Abstract
Interaction between the cytoplasmic domain of GPIbα with its cytoskeletal binding partner, filamin, is a major determinant of platelet size, and deficiency of either protein results in macrothrombocytopenia. To clarify the mechanism by which GPIbα-filamin interactions regulate platelet production, we manipulated the expression levels of filamin and GPIb in cultured embryonic stem cells (ESCs) that were subsequently differentiated into platelets. Knocking down filamins A and B resulted in the production of ESC-derived proplatelets with abnormally large swellings and proplatelet shafts that generated giant platelets in culture. Large platelets could also be generated by overexpressing GPIbα in ESCs, or by overexpressing in vivo a transgene encoding a chimeric protein containing the cytoplasmic domain of GPIbα. To identify the mechanism by which the GPIb:filamin ratio regulates platelet size, we manipulated filamin and GPIbα levels in HEK293T cells and examined the effects of overexpressing either protein on their ability to traffic to the cell periphery. Accumulation of either protein within the endoplasmic reticulum resulted in trapping of the other. Taken together, these data demonstrate that coordinated expression of GPIbα and filamin is required for efficient trafficking of either protein to the cell surface, and for production of normal-sized platelets.
Introduction
Filamin A is a large dimeric 280 kDa multidomain protein that exists as a major component of the membrane skeleton of most cells, and is widely expressed in species ranging from slime molds to humans (for a recent review, see Zhou et al1). Originally described as an actin-binding protein tightly associated with actin-myosin filaments,2,3 filamin A also functions prominently as a scaffolding protein, binding a reported 70+ cytosolic proteins, including transcription factors, GTPase-related proteins, and kinases.1 Encoded by the X chromosome in humans, filamin A is closely related to 2 other members of the filamin family—filamins B and C, the former of which, similar to filamin A, is ubiquitously expressed, and the latter of which is restricted to expression in muscle. In many cells, filamin A is brought close to the inner face of the plasma membrane by virtue of its association with transmembrane receptors of the integrin or Ig superfamily, where it is consequently well-positioned to mediate both signal and force transmission. In blood platelets, filamin A associates predominantly with the cytoplasmic domain of GPIbα4,5—a Type I transmembrane protein that serves, together with GPIbβ, GPV, and GPIX, as a major subunit of the GPIb complex—the platelet adhesion receptor for collagen-bound von Willebrand factor (VWF).6 The nature of the association between filamin A and GPIbα has been extensively studied,7–14 and is currently thought to mainly involve amino acids 556-577 within the central region of the cytoplasmic domain of GPIbα and Ig-like repeat number 17 in the C-terminal half of filamin A.15
The association of filamin A with the GPIb complex has been shown to be important for a number of cellular functions. Early on, filamin A/GPIbα interactions are required for efficient biosynthesis and processing of the GPIb complex, as suggested by the finding that its enforced expression on the surface of melanoma16 and Chinese hamster ovary (CHO)14 cells is diminished in the absence of filamin A, and by the recent observation that GPIb surface expression is significantly decreased in filamin A–deficient murine platelets.17,18 The mechanism by which filamin A promotes cell surface expression of the GPIb complex is not completely understood, but is thought to involve an interaction between filamin and nascent GPIbα within the endoplasmic reticulum (ER) of the cell, which facilitates trafficking of the GPIb complex to the cell surface.14 Whether GPIb conversely affects the cell surface distribution of filamin is not known. Once on the cell surface, GPIb continues to require filamin-mediated attachment to the membrane skeleton to anchor CHO cells11,19 and platelets17 to immobilized VWF under conditions of shear.
In addition to its role in stabilizing GPIb trafficking and function, filamin appears to play a prominent role in regulating platelet size. For example, the macrothrombocytopenia seen in patients with Bernard-Soulier syndrome (BSS)—an inherited bleeding disorder caused by absence or dysfunction of one or more subunits that comprise the GPIb complex—has long been attributed to a failure to anchor the plasma membrane to the underlying membrane skeleton during the process of platelet formation,20 a notion supported by the marked deformability of the plasma membrane of BSS platelets,21 and by the observation that mice genetically engineered to lack either GPIbα22 or filamin A17 both form giant platelets. That the macrothrombocytopenia in BSS mice and humans is primarily because of loss of filamin/GPIbα interactions is supported by the finding that platelets from a BSS patient with a mutation that eliminates only the transmembrane and cytoplasmic domain of GPIbα are as large as those from BSS patients lacking the entire GPIb complex,23 and from the observation that transgenic expression in GPIbα-deficient mice of a chimeric protein consisting of only the transmembrane and cytoplasmic domains of GPIbα fused to the extracellular domain of the IL-4 receptor is able to partially rescue the macrothrombocytopenia, conferring a 40%-50% correction in platelet size.24 Interestingly, platelet size was found to correlate directly, rather than inversely, with the level of expression of the chimeric IL-4Rα/GPIbα protein (eg, transgenic mouse lines expressing more of the transgene produced larger, rather than smaller, platelets).24 The reason increased expression of IL-4Rα/GPIbα should lead to the formation of larger platelets during the process of thrombopoiesis was not determined.
In the present study, we sought to further examine the role of filamin as a determinant of platelet size and production during the process of platelet-poiesis. For this, we used a newly developed model in which the expression levels of filamin A, filamin B, and GPIbα could be manipulated in embryonic stem cells that were subsequently differentiated into embryoid bodies, and then into megakaryocytes, proplatelets, and platelets. We found that, in addition to contributing to megakaryocytopoiesis, filamin plays an important role in the process by which platelets are derived from their proplatelet precursors, as filamin-deprived platelet precursors give rise to distended proplatelets that produce giant platelets. We also report that, in addition to the known contribution of filamin in directing GPIbα to the plasma membrane, balanced expression of GPIbα is similarly required to chaperone filamin to the inner face of the plasma membrane, where it can become incorporated into the membrane skeleton and regulate the deformability of the platelet membrane during thrombogenesis. Taken together, these data suggest that, in addition to controlling the absolute levels of expression of GPIbα and filamin, cells must coordinately regulate the ratio at which these 2 proteins become expressed to achieve normal platelet biogenesis.
Methods
Antibodies
Rabbit polyclonal antibody against mouse filamin A (FlnA) was raised by immunizing rabbits with a recombinant, dimerized domain of mouse FlnA. The mouse monoclonal antibody against FlnB (clone D-6), and a mouse monoclonal antibody recognizing the human IL-4 receptor (MAB230; R&D Systems), are all commercially available.
