Abstract
Background
Starch is a main source of glucose and energy in the human diet. The extent to which it is digested in the gastrointestinal tract plays a major role in variations in postprandial blood glucose levels. Interactions with other biopolymers, such as dairy proteins, during processing can influence both the duration and extent of this postprandial surge.
Objective
To evaluate the effect of the addition of bovine α- or β-casein to waxy maize starch on changes in postprandial blood glucose, insulin, and incretin hormones [glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1)] in 30 kg pigs used as an animal model for humans.
Design
Gelatinised starch, starch gelatinised with α-casein, and starch gelatinised with β-casein were orally administered to trained pigs (n = 8) at a level of 60 g of available carbohydrate. Pre- and postprandial glucose measurements were taken every 15 min for the first hour and every 30 min thereafter up to 180 min. Insulin, GIP, and GLP-1 levels were measured in plasma samples up to 90 min postprandial.
Results
Starch gelatinised with α-casein had a significantly (p < 0.05) lower peak viscosity on pasting and resulted in significantly lower glucose release at 15, 30, and 90 min postprandial compared to starch gelatinised with β-casein. During the first 45-min postprandial, the area under the glucose curve (AUC) for starch gelatinised with α-casein was significantly (p < 0.05) lower than that for starch gelatinised with β-casein. There was also a significant (p < 0.05) difference at T30 in GIP levels in response to the control compared to starch gelatinised with α- or β-casein. Significant (p < 0.05) increases in several free amino acid concentrations were observed on ingestion of either α- or β-casein gelatinised with starch at 30 and 90 min postprandial compared to starch alone. In addition, plasma levels of six individual amino acids were increased on ingestion of starch gelatinised with α-casein compared to ingestion of starch gelatinised with β-casein.
Conclusion
The presence of casein fractions (α- or β-casein) in gelatinised waxy maize starch affects swelling characteristics, viscosity, and subsequent in vivo digestion as determined by glucose levels in blood postprandial.
Keywords: waxy maize starch, glucose and insulin, incretin hormones, digestion
There is increasing interest in the role of carbohydrate (CHO) ingestion in the metabolic syndrome and subsequent health effects relating to glycemia and type 2 diabetes (1). Ingestion of foods, which result in a decreased rate of carbohydrate absorption, leads to a more controlled release of glucose into the bloodstream. These foods have a lower glycemic index (GI) value. The GI system ranks carbohydrate foods based on their postprandial glycemic concentrations (2). High GI foods are associated with a rapid rise in both glucose and insulin in blood after consumption (3). Hyperinsulinemia can reduce insulin efficiency via downregulation of insulin receptors, resulting in insulin resistance (4) which, in addition to impaired pancreatic β-cell function, is a risk factor for type II diabetes (5). Previous studies have reported on the benefits of low GI diets in relation to the metabolic syndrome, i.e. improved blood glucose control and glucose tolerance (6), improvements in insulin sensitivity (7), and a reduction of insulin resistance (8).
Upon ingestion, nutrients stimulate the secretion of various hormones and peptides by specialised enteroendocrine cells in the gastrointestinal tract. This informs the gut and pancreas of the impending arrival of glucose and ensures that insulin is produced and secreted by pancreatic β-cells to maintain glucose homeostasis. This signalling is primarily achieved by the incretin hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), secreted by the gut, which act to stimulate insulin release and suppress glucagon (9). These hormones primarily respond to carbohydrate and fat ingestion but can also respond to proteins and amino acids (10).
It is well established that proteins within a defined food matrix can affect the rate at which carbohydrates are metabolised in vivo (11–14). The authors hypothesise that the addition of dairy protein fractions, α- or β-casein, to waxy maize starch will have a postprandial glucose lowering effect. Previous results from rheological, microscopical, and simulated gastrointestinal digestion studies showed a restriction in granule swelling and decreased maltose and glucose levels when starch is gelatinised with casein fractions. These results were not observed when starch was gelatinised with whey fractions (α-lactalbumin and β-lactoglobulin) (15).
The authors used the pig as an animal model for humans due to its omnivore status and its similarity to humans in digestive physiology, hormonal response, metabolism, and organ size (16, 17). The present study investigates the effect of adding enriched α- or β-casein fractions to gelatinised waxy maize starch on postprandial blood levels of glucose, insulin, and incretin hormones in 30 kg pigs.
Materials and methods
The protein enriched α- and β-casein fractions were sourced from Kerry Ingredients (Listowel Co., Kerry). The protein content of the α-casein was 84.1% and the β-casein 85.6%, as determined by Kjeldahl nitrogen × 6.25 (18). Casein fractions (10% w/w) on a protein basis were made up by dissolving the powders in Milli-Q water at room temperature, with the aid of an overhead stirrer and stored overnight at 4°C to ensure complete hydration.
Waxy maize starch (trade name Amioca Powder TF) was kindly provided by National Starch (Manchester, UK). All trial samples (starch alone or starch gelatinised with α- or β-casein) were gelatinised by heating to 90°C using in-direct steam heating (operating steam pressure of 1 bar) and held for 6 min to ensure complete gelatinisation, then cooled to 20°C in a Stephen cooker (closed system) UMM/SK5 (Stephan U Sohne GMBH + Co., Hameln, Germany) using a mixing baffle operated at 100% capacity and the main motor at 50% capacity. Sample preparation was staggered each day so that each sample was fed to the pigs immediately after the pasting process to reduce retrogradation of the starch.
