Abstract
Cytokines are deregulated in cancers and can contribute to tumor growth. In patients with diffuse large-cell lymphoma (DLBCL), we observed higher levels of JAK/STAT pathway-related serum cytokines (ie, IL-6, IL-10, epidermal growth factor, and IL-2) compared with controls. Of these, only IL-10 activated the JAK2 pathway in lymphoma cells in vitro. Patients with high serum IL-10 had shorter event-free survival (EFS) than patients with low levels (P > .01) and high IL-10 was correlated with high lactase dehydrogenase (P = .0085) and higher International Prognostic Index scores (P = .01). To explore the mechanism by which IL-10 may contribute to an inferior EFS, we investigated the effect of IL-10 on the JAK2 pathway and found that the IL-10/IL-10 receptor complex up-regulated JAK2 signaling. Neutralizing Ab to IL-10 inhibited constitutive and IL-10–induced JAK2/STAT3 phosphorylation. JAK2 inhibition dephosphorylated JAK2 and STAT3 and caused an inhibitory effect on phospho-JAK2–positive DLBCL cells; there was a minimal effect on phospho-JAK2–negative cells. Apoptosis induced by JAK2 inhibition was dependent on inhibition of autocrine IL-10 and c-myc expression and independent of Bcl-2 family expression. These results provide the rationale for testing JAK2 inhibitors in DLBCL patients, and indicate that serum IL-10 may be a biomarker to identify patients more likely to respond to JAK2-targeted therapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent type of nonHodgkin lymphoma (NHL) and is characterized by the activation of several signal-transduction pathways that facilitate tumor-cell survival. Persistent activation of the JAK/STAT pathway is found in a variety of both solid and hematologic malignancies.1,2 For example, breast, lung, and prostate cancer cell lines exhibit activation of the JAK/STAT pathway.3–5 It was reported recently that STAT3 is overexpressed in DLBCL human cell lines6; however, the mechanism(s) of STAT3 activation in DLBCL remains unknown.
The most common mechanism causing abnormal JAK activation in malignant cells is through dysregulated cytokine signaling. The cytokines can be derived from tumor cells themselves (autocrine) or from the microenvironment (paracrine).7–9 A variety of cytokines function through their respective receptors and mediate ligand-dependent activation of the JAK family. In mammals, the JAK family comprises 4 members: JAK1, JAK2, JAK3, and TYK2.10 JAKs are often physically associated with cytokine and growth factor receptors, which, after cytokine-induced receptor dimerization and aggregation, become activated through autophosphorylation. Active JAKs are then able to phosphorylate specific tyrosine residues on cytokine receptors that serve as docking sites for multiple proteins, including STATs.11 JAK-phosphorylated STATs dimerize and become active transcription factors and drive the expression of multiple genes important for cell activation, localization, survival, and proliferation.11
In the present study, we investigated the mechanism of STAT3 activation in DLBCL tumors. Understanding this mechanism(s) will provide important information regarding the potential use of agents to target this pathway in lymphoma. We measured serum cytokine levels from DLBCL patients and compared these with patient outcome. We also determined that IL-10/IL-10 receptor (IL-10R) is the major cytokine involved in the activation of JAK2 in DLBCL cells. Promising results with JAK2 inhibitors in myeloproliferative neoplasms (MPNs)12,13 suggest that they may also be effective in DLBCL. We used a specific JAK2 inhibitor, TG101348 (also known as SARS302503), to determine whether IL-10 and JAK2 are responsible for constitutive activation of STAT3 in lymphoma cells and if inhibition of this signaling axis could inhibit the survival of pJAK2-positive DLBCL cells.
Methods
Reagents
The JAK2 inhibitor TG101348 (TG, also known as SARS302503) was a gift from TargeGEN Pharmaceuticals (now Sanofi-Aventis). Annexin V–FITC was obtained from BD Biosciences. Abs to STAT3, STAT1, STAT5, JAK2, JAK1, bcl-2, bcl-XL, mcl-1, c-myc, and survivin were all purchased from Cell Signaling Technologies. Phospho-specific Abs for JAK2 (tyrosine 1007/1008), JAK1 (tyrosine 1022/1023) STAT3 (tyrosine 705), STAT3 (serine 727), STAT1 (tyrosine 701), and STAT5 (tyrosine 694) were also obtained from Cell Signaling Technologies. Phospho-tyrosine Ab was from Millipore. Recombinant human IL-10 (rIL-10) and Abs to IL-10, IL-10Rα, IL-10Rβ, and isotype control for flow cytometry were from R&D Systems. The IL-10 ELISA kit was from R&D Systems. Doxorubicin was from Sigma-Aldrich.
Lymphoma samples
Frozen tumor samples (n = 6), paraffin-embedded tumor samples (n = 12), normal serum controls, and normal blood B-cell controls were obtained through the University of Iowa/Mayo Lymphoma SPORE Biospecimens Core and the Predolin Foundation Biobank at the Mayo Clinic. Additional paraffin-embedded tumors and pretreatment serum samples were obtained from 70 untreated DLBCL patients participating in a clinical trial of epratuzumab and rituximab immunotherapy combined with standard CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone/prednisolone) chemotherapy (N0489; NCT00301821).14 That study was approved by the Mayo Clinic Institutional Review Board and all patients signed informed consent to provide samples for research in accordance with the Declaration of Helsinki. DLBCL cases were subgrouped into the germinal center B-cell (GCB) or non-GCB molecular type based on the Hans immunohistochemistry (IHC) algorithm applied to paraffin-embedded tumor samples.15 The SUDHL6 (DHL6), OCILy19 (Ly19), OCI-Ly3 (Ly3) OCI-Ly10 (Ly10), SUDHL2 (DHL2), and HBL1 DLBCL cell lines were a kind gift from Dr Louis Staudt (National Cancer Institute, Bethesda, MD). All cell lines were cultured in IMDM supplemented with 20% human serum. CD19+ normal B cells were extracted from PBMCs.
IHC
Sections (4 μm thick) were cut from B5-fixed paraffin-embedded tissue blocks and stained as described previously with Abs to phosphoJAK2 (pJAK2).16 Rabbit IgG was used as a negative control for pJAK2 staining. pJAK2 staining was assessed semiquantitatively as follows: negative, < 10% (−); low, 10%-30% (+); moderate, 30%-80% (++); and high, > 80% (+++).