Vector construction
CMV promoter-EGFP-short hairpin RNA (shRNA) vector. The target sequence for mouse FlnA and mouse FlnB (tac agg caa tat ggt gaa gaa g) was converted into shRNAs based on human pri-miR30 precursor using RNAi Codex (http://cancan.cshl.edu/cgi-bin/Codex/Tools.cgi). A Not/XhoI fragment of a synthesized mini-gene having this shRNA sequence, and a HindIII/NotI fragment of the pEGFP-N1 plasmid vector (Invitrogen) were cloned into pcDNA3.1/Zeo(+) (Invitrogen). hTPO expression vector: full-length cDNA obtained from Open Biosystems was cloned into pcDNA3.1/Zeo 3(+) using its EcoRI site. Expression vectors encoding the human GPIb complex and human filamin A: cDNAs encoding GPIbα, GPIbβ, and GPIX24,25 were subcloned into the eukaryotic expression vector pcDNA3.1/Zeo(+) (Invitrogen), as previously described.24,25 Vectors encoding GPIbα-EGFP (carboxyl-terminal of GPIbα fused to EGFP) and FlnA-DsRed (carboxyl-terminus of filamin A fused to DsRed mono N1) were generated by PCR mutagenesis. cDNA for human FlnA subcloned into pREP4 (Invitrogen), was provided by Y. Ohta (Kitasato University, Kanagawa, Japan).
Generation of transgenic mice expressing an IL4R-GPIbα chimeric protein
IL4R-Ibα Tg33 and Tg51 transgenic mice expressing the extracellular domain of the IL-4 receptor fused to the transmembrane and cytoplasmic domain of GPIbα have been previously described.24 All mice were produced or bred onto a GPIbα-null background.22
Blood cell analysis
Blood samples were collected from the retro-orbital venous plexus using heparin-coated capillary tubes. Blood cell parameters including platelet size, platelet number, and the mean platelet volume (MPV) were analyzed using a Scil Vet abc Counter (Scil Animal Care Company).
Cell culture
Murine embryonic stem cells: the ES cell line E14tg2a (kindly provided by Dr Hitoshi Niwa, Center for Developmental Biology, RIKEN, Kobe, Japan), was maintained on plates coated with 0.1% gelatin and in Glasgow Minimum Essential Medium (GMEM; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS), 100μM nonessential amino acids, 1mM sodium pyruvate, 2mM glutamate, 1% penicillin streptomycin, 100μM β-mercaptoethanol plus 1000−1 U/mL ESGRO (Millipore). Stromal cells: hTERT stromal cells transduced with the human telomerase catalytic subunit (kindly provided by Dr Hirofumi Hamada, Department of Molecular Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan) were grown in long-term culture (LTC) medium containing minimum essential medium-α supplemented with 12.5% FBS (Invitrogen), 12.5% horse serum (Invitrogen), 1 × 10−6M hydrocortisone (Sigma-Aldrich), and 1 × 1−4M β-mercaptoethanol (Sigma-Aldrich).
Establishment of stable cell lines
FlnAB knockdown (FlnABLow ES), hTPO-producing hTERT stromal cells (hTERT-TPO stromal cells), FlnA overexpressing HEK293T cells (OXF), and FlnA-DsRed expressing HEK293T cells were all established by transfection in 6-well tissue culture plates using Lipofectamine LTX with PLUS reagent (Invitrogen). The protocol was performed as per the supplier's directions. Cells were harvested 2 days after transfection, reseeded on 10-cm plates, and cultured for an additional 6 to 9 days in the presence of antibiotics, after which time single clones were removed by micropipette aspiration.
Differentiation of embryonic stem cells
Embryoid body (EB) formation: 2.5 × 105 embryonic stem cells (ESCs) were placed in ultra-low attachment 6-well plates (Corning) containing Iscove modified Dulbecco medium (IMDM) supplemented with a cocktail of 300 μg/mL human transferrin, 0.45mM monothioglycerol, 50 μg/mL ascorbic acid, and penicillin-streptomycin/l-glutamine solution (Invitrogen) as previously described.26 Megakaryocyte-platelet generation: day 1: hTPO-TERT stromal cells (6-9 × 104) were seeded into 6-well dishes. Day 0: 6 (or 8)–day-old EBs were dissociated with 0.25% trypsin/EDTA, and incubated with PE-labeled anti–mouse CD41 antibody (MWReg 30; BD Pharmingen). CD41-positive cells were isolated using anti-PE antibody-conjugated magnetic immunobeads (Miltenyi Biotec). CD41-positive cells (3 × 104) were suspended in IMDM containing 15% FBS with 40 ng/mL hTPO (PeproTech) and transferred to hTPO-TERT stromal cells. Alternatively, 3 × 105 CD41-positive cells were plated per well in 12-well dishes in the presence of 50 ng/mL hTPO. Day 6: analyzed megakaryocytes and platelets.
Immunoprecipitation analysis
Platelets (5 × 108) were suspended in 500 μL modified Tyrode buffer, and then mixed with equal volumes of solubilization buffer (2% Triton X-100, 0.1 mol Tris [tris(hydroxymethyl)aminomethane], 0.01 mol ethyleneglycoltetraacetic acid [EGTA], 0.15 mol NaCl, and 1 tablet of cOmplete ULTRA (Roche) in 10 mL lysis buffer [pH 7.4] and 20μM MG132 (Calbiochem). The lysates (1 mL) were mixed with 50 μL (50% vol/vol) protein G beads (Pierce) and 5μg of the polyclonal Ab against FlnA or monoclonal Ab against FlnA PM6/317 (Sant Cruz) for 90 minutes. The beads were then washed 3 times in the same solubilization buffer containing 1% Triton X-100.
Immunoblotting
Cultured cells or washed platelets were resuspended in phosphate-buffered saline (PBS), and then lysed with equal volumes of T-SDS buffer (50mM Tris-Cl [pH 7.4], 2% SDS) in the presence or absence of β-mercaptoethanol. Platelet lysates or cell lysates were analyzed by electrophoresis in 7.5% or 10% SDS-polyacrylamide gels. The separated proteins were then electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Millipore), blocked with EzBlock (Atto), and then probed with the antibodies previously described. Blots were washed 3 times, incubated with a horseradish peroxidase (HRP)–conjugated secondary antibody, washed again 3 times, developed with Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific), and analyzed using a Kodak 1D imaging system (Kodak Scientific Imaging System).