Pasting behaviour
All samples were pasted as a model system using the same composition as the investigated samples on an AR-G2 Rheometer (TA Instruments, Crawley, UK) fitted with a starch pasting cell (sample size 28 g). The internal diameter of the cell was 36 mm and the diameter of the rotor used was 32 mm with a geometry gap of 550 µm. A thermal cycle was used to provide conditions for gelatinisation, pasting, and subsequent molecular rearrangement on cooling. All samples were tempered at 35°C, heated from 35°C to 95°C at a ramp rate of 14.5°C /min, held for 6 min, subsequently cooled from 95°C to 35°C at a ramp rate of 29°C /min, and finally held for 10 min at 35°C using a shear rate of 16.78 1/s.
Experimental design
A progeny of Large White × Landrace crossbred pigs (n = 8) (reared in the Pig Development Unit) with a body weight of 30 kg (±2) were trained 1 week before trial commencement to eat 10% w/w (600 g) gelatinised starch samples within a 10-min period. The control sample contained 60 g w/w (60 g available CHO) of waxy maize starch in 540 g of water. The starch and protein samples contained 60 g w/w of starch, 71.3 g of α-casein, and 468.8 g of water or 60 g w/w of starch, 70.1 g of β-casein, and 469.9 g of water. (Note: all starch-protein samples were made up on protein basis.) There were no additional ingredients in the sample, i.e. fat, other carbohydrates, or minerals. Additional constituents of a typical pig diet add caloric value and would have an effect on glucose and insulin levels. On completion of training, the pigs were catheterised in the lateral ear vein for blood sampling. Prior to commencement of sampling, a 24-h resting period allowed clearance of the anaesthetics from the body. The pigs were fasted from 16.00 h the previous day after there afternoon meal. Nine pigs were trained for the trial; however, due to problems with one catether, one pig was removed from the trial postcatheterisation. The concentrations of the three semisolid gelatinised foods ingested on a component level were (i) 10% starch (control); (ii) 10% starch + 10% α-casein; and (iii) 10% starch + 10% β-casein. The protein and lipid concentrations naturally present in waxy maize starch were not accounted for as these concentrations are minimal (19). The eight pigs were fed 600 g as a single meal of each gelatinised semisolid food containing 60 g of available CHO. Replicates for each sample were staggered across 3 days to exclude sampling day as a variable. All experiments complied with EU Council Directive 91/630/EEC, which lays down minimum standards for the protection of pigs, and EU Council Directive 98/58/EC, which concerns the protection of animals kept for farming purposes. A licence to perform experiments on live animals under the cruelty of animals act, 1876 regulations 2002 and 2005, was obtained before commencing this work.
Catheterisation
Insertion of catheters was performed on anaesthetised pigs according to Niiyama et al. (20) with the following modifications: pigs were anaesthetised with detomidine (0.13 mg/kg body weight), butorphanol (0.26 mg/kg), and ketamine (5 mg/kg). Prior to anaesthesia, the pigs were fasted for 24 h and the weight of the pigs recorded before administration of the drugs to ensure proper dosage. The lateral auricular vein was selected for catheterisation because of its smooth trajectory. The ear was occluded with a rubber band (at the base of the ear) to dilate the vein and removed after the venous access catheter was in place. Once the pig was under anaesthesia (after 15 min of drug administration), the ear was shaved and cleaned with 70% ethanol, and the vein was dilated by hot water application to the ear. Catheterisation was performed using a 3-FR 55-cm peripherally inserted central catheter (PICC) with trimmable placement wire (Arrow International Inc. Reading, PA, US) comprised of a venous access cannula (VAC) and 17-G peel away catheter over a 19-G needle. Only 40 cm was inserted and the tip of the catheter trimmed off. Venous access was obtained by inserting the VAC in the ear vein and removing the needle. The PICC was then inserted through the 17-G peel away catheter. When the insertion was complete, the guide wire was removed and the correct positioning assessed by sampling 2–3 mL of blood. After insertion, the peel away catheter was removed and the PICC flushed with isotonic saline solution. Pressure was applied at the insertion point to prevent haemorrhaging. The hub of the PICC was secured to the skin by suture with non-resorbable wire and an extension added to minimise the pigs’ manipulation during blood sampling. Several suture points were placed along the neck and back to hold the extension in place. Air was completely removed and the whole system flushed with isotonic saline and primed with Heparin solution (0.01 units/L diluted to 2%). The insertion point was disinfected thoroughly with 70% ethanol and covered with Leukoplast to prevent infection. After the procedure, the pigs were placed in a warm environment in single pens and checked every 30 min until full recovery, usually within 2 h. Every pig was examined at least 3 times daily to ensure that the catheter was in position and to detect any associated health problems early.
