Abstract
As in many human patients with X-linked hypohidrotic ectodermal dysplasia (XHED), XHED dogs are at an increased risk for pulmonary disorders. Localized immune system defects had been suspected previously in affected dogs because of frequent infections and unexpected deaths due to opportunistic respiratory tract infections. Experiments were designed to examine systemic and localized humoral and cellular responses, development and function of T cells, and thymic morphology. All dogs used in these experiments were clinically healthy at the time of examination and their immune responses were compared to normal littermates. Serum immunoglobulin concentrations differed somewhat between normal dogs and dogs affected with XHED but they were all within normal ranges. The XHED dogs responded appropriately to vaccination with tetanus toxoid suggesting normal systemic B and plasma cell function. Thymic morphology was compared between normal and affected dogs and T cells were assessed for functionality. Numbers and phenotypes of T and B cells in blood and thymus of affected dogs were within normal limits suggesting normal development of T cells. Cytotoxic and phagocytic ability of macrophages and neutrophils was also normal in affected dogs. In contrast, the secretory IgA concentrations found in affected dogs were significantly higher than in normal dogs, while lacrimal secretions were significantly decreased. These results suggest a compensatory mechanism for secretory IgA, so that the total amount equals that in normal dogs. The results presented in this study indicate that the XHED dogs have a relatively intact immune system and suggest that the same is true for humans with the homologous form of XHED.
Keywords: Dog, Ectodermal dysplasia, Immune competence, Genetics, Respiratory tract
1. Introduction
In X-linked hypohidrotic ectodermal dysplasia (XHED) in man (MIM #305100; defect in ED1), affected individuals have a developmental disorder characterized by sparse or absent hair, missing and/or malformed teeth, and hypoplastic eccrine glands (Beahrs et al., 1971; Soderholm and Kaitila, 1985; Clarke, 1987; Clarke et al., 1987; Kere et al., 1996). There is significant morbidity and mortality in affected children due to hyperthermia because of their inability to sweat and due to an increased risk of respiratory tract infections (Beahrs et al., 1971; Soderholm and Kaitila, 1985; Clarke et al., 1987; Gilgenkrantz et al., 1989). The human X-linked HED phenotype has been shown to be a result of mutations in the ectodysplasin (ED1) gene (Zonana et al., 1993; Kere et al., 1996; Monreal et al., 1998).
X-linked ectodermal dysplasia has also been described in a variety of dog breeds, cattle and the Tabby mouse and is characterized by hypoplasia of hair and sweat glands, as well as missing and malformed teeth (Drogemuller et al., 2001, 2002, 2003; Moura and Cirio, 2004). Over the past 10 years we have maintained a colony of dogs with XHED derived from an affected male German shepherd that was donated. The XHED male was bred to a Giant Schnauzer and a beagle-cross producing carrier females which in turn have produced all of the dogs in the colony. The affected dogs have all of the classic signs of XHED and the disease is caused by a splice acceptor site mutation in intron 8 of ED1 (Casal et al., 1997, 2004). As in most human patients with XHED, we have found increased morbidity and mortality to other, usually benign, rarely fatal pulmonary infectious diseases. Most affected dogs had chronic nasal and ocular discharge, often accompanied by corneal ulceration, and a small number of the adult dogs had chronic, treatment-resistant demodecosis that in veterinary medicine is associated with a mildly compromised immune system (Caswell et al., 1997).
In humans with XHED, it is thought that the increased occurrences of pulmonary disease are due to the lack of mucous glands in the respiratory tract (Reed et al., 1970; Beahrs et al., 1971; Siegel and Potsic, 1990). To rule out immune system defects as a cause for the increased pulmonary disorders in the XHED dogs, we compared systemic and local immune parameters of clinically healthy, affected dogs to age-matched normal dogs.