Flow cytometry (FACS)
For analysis of ESC-derived proplatelets, the cells were fixed with 1% paraformaldehyde in PBS for 2 hours at 4°C, incubated with rat monoclonal antibody against GPIbβ (Xia.C3, Emfret Analytics GmbH & Co KG) or control rat IgG2a for 20 minutes, followed by Alexa Fluor 647–labeled goat anti–rat IgG. The platelet population was identified by GPIbβ expression or CD41 (αIIb) expression and forward scatter (FSC). The cutoff value for FSC was determined by the size of normal C57BL/6J mouse platelets. For intracellular staining of FlnA and F-actin, whole blood samples were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) after incubation with FITC labeled CD41 antibody (MWReg30), and then incubated with biotinylated rabbit polyclonal antibody against mouse FlnA, followed by Alexa Fluor 647–labeled streptavidin (Invitrogen). F-actin was quantified using Alexa Fluor 647–labeled phalloidin (Invitrogen).
Imaging
A Nikon Eclipse TE200 microscope equipped with a FITC filter, a Cool Snap ES camera (Roper Scientific Photometrics), and Metamorph 7.6 acquisition software (Universal Imaging) were used to acquire images of ESCs, EBs, megakaryocytes, and proplatelets. Immunofluorescent detection was performed by confocal microscopy using an Olympus FV1000-MPE Multiphoton Microscope (Olympus) using a LUMPlanFl 40x W/IR water immersion objective (Olympus).
Statistical analysis
Data are presented as mean ± SD as indicated. Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software).
Results
Generation of embryonic stem cell lines with reduced expression of filamins A and B
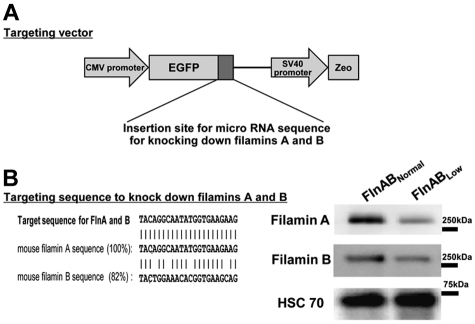
To analyze the function of filamin in vitro megakaryocytopoiesis and platelet production, we constructed a short hairpin RNA (shRNA) vector that targets both filamins A and B under the control of the human cytomegalovirus (CMV) immediate-early promoter and enhancer (Figure 1A). As shown in Figure 1B, the targeting sequence was 100% identical to FlnA and 82% identical to FlnB, and was effective in generating Zeocin resistant, GFP-expressing clones, termed FlnABLow, having reduced expression of both filamin isoforms. These Flns targeting vectors also expressed GFP as a tracer, which could be used to monitor knockdown efficiency. More than 90% of FlnABLow ESCs expressed GFP when first generated (not shown), but lost expression of both GFP and the targeting shRNA, a property that we exploited below to determine the effects of filamin expression in an internally controlled manner.
Figure 1.
Establishing embryonic stem cell lines with reduced expression of filamins A and B. (A) Schematic diagram of the targeting vector used to generate ESCs with reduced expression of both FlnA and FlnB. The vector is under the control of the CMV immediate early promoter, coexpresses GFP as a marker of shRNA expression, and renders cells resistant to Zeocin, allowing for enrichment of transfected cells. (B) shRNA sequence used to knock down both filamins A and B, with corresponding immunoblot of the ES cell line termed FlnABLow. Note that the expression of both filamin isoforms is reduced 70%-80%.
Filamins are required for efficient megakaryocytopoiesis
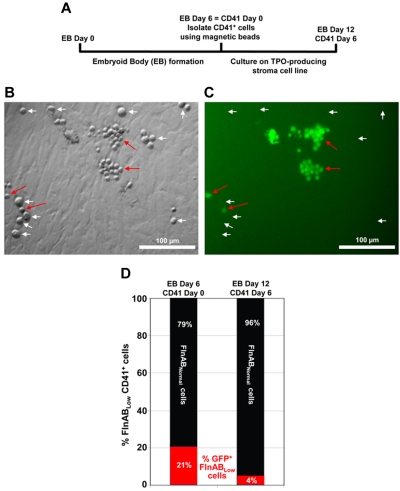
Nishiki et al recently developed an efficient method for generating platelets from ESCs that involves culturing CD41, c-kit double-positive hematopoietic progenitor cells in the presence of OP9 stroma cells to generate hematopoietic progenitor cells.26–28 Transduction of the telomerase gene into bone marrow stroma cells to generate hTERT stroma cells was later found to improve the efficiency of stromal cell help,29 and thrombopoietin has been known not only to be required to drive differentiation down the megakaryocyte/platelet lineage but also to support self-replication of hematopoietic stem cells.30 We combined these features into a newly established TPO-producing, telomerase gene-transduced human stromal cell line, designated hTERT-TPO. As shown schematically in Figure 2A, CD41-positive cells were isolated from embryoid bodies that had been derived from FlnABLow embryonicstem cells and cultured in the presence of TERT-TPO stroma feeder cells for an additional 6 days. Many of these cells lose expression of the GFP-Fln shRNA construct over time, resulting in the appearance of 2 distinct populations of cells by day 4 (Figure 2A-B): (1) numerous large GFP-negative cells that express near-normal levels of these 2 filamin isoforms, and (2) fewer small cells that have remained GFP-positive and FlnABLow. Quantitation of these 2 cell populations (Figure 2D) revealed a 79:21% ratio of GFP-negative:GFP-positive cells at day 0 that further evolved into a 96:4% ratio by day 6, strongly suggesting that expression of filamin confers a maturational, proliferative, and differentiation advantage over those cells that express only low levels of filamins A and B. These data suggest that failure to express filamin results in a delay in megakaryocytopoiesis.
Figure 2.