ELISA for serum cytokines
Serum IL-2, IL-10, epidermal growth factor (EGF), and IL-6 were measured using the Luminex-200 ELISA System Version 2.3 (Invitrogen) and results were generated using STarStation Version 1.8 software.
IL-10 ELISA for cell supernatants
Supernatants from DLBCL cell line cultures were analyzed for IL-10 secretion using the human IL-10 immunoassay kit (R&D Systems). The specimens were run “neat” and the end point read at 450 nm using a SpectraMax190 microplate reader (Molecular Devices).
FACS analysis for the IL-10 receptor
Cells were stained with PE-conjugated anti–human IL-10Rα or anti–human IL-10Rβ. PE-conjugated mouse IgG2a was used as an isotype control. The tubes were incubated at room temperature for 20 minutes while protected from the light. The cells were then washed of excess Ab with 1× PBS and centrifuged for 5 minutes. The samples were run on a FACSCalibur flow cytometer (BD Biosciences) using Cell Quest Pro Version 6.4 software for acquisition. FlowJo software (TreeStar) was used for analysis.
Cell survival and proliferation assay
Cells (0.5 × 106/well) were cultured in the presence of various concentrations of TG for 48 hours or as otherwise indicated, and then analyzed by flow cytometry for cell survival, as described previously.16 Cell proliferation was determined by the tritiated thymidine method, as described previously.17
Western blotting and coimmunoprecipitation assay
Cells (5 × 106) were incubated with various concentrations of TG at 37°C for various lengths of time. Two to 5 μg of the specific Ab was added to the lysates and complexes were allowed to form by incubating with rotation at 4°C. A 50% slurry (25 μL) of protein A-Sepharose or protein G-Sepharose beads was then added and incubated for 2 hours. Immunoprecipitates captured with Sepharose beads were washed 4 times with radioimmunoprecipitation assay buffer and analyzed by Western blotting.16
siRNA transfection
Ly3 (8.0 × 106) DLBCL cells were transfected with siRNAs using the Amaxa Nucleofector Kit (Lonza) according to the manufacturer's protocol. In brief, cells were transfected for 24, 48, and 72 hours with 250 or 500nM siRNA using Amaxa solution and program U15. Nonsilencing siRNA and JAK2 siRNA were from Ambion or Invitrogen.
qRT-PCR
For quantitative RT-PCR (qRT-PCR), cDNA was synthesized using 0.8-3 μg of total RNA isolated from 3-5 million cells with the RNeasy Mini Kit (QIAGEN) and SuperScript III First-Strand Synthesis SuperMix (18080-400; Invitrogen). qRT-PCR was performed on the CFX96 real-time PCR detection system (Bio-Rad).
Primers and probe set for c-myc
The primers and probe set for c-myc were: forward primer, 5′ TTCTTCCTCATCTTCTTGTTCCT 3′; reverse primer, 5′ GATTCTCTGCTCTCCTCGAC3′; and probe, 5′/56-FAM/ TCAGAGTCG/ZEN/CTGCTGGTGGTG/3IABKFQ/ 3′).
Primers and probe set for survivin
The primers and probe set for survivin were: forward primer, 5′ CGCTTCCTATCACTCTATTCTGTC 3′; reverse primer, 5′ CTAAGCACAAAGCCATTCTAAGTC 3′; and probe, 5′/56-FAM/CATCCACCT/ZEN/GAAGTTCACCCCGTT /3IABKFQ/ 3′)
GAPDH
The primers and probe set for GAPDH were: forward primer, 5′ GAAGGTGAAGGTCGGAGTC 3′; reverse primer, 5′ GAAGATGGTGATGGGATTTC 3′; and probe, 5′/HEX/CAAGCTTCCCGTTCTCAGCC/3IABKFQ/ 3′). The program consisted 95°C for 15 minutes, then 40 cycles of 95°C for 10 seconds, and 60°C for 30 seconds. Data analysis was performed by delta delta CT method.
RT-PCR
cDNA was synthesized as described in “qRT-PCR.” IL-10 primers were: forward primer, 5′ TGCCTAACATGCTTCGAGATCTCCG 3′; reverse primer, 5′ TTAGAGGGAGGTCAGGGAAAACAGC 3′. The program was: PCR product 842 bp at 95°C 15 minutes; 95°C for 0.5 minutes, 55°C for 0.5 minutes, and 72°C for 0.8 minutes × 30 cycles; and 72°C for 10 minutes.
JAK2 primers
Primers for JAK2 were: forward primer, 5′ TGTCTTACCTCTTTGCTCAGTGGCG 3′; reverse primer, 5′ CAATGACATTTTCTCGCTCGACAGC 3′. The program was: PCR product 894 bp at 95°C for 15 minutes; 95°C for 0.5 minutes, 58°C for 0.5 minutes, 72°C for 2.5 minutes × 30 cycles; and 72°C for 10 minutes.
GAPDH primers
GAPDH primers were: forward primer, 5′ GAAGGTCGGAGTCAACGGATTTG 3′; reverse primer 5′ ATGGCATGGACTGTGGTCATGAG 3′. The program was: PCR product 531 bp at 95°C for 15 minutes; 95°C for 0.5 minutes, 55°C for 0.5 minutes, 72°C for 0.8 minutes × 30 cycles; and 72°C for 10 minutes.
Results
Serum cytokine profile in untreated DLBCL patients
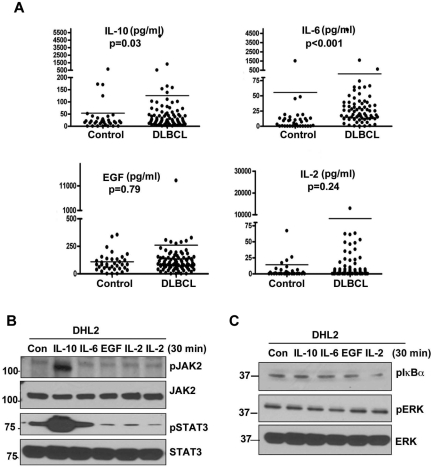
The JAK/STAT pathway–specific cytokines IL-6, IL-10, IL-2, and EGF were measured in pretreatment serum from 70 DLBCL patients and 24 healthy controls (Figure 1A). The median serum values for DLBCL patients and controls, respectively, were: for EGF, 87.4 pg/mL (range, 0-13 120) compared with 84.2 pg/mL (range, 0-501; P = 0.79); for IL-2, 2.7 pg/mL (range, 0-12 930) compared with 0.1 pg/mL (range, 0-123.1; P = .29); for IL-6, 26.9 pg/mL (range, 0-4804) compared with 5.9 pg/mL (range, 0-1502; P < .001); for IL-10, 26.0 pg/mL (range, 0-5030) compared with 18.0 pg/mL (range, 0-1055; P = .03). Of these 4 cytokines, only IL-10 and IL-6 were significantly higher in DLBCL patients compared with controls.