Glucose analysis
Blood glucose was measured using a Glucomen Visio® glucometer (A. Menarini Diagnostics, Firenze, Italy) as per the manufacturer's instructions. The glucometer was calibrated every morning before sampling using the Glucomen Visio control solution provided. The calibration region of the glucometer fell between 4.7 and 6.4 mmol/L. The sampling times for glucose were T0, T15, T30, T45, T60, T90, T120, T150, and T180 min.
Blood sampling
Blood samples were collected at 3 time point intervals: T0, T30 and T90 min, into prechilled vacutainers containing 18.0 mg K2EDTA (BD Biosciences, Oxfordshire, England). For GLP-1 analysis at T0 and T90, Dipeptidyl peptidase IV (DPPIV) inhibitor (Millipore, Ltd., Watford, UK) was added within 30 s of collection, at a concentration of 0.01 µL/L of blood. Tubes were inverted several times to ensure a homogenous mix. Blood samples were kept on ice following collection and then centrifuged at 2500g for 15 min at 4°C within 1 h of collection. Plasma was obtained after centrifugation and stored at −80°C until analyses.
Incretin hormones and insulin assays
Using ELISA commercial kits, plasma concentrations of active GLP-1 [GLP-1 (active) ELISA Kit, Millipore], total GIP [Rat/Mouse GIP (total) ELISA Kit, Millipore], and insulin (Human Insulin ELISA Kit, Millipore) were measured. The sensitivity limits of the ELISA kits were 2.0 nU/L for intact insulin, 8.0 fg/L for total (active and inactive) GIP, and 2.0 fmol/L for active GLP-1. Each plasma sample was assayed in triplicate. The concentrations of insulin, GIP, and GLP-1 were derived by interpolation from a reference curve generated in each individual assay with reference standards of known concentrations of insulin, GIP, and GLP-1, respectively.
Amino acid composition
Plasma samples were deproteinised by mixing equal volumes of 24% (w/v) tri-chloroacetic acid (TCA) and sample, allowed to stand for 10 min and centrifuged at 14,400g for 10 min. Supernatants were removed and diluted with 200 mmol/L sodium citrate buffer, pH 2.2, to give approximately 250 nmol of each amino acid residue. Samples were then diluted 1 in 2 with the internal standard, norleucine, to give a final concentration of 0.125 nmol/L. Amino acids were quantified using a Jeol JLC-500/V amino acid analyser (Jeol, Ltd., Garden city, Herts, UK) fitted with a Jeol Na+ high performance cation exchange column.
Statistical analysis
Data sets were analysed using the mixed models procedure in SAS (Statistical Analysis Systems Institute Incorporated, NC) for an incomplete latin square design. The pig was the experimental unit. The model included the effects for treatment, time of sampling, and the interaction effect between treatment and time of sampling. Animal weight was used as a covariant in the model. Animal was fitted as a repeated effect with compound symmetry (Co) variance structure assumed among records within animal. Treatment effect at particular times of sampling was determined using the slice statement in SAS. The results were presented as least square means ± SEM. Means separation was performed using the Tukey–Kramer test.
Results
Pasting behaviour
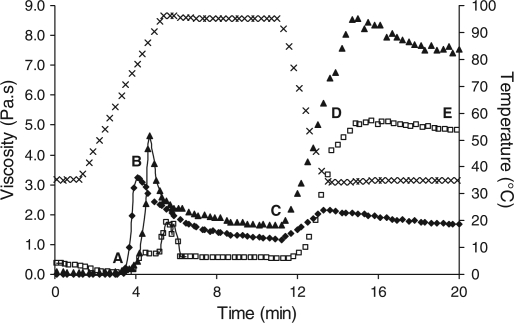
The five stages of the gelatinisation/pasting process are outlined in Fig. 1. Significant (p < 0.05) differences were observed between samples for each stage throughout pasting (Table 1). Significantly higher viscosity values were observed throughout pasting for all samples containing 10% starch gelatinised with 10% β-casein (Table 1) while 10% starch gelatinised with 10% α-casein resulted in a significantly lower peak viscosity (1.7 Pa.s) compared to 10% starch alone (3.2 Pa.s) or 10% starch gelatinised with 10% β-casein (4.6 Pa.s). Starch gelatinised with 10% α-casein had a significantly higher on-set pasting temperature (74.7°C) compared to 10% starch alone (68.4°C) or 10% starch gelatinised with 10% β-casein (64.4°C). The final paste viscosity increased in the following order: 10% starch alone (1.7 Pa.s), 10% starch gelatinised with 10% α-casein (4.8 Pa.s), and 10% starch gelatinised with 10% β-casein (7.6 Pa.s).
Fig. 1.
Gelatinisation profile of 10% starch (♦), 10% starch + 10% α-casein  , and 10% starch + 10% β-casein (▲); (x) represents temperature profile. Point A on the curve represents the on-set stage of pasting, B represents peak viscosity on heating, C represents viscosity at end of heating, D represents viscosity at end of cooling, and E represents the final viscosity of the paste.
, and 10% starch + 10% β-casein (▲); (x) represents temperature profile. Point A on the curve represents the on-set stage of pasting, B represents peak viscosity on heating, C represents viscosity at end of heating, D represents viscosity at end of cooling, and E represents the final viscosity of the paste.
Table 1.