Filamins are required for efficient megakaryocytopoiesis. (A) Schematic diagram of the protocol used to generate megakaryocytes from cultured ESCs. ESCs were differentiated into EBs using standard procedures. The resulting EBs were cultured for 6 days, at which time CD41-positive cells were selected using magnetic beads. CD41-positive EB-derived cells were then cultured for an additional 6 days in the presence of hTERT-TPO stroma cells, which secrete thrombopoietin and help drive differentiation into specific megakaryocyte precursors. (B-C) DIC and fluorescent images of EB-derived CD41+ cells cultured in the presence of TPO-producing stromal cells at day 4. At this time point, 2 distinct populations of cells exist: large, GFP-negative megakaryocyte precursors that have lost expression of the shRNA that keep filamin levels low (white arrows), and much smaller, GFP-positive cells that retain the filamin A/B shRNA (red arrows). An image was chosen that showed relatively equal numbers of both cell types. However, in the total cell culture, there were many more large, filamin-positive GFP-negative megakaryocyte precursors than there were small, filamin-negative, GFP-positive immature cells (quantitation shown in panel C). (D) Megakaryocyte differentiation in this population was confirmed by GPIbβ antibody staining (data not shown). These data suggest that failure to express filamin results in a delay of megakaryocytopoiesis.
Filamins are required for generation of normal size platelets
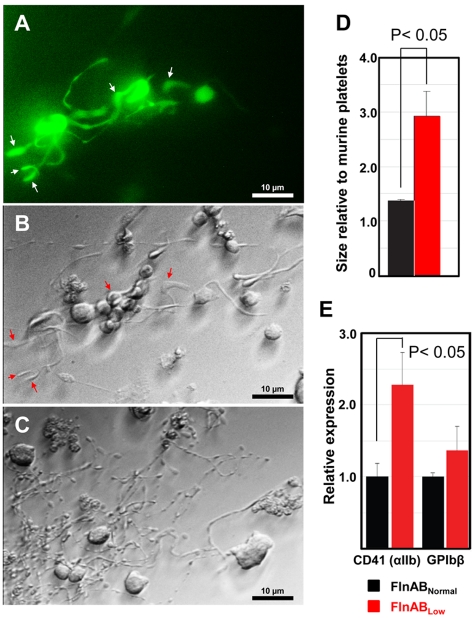
In addition to a role in supporting megakaryocyte differentiation (Figure 2), filamin A has been shown to be important in regulating platelet size in vivo.17 To further examine the importance of filamins A and B in thrombopoiesis, we analyzed the size of the proplatelet projections and ensuing platelets derived from FlnABLow EB-derived cells that had been allowed to mature in the presence of hTERT-TPO stroma cells for 6 days. As shown in Figure 3A and B, GFP-positive FlnABLow proplatelet projections exhibited a distinctly abnormal morphology, characterized by enlarged swellings and thick shafts. In contrast, most of the GFP-negative proplatelets (Figure 3C) had well-extended, noticeably thinner proplatelet shafts decorated with smaller preplatelet swellings. We also evaluated at Day 6 the size of the platelets produced from EB-derived primitive FlnABLow megakaryocytes, which had either become GFP/shRNA-negative (FlnABNormal) or retained GFP and the shRNA to suppress filamin A and B synthesis (FlnABLow). As shown in Figure 3D, FACS analysis of GPIbβ-positive cells revealed that proplatelets that had remained GFP-positive produced platelets 2 to 3 times larger than those derived from FlnABNormal cells. Expression of CD41was proportionally increased, whereas GPIbβ expression, which accurately reports expression of the GPIb complex,26 was proportionally decreased in expression (Figure 3E), consistent with observations made of FlnA-null megakaryocytes and platelets.18 Taken together, these data demonstrate that filamins A and B are required for efficient megakaryocytopoiesis, for efficient trafficking of the GPIb complex to the cell surface, and for production of normal sized platelets.
Figure 3.
Filamins are required for the generation of normal size platelets. (A-C) microscopic images of FlnABLow and FlnNormal ESC/EB-derived cells during the process of proplatelet production. Panel A contains a fluorescent image of GFP-positive, FlnABLow proplatelets, with its corresponding phase-contrast image in panel B. Arrows depict the typical large swellings and shafts produced in the absence of filamin. Compare these structures with those present in GFP-negative, filamin-positive proplatelets (C), which have much thinner swellings and shafts, and numerous small proplatelets coming off of the thin shafts. By CD41 Day 6, the majority of proplatelets produced in the FlnABLow cultures had become GFP and shRNA-negative, and began to reexpress filamins. Platelets shed into the culture media were identified by GPIbβ expression and forward scatter. (D-E) FACS analysis of platelet size and relative expression of CD41 and GPIbβ in the FlnABLow, GFP-positive versus the FlnNormal, GFP-negative population. Note that platelets derived from the proplatelets that remained FlnABLow are approximately twice the size of those that had become FlnABNormal.
ESCs express both filamins A and B,31 although the level of filamin B in platelets is reported to be only ∼ 10% that of filamin A.17 To evaluate the relative contribution of filamin B to these processes, we generated ESCs in which filamin A, but not filamin B, was selectively knocked-down using an shRNA that targets only FlnA (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As shown in supplemental Figure 1C, FlnALow ESCs produced slightly more CD41-positive megakaryocyte precursors than did FlnABLow ESCs (31% vs 21% at day 0, and 10% vs only 4% at day 6; compare Figure 2D with supplemental Figure 1C), indicative of less efficient ESC→megakaryocyte differentiation when both filamins A and B, rather than just filamin A alone, are decreased. In contrast, proplatelet size was not significantly different when they were derived from FlnALow versus FlnABLow ESCs; being approximately twice the size of FlnABNormal platelets in both instances (compare Figure 3D with supplemental Figure 1D). These data suggest that FlnB plays a minor, but measurable, role in megakaryocyte differentiation, but is unable to restore normal platelet size in the absence of filamin A.