Figure 1.
Serum cytokine levels in DLBCL patients. (A) Serum IL-10, IL-6, EGF, and IL-2 levels in DLBCL patients (n = 70) and healthy subjects (n = 24). (B-C) Serum-starved DHL2 cells were incubated with 100 ng/mL of human rIL-10, rIL-6, rIL-2, and rEGF for 30 minutes, and phosphorylation of JAK2 and STAT3 (B) and IkBα and ERK (C) were analyzed by Western blotting.
To identify which of these cytokines are primarily responsible for JAK2 and STAT3 activation in DLBCL cells, we stimulated serum-starved DHL2 cells with 100 ng/mL of each cytokine (IL-10, IL-6, IL-2, and EGF) for 30 minutes. Only IL-10 was able to induce phosphorylation of JAK2 and its downstream effecter STAT3 (Figure 1B) in DHL2 cells. IL-6 also had a minimal effect on pSTAT3. Further experiments demonstrated that IL-10 was unable to stimulate NF-κB or ERK signaling, suggesting that IL-10 specifically stimulates the JAK2-STAT3 pathway (Figure 1C).
Higher serum IL-10 levels are correlated with DLBCL patient EFS
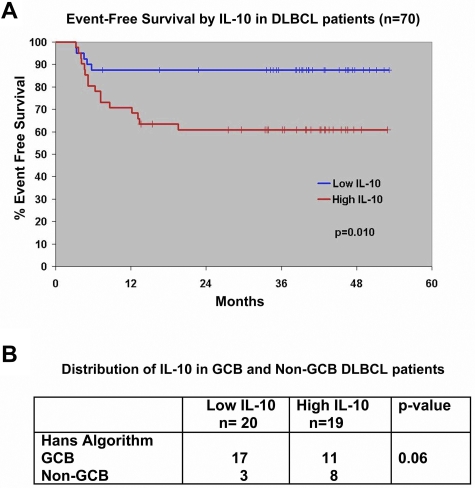
We used the median value for serum IL-10 to separate the patients into high (≥ 26 pg/mL) or low IL-10 (< 26 pg/mL) groups. The 35 patients with high IL-10 had a median IL-10 of 57.7 pg/mL (range, 26.1-503.7); the low group had a median of 16.8 pg/mL (range, 0-25.9; Table 1). Baseline serum IL-10 was correlated with elevated serum lactate dehydrogenase (P = .0085) and International Prognostic Index score (P = .01), but not with other factors (Table 1). Serum IL-10 did not predict the attainment of a complete remission to initial therapy in this trial; 93% (25 of 27) of patients with a low IL-10 entered complete remission, as did 88% (29 of 33) of those with a high IL-10 (P = .68). However, patients with a high serum IL-10 level did have a shorter EFS compared with those with a low serum IL-10 level (P = .01; Figure 2A).
Table 1.
Correlation of low versus high IL-10 pretreatment and DLBCL patient disease characteristics
| Low IL-10 (n = 35) | High IL-10 (n = 35) | P | |
|---|---|---|---|
| Age ≥ 60 y | 20 (50%) | 25 (61%) | .32 |
| Female sex | 18 (45%) | 13 (32%) | .22 |
| PS 0-1 | 36 (90%) | 34 (83%) | .35 |
| Stage III-IV | 30 (75%) | 36 (86%) | .14 |
| Elevated lactate dehydrogenase | 9 (22%) | 20 (50%) | .0085 |
| ≥ 2 extranodal sites | 9 (23%) | 13 (32%) | .35 |
| International Prognostic Index score | |||
| 0-1 | 16 (40%) | 5 (12%) | .027 |
| 2 | 9 (23%) | 9 (22%) | |
| 3 | 11 (28%) | 20 (49%) | |
| 4-5 | 4 (10%) | 7 (17%) | |
| 0-2 | 25 (63%) | 14 (34%) | .011 |
| 3-5 | 15 (38%) | 27 (66%) | |
| B symptoms | 21 (51%) | 27 (68%) | .14 |
| Bulky disease (> 10 cm) | 5 (13%) | 9 (22%) | .26 |
Low IL-10 was < 26.15 pg/mL; high, > 26.15 pg/mL. All values are n (%).
Figure 2.
Survival of untreated DLBCL patients on North Central Cancer Treatment Group Trial N0489 by pretreatment serum IL-10 levels. (A) The median value of serum IL-10 was used to group patients into high and low IL-10 cohorts. The EFS of patients with low serum IL-10 (≤ 26 pg/mL) and high serum IL-10 (> 26 pg/mL) was estimated by the Kaplan-Meier method. (B) Distribution of IL-10 between genetic types of DLBCL as determined by the Hans IHC method. Thirty-nine patients had both serum IL-10 and tissue available for comparison.
On-study tumor tissue from 39 DLBCL patients who also had a serum IL-10 level available were used to classify the DLBCL into GCB and non-GCB genetic types with the Hans IHC method.15 Patients with non-GCB–type DLBCL were more likely to have a high IL-10 level (P = .06; Figure 2B).