Pasting viscosities of treatments throughout gelatinisation.
| n | On-set pasting temp (°C) (a) | Peak viscosity on heating (Pa.s) (b) | End of heating (Pa.s) (c) | End of cooling (Pa.s) (d) | Final viscosity (Pa.s) (e) | |
|---|---|---|---|---|---|---|
| 10% starch | 3 | 68.4b | 3.2b | 1.2b | 2.1c | 1.7c |
| 10% starch + 10% α-casein | 3 | 74.7a | 1.7c | 0.5c | 4.3b | 4.8b |
| 10% starch + 10% β-casein | 3 | 64.4 | 4.6a | 1.7a | 6.7a | 7.6a |
| df* | 8 | 8 | 8 | 8 | 8 |
a–cValues within column with different lowercase letters are significantly different (p < 0.05; Fishers’ test).
*degrees of freedom.
Glucose levels
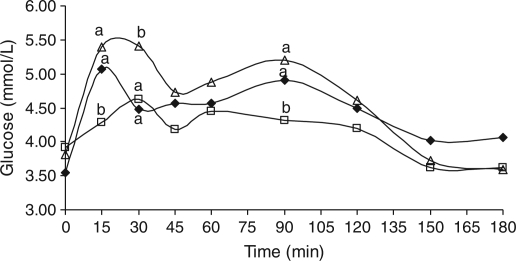
The pre- and postprandial blood glucose concentrations in response to (i) 10% starch, (ii) 10% starch gelatinised with 10% α-casein, or (iii) 10% starch gelatinised with 10% β-casein over 3 h are illustrated in Fig. 2. A rapid increase in glucose concentration was observed 15 min postprandial in response to gelatinised 10% starch, rising to a peak of 5.1 mmol/L. After the initial increase, an additional peak of 4.9 mmol/L was observed at T90 with a subsequent decrease to 4.1 mmol/L at 180 min postprandial. Ingestion of 10% starch gelatinised with 10% β-casein also resulted in a rise in blood glucose concentration within 15 to 30 min, with a peak value of 5.4 mmol/L. A second peak in glucose concentration was observed at T90 (5.2 mmol/L) with a decline to 3.6 mmol/L at T180 postprandial. In contrast, ingestion of 10% starch gelatinised with 10% α-casein resulted in a more gradual increase in blood glucose concentration reaching a peak of 4.6 mmol/L at T30. From T60 to T180, postprandial glucose concentrations decreased to 3.6 mmol/L. Starch gelatinised with α-casein resulted in significantly (p < 0.05) lower glucose release at 15, 30, and 90 min postprandial compared to starch gelatinised with β-casein.
Fig. 2.
Postprandial blood glucose concentrations following ingestion of 10% starch (♦), 10% starch + 10% α-casein  and 10% starch + 10% β-casein (Δ). (a-b)Values with different lowercase letters are significantly different (p < 0.05).
and 10% starch + 10% β-casein (Δ). (a-b)Values with different lowercase letters are significantly different (p < 0.05).
AUC values for the initial rise (T0–T45) in blood glucose concentrations were 204, 194, and 226 mmol/(L min) on ingestion of 10% starch, 10% starch gelatinised with 10% α-casein, and 10% starch gelatinised with 10% β-casein, respectively. A significant difference (p < 0.05) was observed in AUC (T0–T45) between the 10% starch gelatinised with 10% β-casein and the 10% starch gelatinised with 10% α-casein samples, with the latter being significantly lower. The second peak (T60–T120) resulted in AUC values of 141, 129, and 149 mmol/(L min) for 10% starch, 10% starch gelatinised with 10% α-casein, and 10% starch gelatinised with 10% β-casein respectively and (T0-T180) AUC values of 808, 752, and 846 mmol/(L min), respectively.
GLP-1 analysis
There were no significant differences in postprandial plasma GLP-1 concentrations following ingestion of (i) 10% starch, (ii) 10% starch gelatinised with 10% α-casein, or (iii) 10% starch gelatinised with 10% β-casein (Table 2). Preprandial concentrations of plasma GLP-1 ranged from 6.7 to 7.0 pmol/L. At T90, these levels increased between 7.0 and 7.9 pmol/L.
Table 2.
Plasma GLP-1 concentrations (pmol/L) at T0 and T90 following ingestion of 10% starch, 10% starch + 10% α-casein, and 10% starch + 10% β-casein.