Reciprocal requirement for balanced expression of GPIbα and filamin for trafficking to the cell periphery
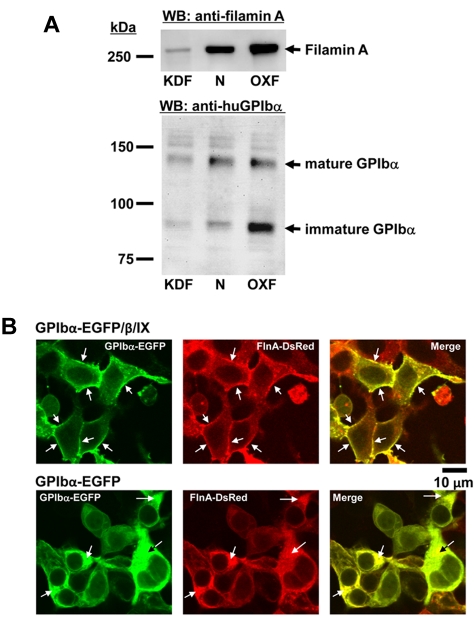
The mechanism by which filamin functions as a determinant of platelet size requires its constitutive association with the GPIb complex not only in mature platelets, but as illustrated in Figure 3, during the process of thrombogenesis. Loss of either protein results in failure to properly anchor the plasma membrane to the underlying membrane skeleton, resulting in deformable, thickened, and distended proplatelet shafts and macrothrombocytopenia. In support of this model, previous studies have shown that filamin associates with GPIbα within the endoplasmic reticulum of the cell during the early stages of their biogenesis, serving as a molecular chaperone to ensure efficient trafficking of the GPIbα/filamin complex to the cell surface.14,16–18 To examine whether balanced expression of these 2 proteins might be required for efficient trafficking of the ER, we established 3 stable lines of HEK293T cells, one transfected with a filamin-specific shRNA to knockdown filamin (KDF), and the other transfected with a filamin-expressing plasmid to overexpress the protein (OXF), and untransfected cells (N) with intermediate levels of filamin A (Figure 4A top panel). GPIbα was then transfected into all 3 cell lines, and its ability to traffic of the ER assessed by Western blot analysis. As shown in the bottom panel of Figure 4A, overexpression of filamin, similar to the lack of filamin expression,14,16–18 results in retention of GPIbα within the ER, as shown by the accumulation of its low molecular weight, endo H-sensitive form (not shown).
Figure 4.
Reciprocal requirement for balanced expression of GPIbα and filamin for efficient trafficking to the plasma membrane. (A) Overexpression of filamin traps GPIbα in the ER and prevents its redistribution to the plasma membrane. Stable HEK293T cell lines were established in which filamin A was either knocked-down ∼ 90% (KDF), expressed at normal levels (N), or overexpressed 2 to 3-fold (OXF), as shown by filamin immunoblot analysis in the top panel. Each of these 3 cell lines were then transfected with a cDNA encoding GPIbα, and the ability of GPIbα to traffic from the ER to the cell surface evaluated by immunoblot analysis using an antibody that recognizes the extracellular domain of GPIbα. As shown in the bottom panel, cells expressing excess filamin (OXF) accumulated noticeably more GPIbα in the ER, as reported by the persistence of a lower, immature, Endo H-sensitive form of the subunit. Note that similar transfection efficiency was observed when GFP expression vector was transfected into these 3 cell lines. (B) Forced accumulation of GPIbα within the ER traps filamin and prevents its redistribution to the membrane skeleton of the cell. HEK293T cells that stably express FlnA-DsRed were transiently transfected with GPIbα-EGFP alone or together with GPIbβ and GPIX expression plasmids. Colocalization of GPIbα (green) with filamin A (red) was analyzed by confocal laser scanning microscopy. Top 3 panels: GPIbα, when expressed with the other subunits of the GPIb complex, becomes efficiently expressed on the cell surface, and chaperones filamin to the inner face of the plasma membrane. Bottom 3 panels: when expressed without GPIbβ and GPIX, GPIbα becomes largely trapped in the ER, trapping filamin as well, suggesting that they need to associate in nearly equimolar amounts for efficient trafficking of both components to the cell surface.
To explore whether GPIb serves a complementary role in chaperoning filamin to the membrane skeleton, we expressed a GPIbα-EGFP26 fusion protein (either alone or together with GPIbβ and GPIX) in HEK293 cells that constitutively expressed filamin, and examined whether conditions that favor trapping of GPIbα within the ER of the cell might also result in the retention of filamin. As shown in the top 3 panels of Figure 4B, when GPIbα was expressed together with GPIbβ and GPIX, both it and filamin became predominantly localized at the cell periphery. In contrast, when GPIbα was expressed without the other 2 subunits of the GPIb complex, conditions under which it is largely retained within the ER,32 filamin became similary trapped, with little or no plasma membrane staining (Figure 4A bottom 3 panels). Thus, forced accumulation within the ER of either filamin (Figure 4A) or GPIbα (Figure 4B) results in retention of the other, strongly suggesting that balanced, stoichiometric expression of filamin and GPIbα is required for their optimal trafficking to the margin of the cell.
Disproportionate expression of GPIbα results in macrothrombocytopenia in vitro and in vivo
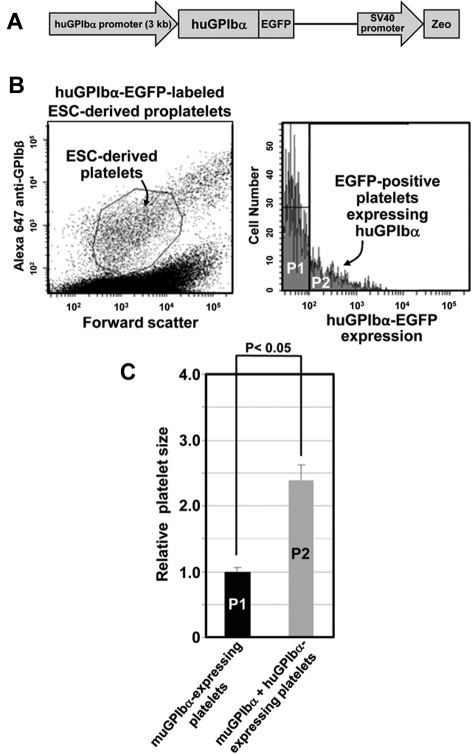
To determine the physiologic importance of balanced expression of GPIb and filamin for producing normal size platelets, 2 different complementary strategies were used. In the first, we generated ESCs expressing, in addition to their own GPIbα, a chimeric protein composed of GPIbα fused at its C-terminus to EGFP26 under the control of the platelet-specific human GPIbα promoter33 (shown schematically in Figure 5A). When cultured in the presence of hTERT-TPO stromal cells to induce proplatelet production using the same procedures described in Figures 2 and 3, these cells generated platelets that could be identified by their forward scatter properties, and by their expression of GPIbβ (Figure 5B left panel). Interestingly, when the ESC-derived platelets were analyzed according to their expression of the human GPIbα-EGFP fusion protein, 2 populations were observed (Figure 5B right panel): population P1, characterized by normal expression of murine GPIb and little or no expression of human GPIbα, and population P2, characterized by normal expression of murine GPIb together with moderate to high expression of human GPIbα. When each of these 2 populations were back-gated and analyzed for their forward-scatter properties, the human GPIbα/GFP-positive cells were found to be more than twice as large as those derived from GFP-negative ES cells (Figure 5C). Taken together with the observation that excess GPIbα results in retention of GPIbα/filamin complexes in the ER (Figure 4B top panel), these data reinforce the notion that balanced expression of GPIb and filamin is required for the complex to be effective in regulating normal platelet biogenesis.