2. Materials and methods
2.1. Dogs and tissues
Medical records from 30 XHED dogs (23 males and 7 females – produced by breeding affected males to carrier females) and 59 non-affected littermates (29 males and 30 females) were reviewed for morbidity and mortality rates. The dogs’ genotypes were determined by assessing their phenotype at birth (Casal et al., 1997) and later confirmed by PCR (Casal et al., 2004). Depending on the assays to be performed, three to nine affected dogs and 3–10 age-matched normal dogs were used for each assay. At the time of sampling, all dogs were clinically healthy to prevent alteration of immune parameters through other disease processes. Three affected and three normal littermates were euthanized for evaluation of the immune and hematopoietic organs. The thymus was carefully removed from each dog to obtain the whole organ for weighing and histological appearance. In addition to the thymus, the spleen, submandibular lymph node, tonsils, and bone marrow were obtained from each dog for determination of total T cell percentage (CD3+) and B cells (CD21+) of each organ, the percentage of CD4+ and CD8+ cells within the CD3 population, and the total percentage of double-positive T cells (CD4+8+) in the thymus. Pulmonary cells were obtained by transtracheal washing immediately after death using 250 ml of sterile saline. The fluid that was recovered was centrifuged, the cells removed, and used for evaluation by cytospin and measurement of TNF-alpha production. All animals were cared for according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals and in the International Guiding Principles for Biomedical Research Involving Animals.
2.2. Immune parameters
Blood was drawn from five XHED affected dogs and from ten normal, age-matched dogs for complete blood cell counts and to study the phenotype of peripheral blood mononuclear cells through flow cytometry according to standard practice in the laboratory (Felsburg et al., 1998). The isotype control tube contained anti-mouse IgG-FITC and anti-mouse IgG2a-PE. Leukocytes were selected by using anti-human CD14-FITC and anti-canine CD45-PE to determine the hematopoietically derived cell and monocyte population. After staining with anti-canine CD3-FITC, anti-canine CD4-PE, anti-canine CD8-PE, and anti-canine CD21-PE, T and B cells were analyzed on a BD FACScaliber (Becton, Dickinson, and Company, Franklin Lakes, NJ). Thymic cells were characterized using the same protocol.
Serum immunoglobulins from nine affected and nine normal dogs were quantified through radial immunodiffusion (Bethyl Laboratories, Inc., Montgomery, TX). The appropriate amount of serum was added to the wells of IgG, IgG1, IgG2, IgA and IgM plates and the amount of immunoglobulin in the serum of each sample was quantified by measuring the precipitin rings surrounding the wells. The diameter of each ring was compared to a standard curve created from controls of known immunoglobulin concentration. Serum complement 3 (C3) was also measured in eight normal and three affected age-matched dogs using a radial immunodiffusion assay. Briefly, 5 ml of 1% agarose containing 2% goat anti canine C3 were poured in plastic plates and wells of 3 mm in diameter were made using the enclosed template (ICN Pharmaceuticals, Inc., Costa Mesa, CA). After 1:25 dilution in PBS, the serum samples were added to the plates at 10 μl per well. The plates were then incubated at room temperature and the resulting rings measured after 12 h. All samples were run in duplicates.
Specific antibody production was measured in 8-week-old affected (N = 3) and age-matched normal (N = 3) dogs. Three affected and three normal dogs were immunized intramuscularly with 0.5 ml tetanus toxoid. The immunizations were repeated 2 weeks later. Four normal dogs were used as unvaccinated controls. Blood was drawn at weekly intervals for 5 weeks from all vaccinates and non-vaccinates beginning 1 week prior to vaccination and the serum samples were collected and frozen. An ELISA was performed to determine the tetanus specific IgG in the serum samples (Hartnett et al., 2002). The plates were evaluated on an ELISA plate reader at 490 nm. Samples were run in duplicates.