JAK2 is constitutively activated in patients with DLBCL
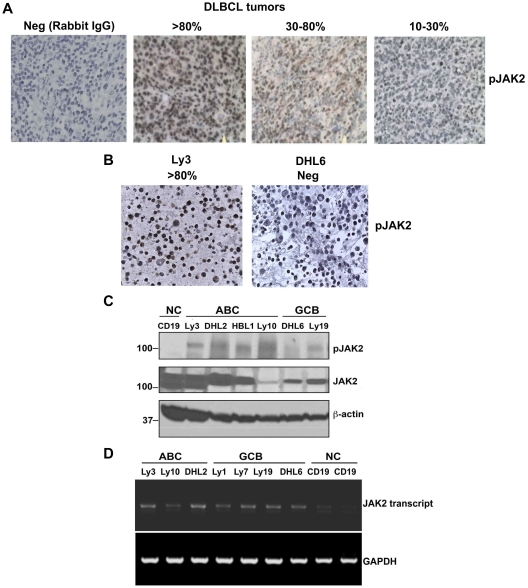
The clinical data suggested that elevated serum IL-10 was common in untreated DLBCL and was correlated with clinical aggressiveness. Because IL-10 signals through the JAK/STAT pathway, we sought to determine whether IL-10 is responsible for constitutively activated JAK2 in DLBCL cells. Twelve patient samples of DLBCL from the SPORE Biobank were analyzed by IHC for pJAK2 and genotyped with the Hans IHC method.15 Rabbit IgG control Ab was used as negative control for pJAK2 staining in DLBCL tissues (Figure 3A). All 12 patients had some level of pJAK2 tumor cell expression (Figure 3A); 1 patient had a high level, 6 had a moderate level, and 5 had a low level. Furthermore, as also shown by IHC, the DHL6 cell line was negative for pJAK2 staining, whereas the Ly3 cell line was strongly positive for pJAK2 (Figure 3B).
Figure 3.
Constitutive activation of JAK2 in patient DLBCL tumors (n = 12) and cell lines. (A) IHC detection (400× magnification) of activated JAK2 protein in DLBCL tissue. (B) IHC detection of activated JAK2 in DLBCL cell lines. (C) Phosphorylation levels of JAK2 in human DLBCL cell lines (ABC and GCB) were analyzed by Western blotting. CD19+ B cells were used as a control. (D) JAK2 mRNA expression was analyzed in DLBCL lines by RT-PCR.
We then characterized JAK2 expression and phosphorylation status in vitro in a panel of DLBCL lines, along with CD19+ normal B cells. The CD19+ normal B cells were pJAK2 − (Figure 3C); however, all of the 4 ABC (activated B-cell) DLBCL cell lines were found to be positive for pJAK2. The GCB cell lines had low-level expression of pJAK2. The JAK2 mRNA level was comparable between non-GCB and GCB DLBCL cells (Figure 3D). These data suggest that JAK2 is constitutively activated in some DLBCL patient tumors and might be responsible for aberrant STAT3 activation in these patients.
IL-10 is the major autocrine factor for JAK2 tyrosine phosphorylation
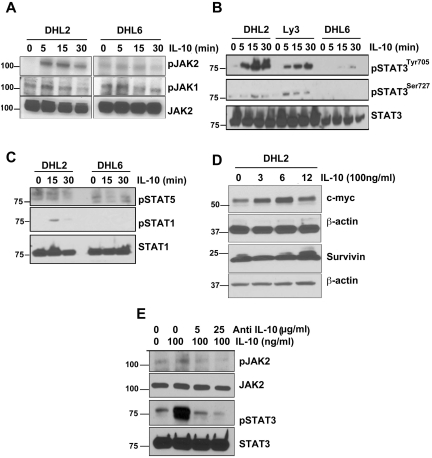
The effect of IL-10 on JAK2 and its downstream signaling was studied in both pJAK2+ and pJAK− DLBCL cells. DHL2 (pJAK2+) and DHL6 (pJAK2−) cells were serum starved overnight and then pulsed with IL-10 for 5, 15, or 30 minutes. IL-10 stimulated the phosphorylation of JAK2 at the tyrosine 1007/1008 residue as soon as 5 minutes in DHL2 cells; however, no stimulation of JAK2 was observed in DHL6 cells (Figure 4A). IL-10 was unable to stimulate JAK1 phosphorylation in either DHL2 or DHL6 cells (Figure 4A).
Figure 4.
IL-10 induces JAK2-pathway activation in DLBCL cells. (A) Serum-starved DHL2 and DHL6 cells were treated with 100 ng/mL of rIL-10 for various times as indicated and phosphorylation of JAK2 and JAK1 was assessed by Western blot. (B) Serum-starved DHL2, Ly3, and DHL6 cells were treated with rIL-10 for 5, 15, and 30 minutes and STAT3 tyrosine and serine phosphorylation was assessed by Western blot. (C) Serum-starved DHL2 and DHL6 cells were treated with 100 ng/mL of rIL-10 and phosphorylation of STAT1 and STAT5 were analyzed. (D) Serum-starved DHL2 cells were treated with 100 ng/mL of rIL-10 for various times and expression of c-myc and survivin were analyzed by Western blot. (E) Serum-starved DHL2 cells were treated with neutralizing IL-10 Ab, followed by IL-10, and JAK2 and STAT3 phosphorylation was analyzed.
We also determined the effect of IL-10 on STAT3 activation in STAT3+ versus STAT3− DLBCL cells. Serum-starved pJAK2+ DLBCL cells (DHL2 and Ly3) and pJAK2− DLBCL cells (DHL6) were treated with rIL-10 for various times point and STAT3705 activation was assayed by Western blotting. IL-10 was able to stimulate STAT3 at the tyrosine residue as soon as 5 minutes with an increase (in a time-dependent manner) in both pSTAT3+ cell lines (Figure 4B). In contrast, IL-10 showed little effect on STAT3 phosphorylation at the serine residue (Figure 4B). The pSTAT3− cell line did not respond to IL-10 except for a slight activation at 30 minutes in pSTAT3 tyrosine (Figure 4B). Additional experiments revealed that IL-10 was not able to stimulate other STATs such as STAT1 or STAT5 in these cells, suggesting that IL-10 selectively activates STAT3 in some DLBCL cells (Figure 4C).
We next sought to determine whether IL-10 is indeed able to induce the expression of the known downstream targets of JAK2-pathway signaling: mcl-1, bcl-2, bcl-xl, c-myc, and survivin. Serum-starved DHL2 cells were induced with rIL-10 for various times and protein lysates were subjected to Western blotting. IL-10 had no effect on the antiapoptotic proteins mcl-1, bcl-2, and bcl-xl (data not shown); however, IL-10 did induce the c-myc protein in a time-dependent manner, with a maximal increase observed at 6 hours (Figure 4D). The effect of IL-10 on survivin protein was seen only at the 12-hour time point, and this effect was minimal (Figure 4D). Studies of the effect of IL-10 on c-myc mRNA expression by real-time RT-PCR also demonstrated an increase in the mRNA level of c-myc at 3 and 6 hours (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Neutralizing Ab to IL-10 inhibits IL-10–mediated JAK2 and STAT3 activation
To ascertain whether IL-10 was indeed responsible for JAK2 and STAT3 phosphorylation in pSTAT3+ cells, DHL2 cells were treated with various doses of neutralizing Ab to IL-10 and then pulsed with IL-10. Anti–IL-10 Ab decreased IL-10–induced JAK2 and STAT3 tyrosine phosphorylation in a dose-dependent manner (Figure 4E).