Figure 5.
Overexpression of GPIbα in ESCs results in the generation of giant platelets in vitro. (A) Schematic diagram of the vector used to generate ESCs that express a human (Hu) GPIbα-EGFP fusion protein. Platelet-specific expression was driven by the human GPIbα promoter. (B) Transfected ESCs were selected with Zeocin and further differentiated into megakaryocytes, proplatelets, and platelets as described in the legends for Figures 2 and 3. Left panel: representative FACS profile of platelets present in the culture media of ESC/EB-derived proplatelet cultures, as defined by forward scatter and expression of GPIbβ. Right panel: expression of huGPIbα in ESC/EB-derived platelets. As shown, most proplatelets lose expression of huGPIbα with time and become GFP-negative (population P1). A minority population (P2, ∼ 10%-20%) remain GFP-positive and continue to express the huGPIbα-EGFP fusion protein. When these 2 populations were back-gated and analyzed for platelet size, GFP-positive platelets were found to be approximately twice the size of GFP-negative platelets, demonstrating that overexpressing GPIbα has the same effect on platelet size as does loss of filamin, and suggesting that the ratio of GPIbα:filamin expression may be an important determinant of platelet size during thrombopoiesis.
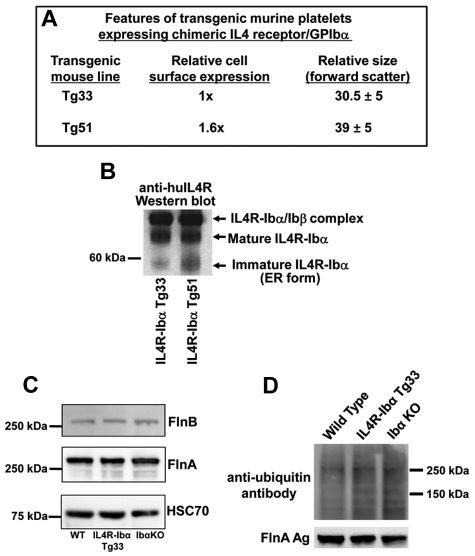
To confirm that the GPIbα cytoplasmic domain is responsible for the observed effects on platelet size of manipulating GPIbα/filamin ratios, we reexamined the relative size of platelets, generated in vivo, from 2 mouse strains, Tg33 and Tg51, that expressed different levels of a chimeric protein composed of the extracellular domain of the IL-4 receptor fused to the transmembrane and cytoplasmic domains of GPIbα (IL4R-GPIbα).24 As shown in Figure 6A, although the Tg51 transgenic line expressed 1.6 times more IL4R-GPIbα on the surface of their platelets than the Tg33 mouse line, their platelets were ∼ 30% larger, not smaller, consistent with the possibility that excess GPIbα might be accumulating in the ER, resulting in the observed phenotype. Western blot analysis of detergent lysates of platelets from Tg33 and Tg51 transgenic mice (Figure 6B) confirmed this suspicion, as there was noticeably more of the ER-trapped form of the chimeric IL4R-GPIbα protein in platelets derived from Tg51, relative to Tg33 mice. Neither the level of expression nor their degree of ubiquitination of filamins A and B differed noticeably between wild-type, Tg33, and GPIb-deficient platelets (Figure 6C-D), consistent with the notion that the subcellular location of filamin, and not its absolute expression level, is a more important determinant of platelet size. The content of F-actin in the platelets from these 3 mouse lines was also proportional to their size (supplemental Figure 2).
Figure 6.
Overexpressed IL4R-Ibα becomes trapped in the ER and results in the generation of large platelets in vivo. (A) Platelets from 2 different transgenic lines, Tg33 and Tg51, have previously been analyzed for cell surface expression of the IL4R-GPIbα chimeric protein and platelet size,24 and the results are summarized here. Note that surface expression of IL4R-Ibα in Tg51 mouse is approximately 1.6 times higher than that of Tg33 mouse, yet the resulting platelets are 30% larger. (B) Immunoblot analysis of the IL4R-Ibα chimeric protein from IL4R-Ibα Tg33 and Tg51 mouse platelet lysates. The samples were subjected to SDS-PAGE under nonreducing conditions and probed using an anti-hIL4R mAb. The IL4R-Ibα chimeric protein from these platelets exists in 3 forms; as a disulfide-linked dimer with GPIbβ, as a mature uncomplexed protein, and as an immature incompletely glycosylated protein present in the ER of the cell. Note that there is more of the ER-trapped form in the IL4R-Ibα Tg51 mouse platelet lysate. (C) Immunoblot analysis of filamin A and B content in platelets from wild-type, Tg33, and GPIb knockout mice, showing similar expression in all 3 lines. HSC70 was used as a loading control. (D) Analysis of the filamin ubiquitination status in platelets from wild-type, Tg33, and GPIb knockout mice. Filamins A was immunoprecipitated as described in “Methods” and their ubiquitination status probed using mAb P4D1. Overall ubiquitination profiles of platelet lysates among these platelets were similar, although ubiquitination of FlnA was barely detectable.
Discussion
Filamins are large (280 kDa) actin-binding proteins composed of an N-terminal F-actin-binding domain, 23 Ig-like domains that organize into a bent rod, and a C-terminal Ig-like domain that mediates its dimerization. The mammalian filamin family is composed of 3 homologous members: filamins A, B and C, with filamin A being the most abundant. In addition to their role in organizing F-actin networks, controlling cell shape, and regulating locomotion, filamins have been reported to bind a diverse array of proteins, including transmembrane adhesion molecules, transcription factors, and cytosolic signaling molecules. The bivalent nature of the molecule, together with its multiple protein interaction sites, allows filamin to serve as a cellular scaffold capable of integrating multiple cellular events.1,34
In human and murine blood platelets, filamin and spectrin35 comprise the major filamentous components of the membrane skeleton,36 and via its association with the cytoplasmic domain of GPIbα, filamin also serves the role of linking the membrane skeleton to the plasma membrane. Constitutive interaction between GPIbα and filamin has been shown to help maintain the stability and integrity of the plasma membrane under both resting conditions21 and under conditions of high shear.37 Association of these 2 proteins takes place early on during the process of megakaryocyte maturation,14 and based on studies presented herein appears to be important not only for efficient megakaryocytopoiesis (Figure 2), but also for supporting the morphology of proplatelet projections that precede platelet production (Figure 3). Our findings in ESC-derived megakaryocytes and their progeny are similar to those recently observed in vivo by Jurak-Begonja et al, who found that filamin A–null megakaryocytes prematurely release large, fragile platelets into circulation that are prone to disintegration and clearance.18 Interestingly, Patel-Hett et al recently reported that the other major component of the platelet membrane skeleton, spectrin, also plays an important role in stabilizing the membranous systems of megakaryocytes, proplatelets, and platelets,38 as blebbing and swelling of proplatelet extensions reminiscent of those observed in cells lacking filamins A and B (Figure 3) were also observed in spectrin-null cells. Whether spectrin also functions in early megakaryocytopoiesis, and whether it interacts with the GPIbα/filamin network to support formation of proplatelet structures involved in the terminal stages of platelet production remains to be determined.