Secretary IgA was measured from lacrimal secretions to assess the mucosal immunity of XHED (N = 5) and age-matched control dogs (N = 6). Schirmer Tear Tests (Schering-Plough, Union, NJ) were inserted into the medial canthus of each eye for exactly 1 min to collect lacrimal fluid and to measure tear production. The lacrimal secretions were centrifuged out of the tear strips and frozen at −70 °C until all samples were collected. IgA was measured with the Dog IgA ELISA Quantitation Kit (Bethyl Laboratories, Inc., Montgomery, TX). Lacrimal IgA measurements were performed in duplicates.
TNF-alpha production was determined in five affected and five normal age-matched dogs by stimulating macrophages with LPS at 5 μg/ml at 37 °C for 6–20 h and then incubating the supernatant with WEHI-164 cells according to previously described methods (Campbell et al., 1997). Samples were analyzed in duplicates.
Neutrophils were obtained from four affected and five age-matched control dogs to assess neutrophil function. The capacity of neutrophils to phagocytize opsonized, fluorescent Escherichia coli was assessed using the Phagotest (OrpegenPharma, Germany) and analyzed by flow cytometry. The cytolytic ability of the neutrophils was evaluated using the oxidative burst assay (Felsburg et al., 1998). All samples were run in duplicates.
Statistical analyses were performed using the Mann–Whitney test for small sample sizes that were not distributed normally (InStat® for Macintosh 2.03, GraphPad Software).
3. Results
3.1. Dogs
Of the 89 offspring born to carrier females and normal or affected males, 30 dogs were affected (23 males and 7 females) and 59 were carrier or normal dogs (29 males and 30 carrier females). There was no phenotypical difference between affected males and affected females. About 30% of the affected pups died within the first 3 days of life while only 10% of the non-affected pups died in the perinatal period (normal losses are 7–10%). Two affected pups and one normal pup had severe cleft palates, but no overt cause of death could be determined for the rest that died at or shortly after birth. Over 50% of the post-weaning survivors had severe forms of bronchopneumonia, which was fatal in 27% of these dogs. Histology of the lungs of two XHED dogs that died of severe pneumonia demonstrated a lack of appropriate numbers of macrophages that would be expected for the extent of disease.
To examine cellular components of the pulmonary defense mechanisms, tracheal washes were performed in three clinically healthy affected dogs and three age-matched control dogs. There appeared to be more macrophages and fewer neutrophils in the tracheal wash fluids obtained from affected dogs compared to that obtained from the normal age-matched controls (Table 1).
Table 1.
Distribution of white blood cell types (in %) harvested by transtracheal washes from the lungs of normal (N = 3) and affected (N = 3) dogs
| Normal dogs | XHED dogs | |
|---|---|---|
| Macrophages | 83.2 (81.1–86.8) | 91.5 (91.2–92.4) |
| Neutrophils | 4.2 (1.9–5.9) | 1.2 (0–1.8) |
| Lymphocytes | 8.9 (3.6–10.7) | 4.5 (3.5–7.9) |
| Eosinophils | 1.6 (0.5–4.9) | 0.9 (0.3–2.7) |
| Plasma cells | 0.9 (0.3–4.9) | 0 (0–2) |
| Mast cells | 0.9 (0–2.1) | 0 (0–0) |
Median and range are given. Statistical analysis could not be performed, as the sample sizes were too small.
3.2. Lymphoid tissue and peripheral blood mononuclear cell (PBMC) characterization
Blood was drawn from five adult affected dogs to compare phenotypes of the PBMC to normal adult ranges and 10 normal dogs housed in the same facility. Comparison of complete blood cells counts revealed that all total white blood cell, neutrophil, lymphocyte, monocyte and eosinophil counts were within normal ranges established in the laboratory and did not differ significantly between normal and affected dogs (Table 2). Each sample was stained using canine-specific antibodies CD3, CD4, CD8, CD14, CD21, and CD45RA. No statistically significant differences were found between the percentages of the cells examined from normal and affected dogs except for decreased CD45RA positive cells in the affected dogs (Table 3).
Table 2.