JAK2 activation is correlated with levels of IL-10 and IL-10R
DLBCL lines were tested for intracellular IL-10 mRNA by RT-PCR. The lines with higher levels of pJAK2 (Ly3 and DHL2) had higher levels of IL-10 mRNA (Figure 5A) than the pJAK2− lines (Ly19 and DHL6). Supernatants from the DHL6, Ly19, Ly3, and DHL2 cell lines were collected and assayed for IL-10, EGF, IL-6, and IL-2 by a multiplex ELISA. Both pJAK2+ cell lines secreted high amounts of detectable IL-10 (range, 600-800 pg/mL), whereas IL-10 production was negligible in the pJAK2− DLBCL lines (Figure 5B). Secretion of the other JAK/STAT pathway–related clinically relevant cytokines (EGF, IL-6, and IL-2) were very low in the supernatants of the DLBCL cell lines and not different between the pJAK2+ and pJAK2− DLBCL cell lines (data not shown). These data suggest that these DLBCLs are capable of autocrine secretion of IL-10. Moreover, exogenous IL-10 was able to increase the proliferation of pJAK2+ DHL2 and Ly10 cells (supplemental Figure 2).
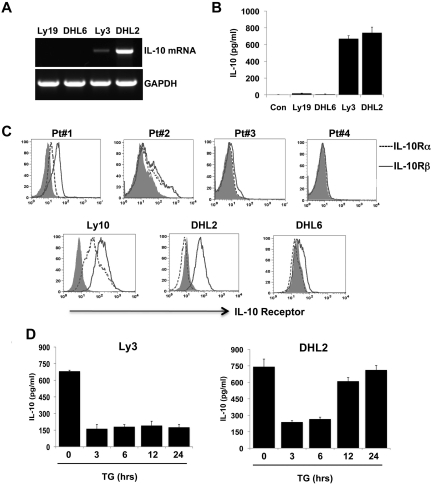
Figure 5.
Autocrine secretion of IL-10 and expression of IL-10 receptors on DLBCL cells. (A) IL-10 mRNA in DLBCL lines was detected by RT-PCR. (B) Cell-culture supernatants were collected from various DLBCL cell lines and subjected to IL-10 ELISA. Data represent means ± SD of 3 independent experiments. (C) Surface IL-10 receptors were analyzed in DLBCL patient cells (top panel) and DLBCL cell lines (bottom panel) by flow cytometry. (D) DHL2 and Ly3 cell lines were treated with a 4μM concentration of the JAK2 inhibitor TG for various times and the supernatants were collected and subjected to IL-10 ELISA. Means ± SD from 4 determinations obtained in 2 separate experiments are shown.
IL-10 binds to IL-10 cell-surface receptors and signals through the JAK/STAT pathway.18 Because we demonstrated that DLBCL cells could synthesize and secrete IL-10, we investigated whether DLBCL cells also express the surface IL-10R (IL-10Rα and IL-10Rβ). Using 6 patient samples, IL-10Rs were expressed in 4 of the 6 cases. The expression was variable in intensity and isoform expression. In 2 cases, the cells expressed both IL10Rα and IL-10β; in 2 others, only IL-10β was expressed and the other 2 were negative for both IL-10Rα and IL-10Rβ (Figure 5C upper panel). In cell line experiments, the DLBCL line Ly3 expressed high levels of both IL-10Rα and IL-10Rβ, but the DHL2 cell line expressed only large amounts of IL-10Rβ; however, DHL6 does not express IL-10Rα and has very low levels of IL-10β (Figure 5C lower panel). In the cell lines, the IL-10Rα or IL-10Rβ expression level was correlated with pJAK2 expression. Both the Ly10 and DHL2 cell lines were positive for pJAK2, as shown by Western blotting (Figure 3C), and had surface IL-10R. Conversely, DHL6 cells were negative for pJAK2 (Figure 3C) and expressed very low levels of IL-10Rβ. We also examined the correlation of IL-10R and pJAK2 in the DLBCL patient samples. Of the 3 patients whose cells were IL-10R+, 2 expressed pJAK2 (data not shown).
JAK2 inhibition inhibits autocrine IL-10 secretion
We evaluated the impact of JAK2 inhibition on IL-10 production in DHL2 and Ly3 cells, which constitutively secrete IL-10 (Figure 5B). We measured the levels of IL-10 by ELISA in culture supernatants treated with the JAK2 inhibitor TG at various time points. TG (SARS302503) is currently being tested as a daily oral agent in advanced clinical trials for MPN.12 Figure 5D shows that TG causes a time-dependent inhibition of autocrine IL-10 production in DHL2 and Ly3 cells compared with cells grown in medium alone. In Ly3 cells, TG was able to block IL-10 production as soon as 3 hours and the inhibition was sustained for up to 24 hours. In contrast, in DHL2 cells, the inhibition of IL-10 by TG was dramatic at early time points (ie, 3 and 6 hours).
The JAK2-specific inhibitor TG inhibits constitutive and IL-10–induced JAK2 and STAT3 phosphorylation
We next studied the effect of JAK2 inhibition by TG on constitutive JAK2 signaling in DLBCL cells. Treatment of DHL2 cells with increasing doses of TG resulted in a concentration-dependent inhibition of phosphorylation of JAK2 and STAT3, but not JAK1 (Figure 6A). Incubation with TG abrogated tyrosine phosphorylation of STAT3 as soon as 2 hours after beginning treatment, with no effect on STAT3 serine phosphorylation (Figure 6B). Exogenous treatment of Ly3 and DHL2 cells with anti–IL-10–neutralizing Ab decreased constitutive activated STAT3 (tyrosine) phosphorylation (Figure 6C), confirming that in pSTAT3+ DLBCL cells, JAK2-pathway activation is dependent on autocrine IL-10 secretion. To determine the effect of the JAK2 inhibitor TG on tyrosine activity, we immunoprecipitated endogenous JAK2, JAK1, and STAT3 from DHL2 cells and blotted with phosphotyrosine Ab. TG treatment at 2 and 4μM resulted in a reduction of the phosphotyrosine level of JAK2 and STAT3, but not JAK1 (Figure 6D-F). These results were only performed in cell lines because of limited availability of fresh DLBCL patient samples.