Major findings of the present investigation were the reciprocal requirement for filamin to associate with an intact GPIb complex for travel to the cell periphery, and cells must coordinately regulate the ratio at which these 2 proteins become expressed to achieve normal platelet biogenesis. In the case of filamin, it had previously been established,14 and confirmed by us in this study (Figure 4A), that filamin promotes efficient trafficking of the GPIb complex to the cell surface; however, it was not previously appreciated that the level of filamin expression appears to be as important as expression itself, as reported by the observation (Figure 4A) that overexpression of filamin also results in the trapping of nascent GPIbα within the ER. The mutual requirement for GPIb to chaperone filamin to the periphery of the cell was also somewhat unexpected. In the absence of GPIbβ and GPIX, GPIbα forms an unproductive association with filamin, which traps both proteins within the endoplasmic reticulum of the cell (Figure 4B) with reduced expression of both components at the cell margin. Although it cannot be shown directly, similar unproductive associations probably occur when GPIbα is expressed without the other components of the GPIb complex in ESC-derived proplatelets (Figure 5), or when the GPIbα cytoplasmic domain is overexpressed in vivo (Figure 6). In each case, larger-than-normal platelets were generated. Taken together, the inability of the cell to anchor the plasma membrane to the underlying cytoskeleton has profound effects on megakaryocyte maturation, proplatelet morphology, and ultimately generation of normal sized platelets—both in vitro and in vivo.
Exactly how the GPIbα/filamin complex regulates platelet size during the final stages of plateletpoiesis is still not well understood. Chang et al observed that inhibition of myosin light chain 2 phosphorylation by inhibitors of Rho, Rho associated kinase, or myosin light-chain kinase reduced contractility although increasing proplatelet formation.39 Chen et al reported that loss of myosin-IIA accelerates proplatelet formation, whereas constitutive phosphorylation of myosin light-chain significantly attenuated proplatelet formation.40 Jurak-Begonja et al showed that expression of FlnA, GPIb, and actin dramatically increased with megakaryocyte maturation.18 We speculate that down-regulation of ASB2, which targets filamin for proteasomal degradation,41 contributes to increase FlnA during the process of megakaryocyte maturation. Expression of myosin is also reported to be up-regulated.39 The increased contractile forces enabled by up-regulating filamin and myosin, combined with the known interactions of GPIb, filamin, actin, and myosin may very well regulate platelet production via their ability to limit the size of proplatelets during their formation. In the absence of either filamin or GPIbα, megakaryocytes may not possess sufficient contractile force to restrict proplatelet size, resulting in aberrant, early release of large platelets from their proplatelet precursors. Further studies will be required to address this important and fascinating aspect of platelet and megakaryocyte cell biology.
Supplementary Material
Acknowledgments
The authors thank Dr Hidekazu Nishikii (University of Tsukuba) and Dr Koji Eto (Kyoto University) for technical advice for generating ESC-derived platelets and Susan Russell for platelet sample preparation. They also acknowledge the contributions of Dr Naotaka Hamasaki (Nagasaki International University).
This work was supported by Program Project Grant HL-44612 from the National Heart, Lung, and Blood Institute of the National Institutes of Health (P.J.N.), HL-50545 (J.W.), and the Japan Society for the Promotion of Science and the Futamata Memorial Foundation (T.K. and T.O.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.K. designed and performed experiments, interpreted data, and wrote the paper; J.W. and T.O. contributed vital reagents and reviewed the paper; and P.J.N. supervised experiments, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taisuke Kanaji MD, PhD, Blood Research Institute, BloodCenter of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53266-3548; e-mail: Taisuke.Kanaji@bcw.edu.