| 10% starch (pmol/L) | 10% starch +10% α-casein (pmol/L) | 10% starch +10% β-casein (pmol/L) | |
|---|---|---|---|
| T0 | 7.01 | 6.78 | 6.65 |
| T90 | 7.87 | 7.34 | 7.04 |
GIP analysis
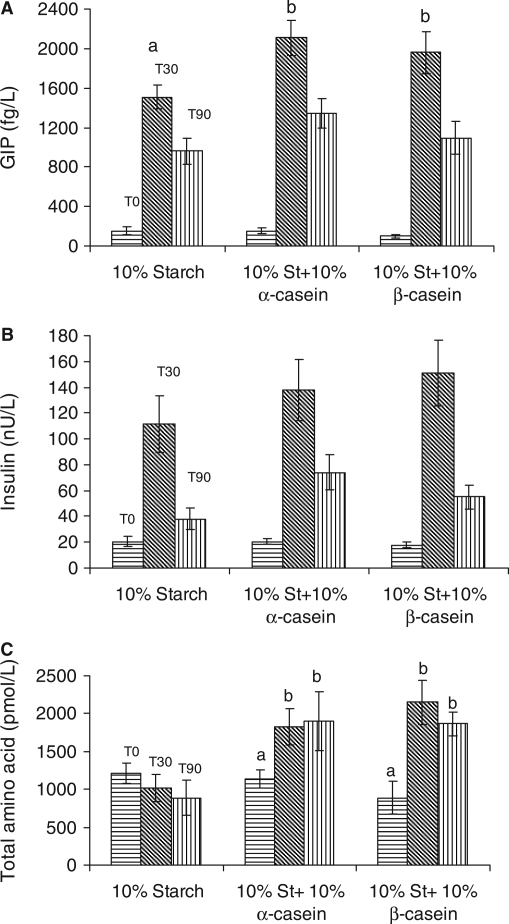
Postprandial GIP concentrations in plasma over 90 min in response to (i) 10% starch, (ii) 10% starch gelatinised with 10% α-casein, and (iii) 10% starch gelatinised with 10% β-casein are detailed in Fig. 3A. Preprandial plasma concentrations of GIP was 151 (SE = 39) fg/L. The GIP concentration increased to 1,509 (SE = 117) fg/L at T30 and decreased to 959 (SE = 135) fg/L at T90 in response to 10% starch alone. Ingestion of 10% starch gelatinised with 10% α-casein resulted in GIP plasma concentrations of 1,959 (SE = 212) fg/L at T30 and 1093 (SE = 167) fg/L at T90. Ingestion of 10% starch gelatinised with 10% α-casein resulted in plasma GIP concentrations at T0, T30, and T90 of 155 (SE = 24), 2,109 (SE = 180), and 1,348 (SE = 149) fg/L, respectively. There was a significant (p < 0.05) difference at T30 in GIP levels in response to the control compared to starch gelatinised with α- or β-casein.
Fig. 3.
(A) Plasma GIP concentrations following ingestion of 10% starch, 10% starch + 10% α-casein, and 10% starch + 10% β-casein. Preprandial GIP concentrations [horizontal], 30 min postprandial [slanted], and 90 min postprandial [vertical]. (B) Insulin concentrations (in plasma) following ingestion of 10% starch, 10% starch + 10% α-casein, and 10% starch + 10% β-casein. Preprandial insulin concentrations [horizontal], 30 min postprandial [slanted], and 90 min postprandial [vertical]. (C) Total amino acid levels of 10% starch, 10% starch + 10% α-casein, and 10% starch + 10% β-casein postprandial at T30 (slanted line) and T90 (vertical line). (a-b)Values with different lowercase letters are significantly different from each other (p < 0.05).
Insulin analysis
Postprandial plasma concentrations of insulin in response to (i) 10% starch, (ii) 10% starch gelatinised with 10% α-casein, and (iii) 10% starch gelatinised with 10% β-casein are detailed in Fig. 3B. Ingestion of 10% starch resulted in a rise from 21 (SE = 3) nU/L to 111 (SE = 21) nU/L in 30 min. This was followed by a decline to 38 (SE = 8) nU/L at T90. Ingestion of 10% starch gelatinised with 10% β-casein resulted in a rise from 18 (SE = 2) nU/L to 151 (SE = 25) nU/L within 30 min and decreased to 55 (SE = 9) nU/L at T90. Ingestion of 10% starch gelatinised with 10% α-casein resulted in a rise from 20 (SE = 2) nU/L to 138 (SE = 23) nU/L within 30 min, decreasing to 74 (SE = 13) nU/L at T90. Postprandial insulin levels at T30 tended (p < 0.1) to be higher for starch gelatinised with β-casein compared to 10% starch and at T90 tended (p < 0.1) to be higher for 10% starch gelatinised with 10% α-casein compared to 10% starch. Plasma levels for both GIP and insulin peaked at the same time point (T30).
Amino acid composition
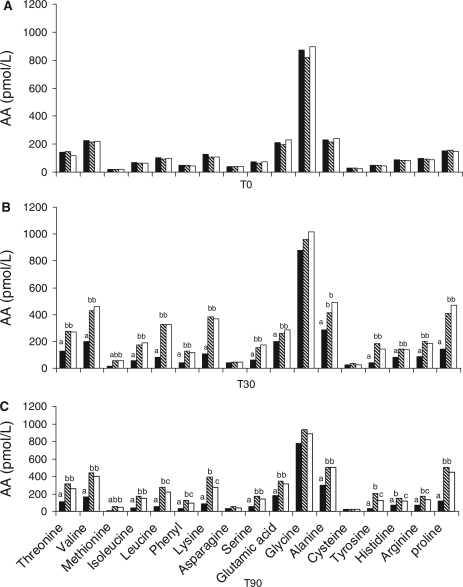
Total amino acid concentration in plasma (Fig. 3C) decreased on ingestion of 10% starch alone from a T0 concentration of 1,213 pmol/L to a T90 concentration of 887 pmol/L. There was a significant (p < 0.05) increase in plasma amino acid concentration at T30 and T90 postprandial in response to 10% starch gelatinised with 10% α-casein and 10% starch gelatinised with 10% β-casein compared to T0 levels. Not surprisingly, there was a significant (p < 0.05) increase in plasma concentrations of individual free amino acids, postprandial in response to 10% starch gelatinised with 10% α-casein and 10% starch gelatinised with 10% β-casein at T30 and T90 (Figs 4B and 4C) compared to basal levels. Exceptions to this were levels of glycine, cysteine, and asparagine. Moreover, there was a significant (p < 0.05) increase in T90 plasma levels of leucine, phenylalanine, lysine, tyrosine, histidine, and arginine postingestion of 10% starch gelatinised with 10% α-casein compared to 10% starch gelatinised with 10% β-casein (Fig. 4C).
