulate immune cell function. Thus, the highly charged ODN might nonspecifically interfere with several cellular processes. Although the ODN did not specifically target TLR9, it remains possible that the ODN might have interfered with the cellular trafficking of TLR9 or the enzymatic cleavage of TLR9 in the endolysosome.24
Although our studies on the use of ODN have been entirely performed in vitro, interaction of Tregs with DNA released from dying cells during the course of an inflammatory response may also function by a similar pathway to stabilize Foxp3/Helios expression and potentiate Treg function. Conversely, DNA released from dying cells in the tumor microenvironment could also stabilize Foxp3/Helios expression and result in Treg-mediated inhibition of antitumor Teff cells. Lastly, as ODNs are already available for clinical use and can be removed from the cultures after a few days with no negative effects on expansion, it is probable that this protocol can readily be adapted for the preparation of clinical-grade Tregs. Indeed, expansion of Tregs isolated by MACS bead purification and expanded in the presence of ODN also expressed high percentages of Foxp3+ Helios+ cells (supplemental Figure 6).
Supplementary Material
Acknowledgments
This study was supported by funds from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.C.K. designed all experimental strategies, performed most of experiments, and wrote the paper; R. B. contributed Figure 3C; J.Y. contributed Figure 6F; A.G. contributed to all experimental designs for stimulation and expansion of human regulatory T cells; A.M.T. contributed to all experimental designs related to Helios expression; D.Q.T. contributed to experimental setup of human Treg isolation and culture in vitro; and E.M.S. supervised the project, interpreted the data, and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ethan M. Shevach, Laboratory of Immunology, NIAID, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 11N315, Bethesda, MD 20892; e-mail: eshevach@niaid.nih.gov.
References
- 1.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 3.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trzonkowski P, Bieniaszewska M, Juœciñska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133(1):22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 8.Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol. 2008;38(4):915–917. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippen KL, Merkel SC, Schirm DK, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyara M, Yoshioka Y, Kitoh A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floess S, Freyer J, Siewert C, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103(18):7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Kim YCC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186(11):6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309(5739):1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines on in vitro expansion. Blood. 2006;108(13):4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann P, Boeld TJ, Eder R, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39(4):1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 19.Peters JH, Preijers FW, Woestenenk R, et al. Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS One. 2008;3(9):e3161. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41–83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186(7):3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golovina TN, Mikheeva T, Brusko TM, et al. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS One. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltinger C, Saragovi HU, Smith RM, et al. Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J Clin Invest. 1995;95(4):1814–1823. doi: 10.1172/JCI117860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewald SE, Lee BL, Lau L, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.