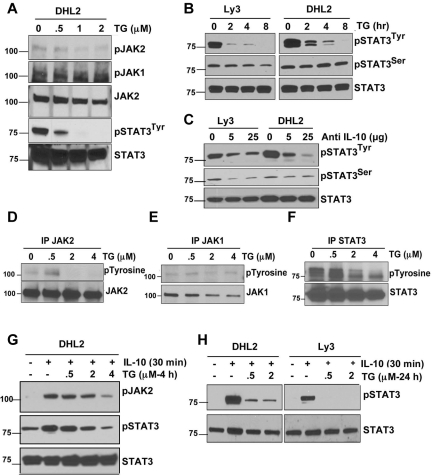
Figure 6.
Effect of JAK2 inhibition on JAK/STAT signaling. (A) DHL2 cells were treated with various doses of the JAK2 inhibitor TG for 24 hours and phosphorylation of JAK1, JAK2, and STAT3 was analyzed. (B) Ly3 and DHL2 cells were treated with a 4μM concentration of TG for 2, 4, and 8 hours and phosphorylation of STAT3 was performed by Western blotting. (C) Ly3 and DHL2 cells were treated with neutralizing IL-10 Ab for 24 hours and STAT3 phosphorylation was detected. (D-F) DHL2 cells were exposed to various concentrations of TG for 8 hours and then lysed. Cell lysates were then immunoprecipitated with anti-JAK1, anti-JAK2, or anti-STAT3 and blots were probed with phosphotyrosine Ab. DHL2 (G) and DHL2 and LY3 (H) cells were pretreated with 100 ng/mL of rIL-10 for 30 minutes and then treated with various concentrations of TG for 4 hours (G) and 24 hours (H), and JAK2 and STAT3 phosphorylation was analyzed by Western blotting.
We also determined whether JAK2 blockade with the JAK2 inhibitor TG could abrogate IL-10–induced JAK2 and STAT3 activation. We pretreated DHL2 cells with various concentrations of TG for 4 hours and then added IL-10 for 30 minutes. TG induced a dose-dependent inhibition in IL-10–induced pJAK2 and pSTAT3 (tyrosine) activation (Figure 6G). Longer exposure (24 hours) to TG had a much more inhibitory effect on IL-10–induced STAT3 phosphorylation. Even 0.5μM TG was able to dramatically inhibit IL-10–induced STAT3 phosphorylation in DLBCL cells (Figure 6H).
JAK2 inhibition suppresses the growth of DLBCL cells with constitutive JAK2 activity
STAT3 transmits cell-survival signals and protects cells from apoptosis.19 We investigated whether decreasing JAK2 or STAT3 phosphorylation by the JAK2 inhibitor TG (Figure 6A-B) is associated with apoptosis of the cells. We treated a series of pSTAT3+ and pSTAT3− cell lines with increasing doses of TG for 48 hours and assessed survival. TG inhibited the survival of all 3 pJAK2+ cell lines (Figure 7A). The IC50 value for TG in these cell lines was between 2 and 3μM. A limited effect of TG on pJAK2− DLBCL cell survival was observed (Figure 7B). These results indicate that survival of pSTAT3+ DLBCL cells is indeed dependent on JAK2-pathway activation.
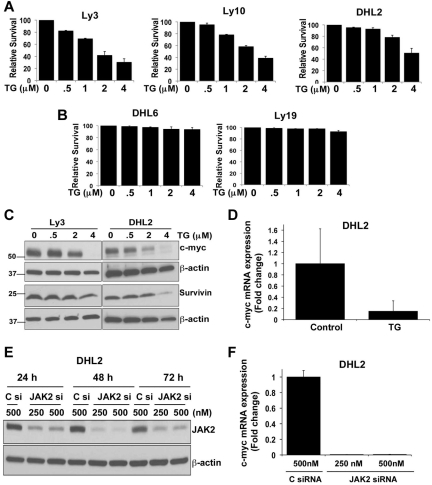
Figure 7.
Effect of the JAK2 inhibitor TG on DLBCL-cell survival. (A-B) Ly3, Ly10, DHL2, DHL6, and Ly19 DLBCL cells were treated with various doses of the JAK2 inhibitor TG for 48 hours. Survival was then assessed by flow cytometry. Bars represent means ± SD from 3 experiments. (C) Ly3 and DHL2 cells were exposed to various concentrations of TG for 24 hours and expression of c-myc and survivin was analyzed by Western blotting. (D) c-myc mRNA expression was analyzed by qRT-PCR (as described in “qRT-PCR”) in TG-treated DHL2 cells. Bars represent means ± SD from 3 experiments. (E) siRNA (250 and 500nm) targeting JAK2 and a nonsilencing control siRNA were transfected as described in “siRNA transfection” and DHL2 cells were harvested 24, 48, and 72 hours later. Cell lysates were immunoblotted with JAK2 and β-actin Abs. (F) c-myc expression in JAK2 siRNA- (250 and 500nM) and control siRNA (500nM)–transfected DHL2 cells was analyzed at 72 hours by qRT-PCR.
JAK2 inhibition inhibits c-myc expression
To better understand the mechanisms of JAK2 inhibitor–induced apoptosis, we sought to determine whether JAK2 signaling regulates downstream apoptotic targets in DLBCL. Activation of STAT3 signaling up-regulates the expression of various genes involved in cell survival and proliferation, such as bcl-xl, bcl-2, mcl-1, and c-myc.20–24 Immunoblotting failed to demonstrate TG-induced down-regulation of mcl-1 and bcl-XL proteins in pSTAT3+ DLBCL cells (supplemental Figure 3). TG induced a dose-dependent decrease in c-myc protein in both Ly3 and DHL2 cells (Figure 7C). The most potent inhibition was seen at a 4μM concentration, which completely abrogated c-myc protein expression. Recent studies have shown clinical correlation of STAT3 and the survivin protein.25,26 However, we saw little inhibitory effect of TG on survivin protein levels in DLBCL cells (Figure 7C). qRT-PCR demonstrated that TG decreases c-myc–encoding mRNA in DHL2 cells (Figure 7D), with no effect on survivin mRNA (data not shown).