Fig.4. .
(A) Concentrations of free amino acids in plasma preprandial (T0) of 10% starch  , 10% starch + 10% α-casein
, 10% starch + 10% α-casein  , and 10% starch + 10% β-casein
, and 10% starch + 10% β-casein  . (B) Concentrations of free amino acids in plasma postprandial (T30) of 10% starch
. (B) Concentrations of free amino acids in plasma postprandial (T30) of 10% starch  , 10% starch + 10% α-casein
, 10% starch + 10% α-casein  , and 10% starch + 10% β-casein
, and 10% starch + 10% β-casein  . (a-b)Values with different lowercase letters are significantly different from each other (p < 0.05). (C) Concentrations of free amino acids in plasma postprandial (T90) of 10% starch
. (a-b)Values with different lowercase letters are significantly different from each other (p < 0.05). (C) Concentrations of free amino acids in plasma postprandial (T90) of 10% starch  , 10% starch + 10% α-casein
, 10% starch + 10% α-casein  , and 10% starch + 10% β-casein
, and 10% starch + 10% β-casein  . (a,b,c)Values with different lowercase letters are significantly different from each other (p < 0.05).
. (a,b,c)Values with different lowercase letters are significantly different from each other (p < 0.05).
Discussion
Ingestion of starch gelatinised with α-casein resulted in a lower postprandial glucose peak, lower glucose levels over 120 min, and increased levels of several free amino acids than ingestion of starch gelatinised with β-casein. The difference in blood glucose levels may be explained by the lower peak viscosity of starch-α-casein compared to that of the starch-β-casein equivalent. Lower peak viscosity may be indicative of reduced granule swelling. Previous work by Kett et al. showed that the swelling characteristics of starch–protein mixtures were concentration dependent (15). The starch–protein concentration [compared to previous studies at 5% (15)] in this study, i.e. 10%, appeared to result in more extensive restriction swelling in the starch–α-casein mixture. During heating, starch granules absorb water and increase in size resulting in a concomitant increase in viscosity (21). We postulate that granule restriction specific to the starch and α-casein mixture may be due to (i) competition between the starch and α-casein for available water in the mixture or (ii) association of α-casein with the surface proteins/lipids of the starch granules protecting granular structure (22). Protection of starch granules, resulting in increased granular structure, may have resulted in reduced digestion by pancreatic α-amylase activity and delayed glucose release into the blood. Several studies have demonstrated granule restriction of starch in the presence of various polymers including proteins (23, 24). In addition, water competition has been reported in heated solutions of waxy rice starch and sodium caseinate (25). Studies performed by the authors would suggest that reduced granular swelling has a role to play in inhibiting enzymatic hydrolysis, delaying subsequent breakdown of the starch. Confocal and light microscopy indicated that waxy maize starch gelatinised in the presence of α- or β-casein resulted in a significant reduction in granular swelling compared to starch gelatinised alone (15). Studies by Manders et al. (26) showed that, in healthy and diabetic patients, ingestion of a carbohydrate (50:50 glucose–maltodextrin mixture) and casein hydrolysate–amino acid mixture lowers plasma glucose concentration compared to ingestion of carbohydrate alone. Previous work by van Loon et al. (27) with drinks containing CHO (50:50 glucose–maltodextrin mixture) and three different protein hydrolysates (i.e. whey, wheat, and casein) reported that ingestion of the CHO–casein hydrolysate drink resulted in the lowest blood glucose levels 30 min postprandial. In contrast, the presence of β-casein in the gelatinised starch sample provided little protection against amylase degradation in our study.