References
- 1.Zhou AX, Hartwig JH, Akyurek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20(2):113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig JH, Stossel TP. Isolation and properties of actin, myosin, and a new actin-binding protein in rabbit alveolar macrophages. J Biol Chem. 1975;250(14):5696–5705. [PubMed] [Google Scholar]
- 3.Wang K, Ash JF, Singer SJ. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okita JR, Pidard D, Newman PJ, Montgomery RR, Kunicki TJ. On the association of glycoprotein Ib and actin-binding protein in human platelets. J Cell Biol. 1985;100(1):317. doi: 10.1083/jcb.100.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox JEB. Identification of actin-binding protein as the protein linking the membrane skeleton to glycoproteins on platelet plasma membranes. J Biol Chem. 1985;260(22):11970–11977. [PubMed] [Google Scholar]
- 6.Andrews RK, Berndt MC, Lopez JA. The glycoprotein Ib-IX-V complex. In: Michelson AD, editor. Platelets. 2nd ed. San Diego, CA: Academic Press; 2007. pp. 145–164. [Google Scholar]
- 7.Andrews RK, Fox JE. Interaction of purified actin-binding protein with the platelet membrane glycoprotein Ib-IX complex. J Biol Chem. 1991;266(11):7144–7147. [PubMed] [Google Scholar]
- 8.Andrews RK, Fox JEB. Identification of a region in the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX complex that binds to purified actin-binding protein. J Biol Chem. 1992;267(26):18605–18611. [PubMed] [Google Scholar]
- 9.Cunningham JG, Meyer SC, Fox JE. The cytoplasmic domain of the α-subunit of glycoprotein (GP) Ib mediates attachment of the entire GP Ib-IX complex to the cytoskeleton and regulates von Willebrand factor-induced changes in cell morphology. J Biol Chem. 1996;271(19):11581–11587. doi: 10.1074/jbc.271.19.11581. [DOI] [PubMed] [Google Scholar]
- 10.Meyer SC, Zuerbig S, Cunningham CC, et al. Identification of the region in actin-binding protein that binds to the cytoplasmic domain of glycoprotein Ibα. J Biol Chem. 1997;272(5):2914–2919. doi: 10.1074/jbc.272.5.2914. [DOI] [PubMed] [Google Scholar]
- 11.Williamson D, Pikovski I, Cranmer SL, et al. Interaction between platelet glycoprotein Ibα and filamin-1 is essential for glycoprotein Ib/IX receptor anchorage at high shear. J Biol Chem. 2002;277(3):2151–2159. doi: 10.1074/jbc.M109384200. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Resendiz JC, Lu X, Kroll MH. Filamin A binding to the cytoplasmic tail of glycoprotein Ibα regulates von Willebrand factor-induced platelet activation. Blood. 2003;102(6):2122–2129. doi: 10.1182/blood-2002-12-3805. [DOI] [PubMed] [Google Scholar]
- 13.Cranmer SL, Pikovski I, Mangin P, et al. Identification of a unique filamin A binding region within the cytoplasmic domain of glycoprotein Ibα. Biochem J. 2005;387(Pt3):849–858. doi: 10.1042/BJ20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Lu X, Kroll MH. Filamin A binding stabilizes nascent glycoprotein Ibα trafficking and thereby enhances its surface expression. J Biol Chem. 2005;280(8):6709–6715. doi: 10.1074/jbc.M413590200. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura F, Pudas R, Heikkinen O, et al. The structure of the GPIb-filamin A complex. Blood. 2006;107(5):1925–1932. doi: 10.1182/blood-2005-10-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer SC, Sanan DA, Fox JE. Role of actin-binding protein in insertion of adhesion receptors into the membrane. J Biol Chem. 1998;273(5):3013–3020. doi: 10.1074/jbc.273.5.3013. [DOI] [PubMed] [Google Scholar]
- 17.Falet H, Pollitt AY, Begonja AJ, et al. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med. 2010;207(9):1967–1979. doi: 10.1084/jem.20100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurak-Begonja A, Hoffmeister KM, Hartwig JH, Falet H. FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood. 2011;118(8):2285–2295. doi: 10.1182/blood-2011-04-348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cranmer SL, Ulsemer P, Cooke BM, et al. Glycoprotein (GP) Ib-IX-transfected cells roll on a von Willebrand factor matrix under flow. Importance of the GPIb/actin-binding protein (ABP-280) interaction in maintaining adhesion under high shear. J Biol Chem. 1999;274(10):6097–6106. doi: 10.1074/jbc.274.10.6097. [DOI] [PubMed] [Google Scholar]
- 20.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91(12):4397–4418. [PubMed] [Google Scholar]
- 21.White JG, Burris SM, Hasegawa D, Johnson M. Micropipette aspiration of human blood platelets. A defect in Bernard-Soulier syndrome. Blood. 1984;63(5):1249. [PubMed] [Google Scholar]
- 22.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci U S A. 2000;97(6):2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenny D, Newman PJ, Morateck PA, Montgomery RR. A dinucleotide deletion results in defective membrane anchoring and circulating soluble glycoprotein Ibα in a novel form of Bernard-Soulier syndrome. Blood. 1997;90(7):2626–2633. [PubMed] [Google Scholar]
- 24.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100(6):2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 25.Kanaji S, Kanaji T, Furihata K, Kato K, Ware JL, Kunicki TJ. Convulxin binds to native, human glycoprotein Ibα. J Biol Chem. 2003;278(41):39452–39460. doi: 10.1074/jbc.M300199200. [DOI] [PubMed] [Google Scholar]
- 26.Nishikii H, Eto K, Tamura N, et al. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med. 2008;205(8):1917–1927. doi: 10.1084/jem.20071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitajima K, Tanaka M, Zheng J, Sakai-Ogawa E, Nakano T. In vitro differentiation of mouse embryonic stem cells to hematopoietic cells on an OP9 stromal cell monolayer. Methods Enzymol. 2003;365:72–83. doi: 10.1016/s0076-6879(03)65005-6. [DOI] [PubMed] [Google Scholar]
- 28.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265(5175):1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 29.Matsunaga T, Tanaka I, Kobune M, et al. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 2006;24(12):2877–2887. doi: 10.1634/stemcells.2006-0309. [DOI] [PubMed] [Google Scholar]
- 30.Yagi M, Ritchie KA, Sitnicka E, Storey C, Roth GJ, Bartelmez S. Sustained ex vivo expansion of hematopoietic stem cells mediated by thrombopoietin. Proc Natl Acad Sci U S A. 1999;96(14):8126–8131. doi: 10.1073/pnas.96.14.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Chen MH, Moskowitz IP, et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103(52):19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez JA, Leung B, Reynolds CC, Li CQ, Fox JEB. Efficient plasma membrane expression of a functional platelet glycoprotein Ib-IX complex requires the presence of its three subunits. J Biol Chem. 1992;267(18):12851–12859. [PubMed] [Google Scholar]
- 33.Fujita H, Hashimoto Y, Russell S, Zieger B, Ware J. In vivo expression of murine platelet glycoprotein Ibalpha. Blood. 1998;92(2):488–495. [PubMed] [Google Scholar]
- 34.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6(11):1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 35.Fox JE, Reynolds CC, Morrow JS, Phillips DR. Spectrin is associated with membrane-bound actin filaments in platelets and is hydrolyzed by the Ca2+-dependent protease during platelet activation. Blood. 1987;69(2):537–545. [PubMed] [Google Scholar]
- 36.Hartwig JH, DeSisto M. The cytoskeleton of the resting human blood platelet: Structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol. 1991;112(3):407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cranmer SL, Ashworth KJ, Yao Y, et al. High shear-dependent loss of membrane integrity and defective platelet adhesion following disruption of the GPIbα-filamin interaction. Blood. 2011;117(9):2718–2727. doi: 10.1182/blood-2010-07-296194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel-Hett S, Wang H, Begonja AJ, et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood. 2011;118(6):1641–1652. doi: 10.1182/blood-2011-01-330688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang Y, Aurade F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–4236. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Naveiras O, Balduini A, et al. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;110(1):171–179. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heuze ML, Lamsoul I, Baldassarre M, et al. ASB2 targets filamins A and B to proteasomal degradation. Blood. 2008;112(13):5130–5140. doi: 10.1182/blood-2007-12-128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.