To confirm the c-myc dependency on JAK2 signaling in DLBCL cells, siRNA directed against JAK2 was tested to determine whether it could inhibit c-myc expression in DHL2 cells. JAK2 siRNA was able to inhibit JAK2 protein in 24 hours and this effect was sustained for up to 72 hours (Figure 7E). We were able to achieve a more than 90% knockdown of the JAK2 protein at 48 and 72 hours. Reduction of JAK2 expression by siRNA almost completely inhibited c-myc mRNA at 72 hours compared with a nonsilencing control siRNA (Figure 7F). Similar results were found when we transfected another DLBCL line, Ly3, with JAK2 siRNA (data not shown).
Discussion
DLBCL is the most common form of NHL and has variable clinical presentations and responses to standard rituximab-based immunochemotherapy regimens. Although the disease is curable in most patients, approximately 40% of patients still die from their disease.27,28 To further improve the outcome of DLBCL, current research is focusing on the genetic types of DLBCL29 and the signal pathways used by DLBCL cells. For example, the identification of NF-κB as an important signaling pathway in DLBCL led to studies of bortezomib in combination with rituximab plus CHOP for new, untreated DLBCL.30 Others have demonstrated that the JAK2 and STAT3 pathways play an important role in hematologic malignancies.31,32 With the advent of several new oral JAK2 pathway inhibitors for MPN,12,33 it is important to investigate this pathway in lymphoma. In the present study, we demonstrate that JAK2 is constitutively activated in a subset of patient DLBCL tumors. In studies of the mechanism of this activation, JAK2 mRNA data showed that the levels of JAK2 transcripts were not significantly different between pJAK2+ and pJAK2− DLBCL cells. Because no gain-of-function (V617F) JAK2 mutants have been found in Hodgkin lymphoma, primary mediastinal lymphoma,34 or B-cell lymphoma,35 it is important to investigate other factors responsible for JAK2 activation in DLBCL cells.
Cytokines play important roles in the pathogenesis of various cancers via autocrine and/or paracrine mechanisms.36 Cytokine signaling is primarily mediated by tyrosine kinases such as JAKs and their downstream transcription factors, STATs. Using pretreatment sera from patients participating in a recently reported clinical trial for new, untreated DLBCL, we found that the JAK/STAT pathway–relevant cytokines IL-10, IL-6, EGF, and IL-2 were indeed elevated in some DLBCL patients compared with controls. Of these 4 cytokines, we focused on IL-10 because it was the only one that stimulated the JAK2 pathway in DLBCL cells in vitro (Figure 1B). In addition, several studies have provided evidence for the role of human IL-10 in the pathogenesis of malignant B-cell neoplasms.37,38 Increased serum IL-10 levels have been described in patients with untreated CLL and DLBCL and were correlated with an inferior survival.39,40 We have also demonstrated that IL-10 levels were significantly higher in a subset of DLBCL patients, were correlated with other known adverse clinical features, and that patients with elevated IL-10 had a shorter EFS (P = .01). Compared with previous studies, patients in the present study were uniformly treated with a rituximab chemoimmunotherapy program. Further studies of serum IL-10 are needed to define its role as a prognostic and predictive factor in future phase 3 trials in DLBCL.
In vitro and in vivo studies with recombinant cytokine and neutralizing Abs revealed pleiotropic effects of IL-10 on B and T cells.41,42 IL-10 can serve a key function in the induction of tolerance18,43 and therefore may contribute to the DLBCL pathogenesis by directly enhancing the survival and proliferation of tumor cells and by impairing host immune responses.
In human T cells and monocytes, the IL-10/IL-10R complex has been shown to induce tyrosine phosphorylation of TYK2 and JAK1 kinases with subsequent activation of STAT1 and STAT3.44 In DLBCL cells, we found that IL-10 is a major factor for tyrosine phosphorylation of JAK2 and STAT3 but not of JAK1, STAT1, and STAT5. In contrast, IL-10 showed no effect on the activity of ERK or NF-κB, suggesting that IL-10 specifically activates the JAK/STAT pathway in DLBCL cells. Furthermore, we have shown herein that DLBCL tumors with high levels of pJAK2 also secreted more IL-10 than the cells from tumors with no activation of pJAK2. Because cytokine-mediated JAK2 activation induces phosphorylation of STAT1, STAT3, and STAT5,45 we analyzed the phosphorylation of these molecules in pSTAT3+ and pSTAT3− DLBCL cells after IL-10 stimulation. STAT3 tyrosine was significantly activated by IL-10, whereas no phosphorylation of STAT3 serine and STAT1 or STAT5 was detected in pSTAT3+ cells. In pSTAT3− cells, neither STAT3 nor STAT1 was activated by IL-10 treatment. Therefore, the pattern of JAK/STAT signaling on IL-10 stimulation appears to be different between pSTAT3+ and pSTAT3− DLBCL cells. Blockade of IL-10 signaling by IL-10–neutralizing Ab and the JAK2-specific inhibitor TG inhibited constitutive and IL-10–induced JAK2 and STAT3 signaling. This finding is consistent with the results of Alas et al,46 who showed that a different STAT3 inhibitor (piceatannol) was able to inhibit constitutive STAT3 activity in an IL-10–dependent NHL cell line (2F7).
The discovery of the JAK2 V617F mutation in MPN has resulted in the development of several JAK2 inhibitors.33 In the present study, we used TG101348, a compound now known as SARS302503 that is in advanced clinical trials for MPN and demonstrated that it can completely block the phosphorylation of JAK2 and STAT3 in pJAK2+ DLBCL cells. Moreover, we have demonstrated that TG selectively inhibits the survival of pJAK2+ cells (LD50 = 2-3μM). These in vitro doses of TG are achievable in vivo based on pharmacokinetic studies recently reported in MPN patients.12 We have also demonstrated that TG inhibited IL-10 secretion and decreased c-myc expression. This is an important finding because c-myc is a resistance factor in DLBCL47 and agents that down-regulate it are needed in the clinical setting. Our data also demonstrated that the JAK2 inhibitor TG triggered apoptosis without having any effect on Bcl2 family members. This differs from studies by Alas et al with the anti-CD20 Ab rituximab, in which IL-10 secretion and bcl2 expression were inhibited in the 2F7 and 10C9 lymphoma cell lines.48 This difference is likely because of a different mechanism of action of rituximab compared with TG and different lymphoma cell lines.
Although our cell line data support the hypothesis that high IL-10 levels are correlated with pJAK2 expression, we were unable to test this in DLBCL patient tumors in this cohort because of the limited paraffin-embedded tissue available. A correlation between pJAK2 and serum IL-10 will best be found in prospective DLBCL studies in which these biomarkers can be evaluated as response and survival factors.