Although the plasma levels of total amino acids did not differ between starch–casein samples, significant differences at T90 in six (leucine, phenylalanine, lysine, tyrosine, histidine, and arginine) individual amino acids postprandial in response to starch gelatinised with α-casein compared to starch gelatinised with β casein may be due to differences in (i) molecular state (28), (ii) hydrophobicity (29, 30), (iii) amino acid sequence and composition (31), and (iv) serine phosphate groups (32) of the α- and β-casein proteins. Although αs1-casein and β-casein are 31% homologous, αs1-casein consists of 199 amino acid residues (GenBank GI:30794348) whereas β-casein is composed of 209 amino acid residues (GenBank GI:162931) (29). In addition, β-casein has less phosphate groups than α-casein. In acidic conditions, such as in the stomach, partial dephosphorylation of casein occurs, reducing the size of protein precipitates, thus facilitating peptic digestion. Therefore, β-casein with its lower degree of phosphorylation is more susceptible to peptic hydrolysis (33). Evidence of this susceptibility can be seen in Fig. 3C whereby the percentage increase (from T0-T180) in total free amino acids was statistically higher for the starch–β-casein mixture compared to the starch–α-casein mixture. Assuming that the postprandial levels of the significant six amino acids were primarily as a result of the dietary source, ingestion of β-casein within the starch-β-casein mixture resulted in a reduction in the plasma levels of these amino acids after 90 min, which may imply that the starch–β-casein mixture was absorbed from the blood at a faster rate than starch–α-casein equivalents. Of the six amino acids that were significantly different, five are essential amino acids. Adibi et al. reported that essential amino acids were absorbed more rapidly within a mixture of amino acids compared to the non-essential amino acids (34).
The insulinotropic amino acids, arginine, leucine, and phenylalanine (35, 36), were significantly (p < 0.05) higher in plasma 90 min postprandial in response to starch gelatinised with α-casein compared to starch gelatinised with β-casein. van Loon et al. (27) showed that these three amino acids promote insulin production when orally administered with carbohydrate. There was a significant difference at T30 in GIP levels in response to the control compared to starch gelatinised with α- or β-casein. Although there was no significant difference in plasma insulin levels between starch and α- or β-casein, there was an observed tendency (p < 0.1) for an increase in plasma insulin levels 90 min postprandial in response to starch gelatinised with α-casein albeit compared to starch alone.
Not surprisingly the addition of casein to starch resulted in higher levels of free amino acids in the peripheral blood within 30 min compared to starch alone. Nilsson et al. (37) reported that peak amino acid levels in humans for milk proteins occurred 30–45 min postprandial, indicating that milk proteins are highly digestible and result in a rapid release of free amino acids into peripheral blood.
Conclusions
This study demonstrates that individual protein fractions can affect swelling characteristics, viscosity, and subsequent digestion of waxy maize starch. The presence of 10% α-casein during gelatinisation of the starch resulted in a significantly lower peak glucose concentration during subsequent digestion in an in vivo pig model compared to 10% β-casein. The difference in glucose release could be attributed to (i) restricted granule swelling during gelatinisation and/or (ii) differences in viscosity restricting α-amylase activity during digestion. In addition, plasma levels of six individual amino acids were increased following ingestion of starch gelatinised with α-casein compared to ingestion of starch gelatinised with β-casein. These findings suggest that the type of protein used in starch based food systems may influence the ability of the food to modulate glycemia. This is an important consideration in the design of foods with positive health benefits.
Acknowledgement
We would like to thank Stefan Buzoianu and William Ryan for cannulation of the pigs and Paula O. Connor for amino acid analysis. We would like to acknowledge Kerry Dairy Ingredients, Ltd., for supplying the casein fractions used in this study. All authors have read and approved the final manuscript.
Conflicts of interest and funding
The authors have no conflicts of interest to declare. Funding was provided by the Department of Agriculture and Foods’ Food Institutional Research Measure (FIRM) under the National Development Plan 2007–2013. Anthony Kett and Christine Bruen were in receipt of Teagasc Walsh Fellowships.
References
- 1.Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab. 2008;5:1–8. doi: 10.1186/1743-7075-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins DJA, Wolever TMS, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 3.Augustin LSA, Franceschi S, Jenkins DJA, Kendall CWC, Vecchia CL. Glycemic index in chronic disease: a review. Eur J Clin Nutr. 2002;56:1049–71. doi: 10.1038/sj.ejcn.1601454. [DOI] [PubMed] [Google Scholar]
- 4.Virkamaki A, Ueki K, Kahn R. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–43. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijpels G. Determinants for the progression from impaired glucose tolerance to non-insulin-dependant diabetes mellitus. Eur J Clin Invest. 1998;28:8–13. doi: 10.1046/j.1365-2362.1998.0280s2008.x. [DOI] [PubMed] [Google Scholar]