Supplementary Material
Acknowledgments
This work was supported in part by a Career Development Award from the University of Iowa /Mayo Clinic Lymphoma Specialized Program of Research Excellence (P50 CA097274 to M.G.); the National Institutes of Health (R01CA127433 to T.E.W.); the Goodwin Foundation Pilot award (to M.G.); the Predolin Foundation for Hematology Research; and the North Central Cancer Treatment Group (CA25224 and Biospecimen Resource grant CA-114740).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.G. designed the research, analyzed and interpreted the data, produced the figures, wrote the manuscript, and provided the funding; M.G., J.J.H., M.S., L.W., G.H., S.Z., and A.D. performed the experiments; M.M. performed the statistical analysis; and T.E.W. provided the clinical samples, wrote the manuscript, and provided the funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mamta Gupta, PhD, Assistant Professor of Medicine, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gupta.mamta@mayo.edu.
References
- 1.Levy DE, Inghirami G. STAT3: a multifaceted oncogene. Proc Natl Acad Sci U S A. 2006;103(27):10151–10152. doi: 10.1073/pnas.0604042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu LF, Zhu YB, Qiao MM, Zhong J, Tu SP, Wu YL. Constitutive activation and clinical significance of Stat3 in human gastric cancer tissues and cell lines [Article in Chinese]. Zhonghua Yi Xue Za Zhi. 2004;84(24):2064–2069. [PubMed] [Google Scholar]
- 3.Berishaj M, Gao SP, Ahmed S, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9(3):R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao H, Ward PA. STAT3 and suppressor of cytokine signaling 3: potential targets in lung inflammatory responses. Expert Opin Ther Targets. 2007;11(7):869–880. doi: 10.1517/14728222.11.7.869. [DOI] [PubMed] [Google Scholar]
- 5.Gao B, Shen X, Kunos G, et al. Constitutive activation of JAK-STAT3 signaling by BRCA1 in human prostate cancer cells. FEBS Lett. 2001;488(3):179–184. doi: 10.1016/s0014-5793(00)02430-3. [DOI] [PubMed] [Google Scholar]
- 6.Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood. 2008;111(7):3701–3713. doi: 10.1182/blood-2007-09-111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catlett-Falcone R, Dalton WS, Jove R. STAT proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr Opin Oncol. 1999;11(6):490–496. doi: 10.1097/00001622-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 12.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29(7):789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micallef IN, Maurer MJ, Wiseman GA, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118(15):4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 16.Gupta M, Ansell SM, Novak AJ, Kumar S, Kaufmann SH, Witzig TE. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114(14):2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta M, Dillon SR, Ziesmer SC, et al. A proliferation-inducing ligand mediates follicular lymphoma B-cell proliferation and cyclin D1 expression through phosphatidylinositol 3-kinase-regulated mammalian target of rapamycin activation. Blood. 2009;113(21):5206–5216. doi: 10.1182/blood-2008-09-179762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 19.Barré B, Vigneron A, Perkins N, Roninson IB, Gamelin E, Coqueret O. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13(1):4–11. doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Zushi S, Shinomura Y, Kiyohara T, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78(3):326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann S, Schuster I, Walch A, et al. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60(2):173–179. doi: 10.1136/jcp.2005.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke WM, Jin X, Lin HJ, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20(55):7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 23.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 24.Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21(50):7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- 25.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101(4):1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 26.Schlette EJ, Medeiros LJ, Goy A, Lai R, Rassidakis GZ. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin Oncol. 2004;22(9):1682–1688. doi: 10.1200/JCO.2004.10.172. [DOI] [PubMed] [Google Scholar]
- 27.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 28.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 29.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B cell lymphoma. Blood. 2009;113(24):6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol. 2006;71(6):713–721. doi: 10.1016/j.bcp.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Ferrajoli A, Faderl S, Ravandi F, Estrov Z. The JAK-STAT pathway: a therapeutic target in hematological malignancies. Curr Cancer Drug Targets. 2006;6(8):671–679. doi: 10.2174/156800906779010227. [DOI] [PubMed] [Google Scholar]
- 33.Verstovsek S. Janus-activated kinase 2 inhibitors: a new era of targeted therapies providing significant clinical benefit for Philadelphia chromosome-negative myeloproliferative neoplasms. J Clin Oncol. 2011;29(7):781–783. doi: 10.1200/JCO.2010.33.4508. [DOI] [PubMed] [Google Scholar]
- 34.Melzner I, Weniger MA, Menz CK, Moller P. Absence of the JAK2 V617F activating mutation in classical Hodgkin lymphoma and primary mediastinal B-cell lymphoma. Leukemia. 2006;20(1):157–158. doi: 10.1038/sj.leu.2404036. [DOI] [PubMed] [Google Scholar]
- 35.Steensma DP, McClure RF, Karp JE, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20(6):971–978. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19(4):429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nacinović-Duletić A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol. 2008;30(3):230–239. doi: 10.1111/j.1751-553X.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 38.Lech-Maranda E, Baseggio L, Bienvenu J, et al. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood. 2004;103(9):3529–3534. doi: 10.1182/blood-2003-06-1850. [DOI] [PubMed] [Google Scholar]
- 39.Blay JY, Burdin N, Rousset F, et al. Serum interleukin-10 in non-Hodgkin's lymphoma: a prognostic factor. Blood. 1993;82(7):2169–2174. [PubMed] [Google Scholar]
- 40.Fayad L, Keating MJ, Reuben JM, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001;97(1):256–263. doi: 10.1182/blood.v97.1.256. [DOI] [PubMed] [Google Scholar]
- 41.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145(12):4167–4173. [PubMed] [Google Scholar]
- 43.Villagra A, Cheng F, Wang HW, et al. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009;10(1):92–100. doi: 10.1038/ni.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155(3):1079–1090. [PubMed] [Google Scholar]
- 45.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26(47):6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 46.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer. Res. 2003;9(1):316–326. [PubMed] [Google Scholar]
- 47.Slack GW, Gascoyne RD. MYC and aggressive B-cell lymphomas. Adv Anat Pathol. 2011;18(3):219–228. doi: 10.1097/PAP.0b013e3182169948. [DOI] [PubMed] [Google Scholar]
- 48.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's lymphoma to apoptosis. Clin Cancer Res. 2001;7(3):709–723. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.