- 6.Brand J, Colagiuri S, Crossman S, Allen A, Roberts D, Truswell S. Low-glycemic index foods improve long term glycemic control in NIDDM. Diabetes Care. 1991;14:95–101. doi: 10.2337/diacare.14.2.95. [DOI] [PubMed] [Google Scholar]
- 7.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IME, Asp NG, Vessby BOH. Improved glycemic control and lipid profile and nomalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22:10–8. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Bjorck I, Liljeberg H, Ostman E. Low glycemic-index foods. Br J Nutr. 2000;83:149–55. doi: 10.1017/s0007114500001094. [DOI] [PubMed] [Google Scholar]
- 9.Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker PL. The glucagon-like peptides: pleiotropic regulators of nutrient homeostasis. Ann N Y Acad Sci. 2006;1070:10–26. doi: 10.1196/annals.1317.006. [DOI] [PubMed] [Google Scholar]
- 11.Holt S, Miller J, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr. 1997;66:1264–76. doi: 10.1093/ajcn/66.5.1264. [DOI] [PubMed] [Google Scholar]
- 12.Lang V, Bellisle F, Alamowitch C, Craplet C, Bornet F, Slama G, et al. Varying the protein source in mixed meal modifies glucose, insulin and glucagon kinetics in healthy men, has weak effects on subjective satiety and fails to affect food intake. Eur J Clin Nutr. 1999;53:959–65. doi: 10.1038/sj.ejcn.1600881. [DOI] [PubMed] [Google Scholar]
- 13.Nuttall F, Gannon M. Plasma glucose and insulin response to macronutrients in nondiabetic and NIDDM subjects. Diabetes Care. 1991;14:824–38. doi: 10.2337/diacare.14.9.824. [DOI] [PubMed] [Google Scholar]
- 14.Nuttall F, Gannon M, Wald J, Ahmed M. Plasma glucose and insulin profiles in normal subjects ingesting diets of varying carbohydrate, fat and protein content. J Am Coll Nutr. 1985;4:437–50. doi: 10.1080/07315724.1985.10720086. [DOI] [PubMed] [Google Scholar]
- 15.Kett AP, Fitzsimons SM, Morris ER, Fenelon MA. 5th International Symposium on Food Rheology and Structure; pp. 658–659. Zurich, Laboratory of Food Process Engineering. [Google Scholar]
- 16.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 17.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 18.International Dairy Federation. Milk: Determination of the nitrogen content (Kjeldahl method) and calculation of crude protein content; 1993. International Dairy Federation. [Google Scholar]
- 19.Debet MR, Gidley MJ. Three classes of starch granule swelling: influence of surface proteins and lipids. Carbohydr Polym. 2006;64:452–65. [Google Scholar]
- 20.Niiyama M, Yonemichi H, Harada E, Syuto B, Kitagawa H. A simple catheterisation from the ear vein into the jugular vein for sequential blood sampling from unrestrained pigs. Jpn J Vet Res. 1985;33:1–9. [PubMed] [Google Scholar]
- 21.Tester RF, Karkalas J, Qi X. Starch structure and digestibility; enzyme-substrate relationship. World's Poult Sci J. 2004;60:186–95. [Google Scholar]
- 22.Noisuwan A, Hemar Y, Wilkinson B, Bronlund JE. Adsorption of milk proteins onto rice starch granules. Carbohydr Polym. 2011;84:247–54. [Google Scholar]
- 23.Christianson DD, Hodge JE, Osborne D, Detroy RW. Gelatinization of wheat starch as modified by xanthan gum, guar gum and cellulose gum. Cereal Chem. 1981;58:513–7. [Google Scholar]
- 24.Kelly RJ, Wagenberg MV, Latham J, Mitchell JR. A rheological comparison between the effects of sodium caseinate on potato and maize starch. Carbohydr Polym. 1995;28:347–50. [Google Scholar]
- 25.Noisuwan A, Hemar Y, Bronlund JE, Wilkinson B, Williams MAK. Viscosity, swelling and starch leaching during the early stages of pasting of normal and waxy rice starch suspensions containing different milk ingredients. Starch. 2007;59:379–87. [Google Scholar]
- 26.Manders RJ, Wagenmakers AJM, Koopman R, Zorenc AH, Menheere PP, Schaper NC, et al. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr. 2005;82:76–83. doi: 10.1093/ajcn.82.1.76. [DOI] [PubMed] [Google Scholar]
- 27.van Loon LJC, Saris WHM, Verhagen H, Wagenmakers AJM. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr. 2000;72:96–105. doi: 10.1093/ajcn/72.1.96. [DOI] [PubMed] [Google Scholar]
- 28.Horne DS. Casein structure, self-assembly and gelation. Curr Opin Colloid Interface Sci. 2002;7:456–61. [Google Scholar]
- 29.Dalgleish DG. Structure-function relationships of caseins. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Marcel Dekker; 1997. pp. 199–223. [Google Scholar]
- 30.Patino JM, Sánchez CC, Nino MR. Structural and morphological characteristics of β-casein monolayers at the air-water interface. Food Hydrocolloids. 1999;13:401–8. [Google Scholar]
- 31.Swaisgood HE. Chemistry of the caseins. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry. proteins. New York: Kluwer Academic/Plenum Publishers; 2003. pp. 139–201. [Google Scholar]
- 32.Fox PF. Milk proteins: general and historical aspects. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry.proteins. Kluwer academic; 2003. pp. 1–48. [Google Scholar]
- 33.Fox M, Georgi G, Boehm G, Menne D, Fried M, Thumshirn M. Dietary protein precipitation properties have effects on gastric emptying in healthy volunteers. Clin Nutr. 2004;23:641–6. doi: 10.1016/j.clnu.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Adibi SA, Gray SJ, Menden E. The kinetics of amino acid absorption and alteration of plasma composition of free amino acids after intestinal perfusion of amino acid mixtures. Am J Clin Nutr. 1967;20:24–33. doi: 10.1093/ajcn/20.1.24. [DOI] [PubMed] [Google Scholar]
- 35.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Insulin secretion in response to protein ingestion. J Clin Invest. 1966;45:1479–86. doi: 10.1172/JCI105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson M, Stenberg M, Frid A, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]