White blood cell counts (cells/μl) in five affected dogs and ten normal, age-matched control dogs
| Normal dogs | XHED dogs | |
|---|---|---|
| Total WBC | 10990 ± 1371 | 14506 ± 1337 |
| Neutrophils | 7000 ± 1029 | 10460 ± 1277 |
| Lymphocytes | 2800 ± 341 | 2680 ± 362 |
| Monocytes | 624 ± 71 | 846 ± 168 |
| Eosinophils | 566 ± 190 | 520 ± 192 |
There was no statistically significant difference between the affected and normal dogs at p < 0.05 (Mann–Whitney test).
Table 3.
Blood lymphocyte profiles in 5 affected dogs and 10 normal, age-matched control dogs (expressed in % of gated cells ± SEM)
| Normal dogs | XHED dogs | |
|---|---|---|
| B cells | 13.2 ± 4.8 | 16.4 ± 6.4 |
| CD3+ | 76.2 ± 5.8 | 71.6 ± 5.4 |
| CD4+ | 44.5 ± 4.8 | 41.9 ± 7.2 |
| CD8+ | 23.8 ± 3.6 | 25.2 ± 5.4 |
| CD45RA+ | 73.4 ± 3.7 | 61.1 ± 4.4* |
Statistically significant difference (p = 0.001; Mann–Whitney test).
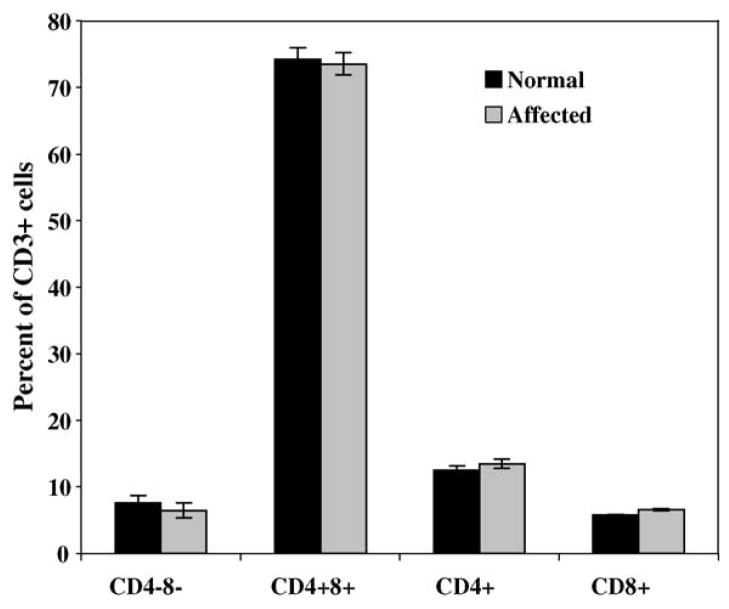
Thymus weights and histological appearance of all lymphoid tissues were compared between three 6-month-old normal and three 6-month-old affected dogs. There were no apparent differences in thymic weights (data not shown) and no differences in the microscopic appearance of lymphoid tissues from affected dogs when compared to the normal controls. Thymus, spleen, submandibular lymph node, tonsils, and bone marrow were also analyzed for total T cell percentage (CD3+) and B cells (CD21+), the percentage of CD4+ and CD8+ cells within the CD3+ population and the total percentage of double-negative (CD4−8−) and double-positive T cells (CD4+8+) in the thymus. Tonsils from affected dogs appeared to have a slightly lower percentage of CD3+ cells than the same tissues from the normal counterparts. The percentage of CD8+ cells was slightly lower in lymph nodes from affected dogs compared to normal age-matched controls (not shown). There were no apparent differences within any of the cell lineages from affected or normal thymi (Fig. 1).
Fig. 1.
Flow cytometric analysis of the thymic lymphocyte population. The standard deviation is indicated on the top of each bar; its absence indicates that the standard deviation was too small to be visible on the graph. No statistical analysis was performed, as the sample sizes were too small.
3.3. Humoral immunity
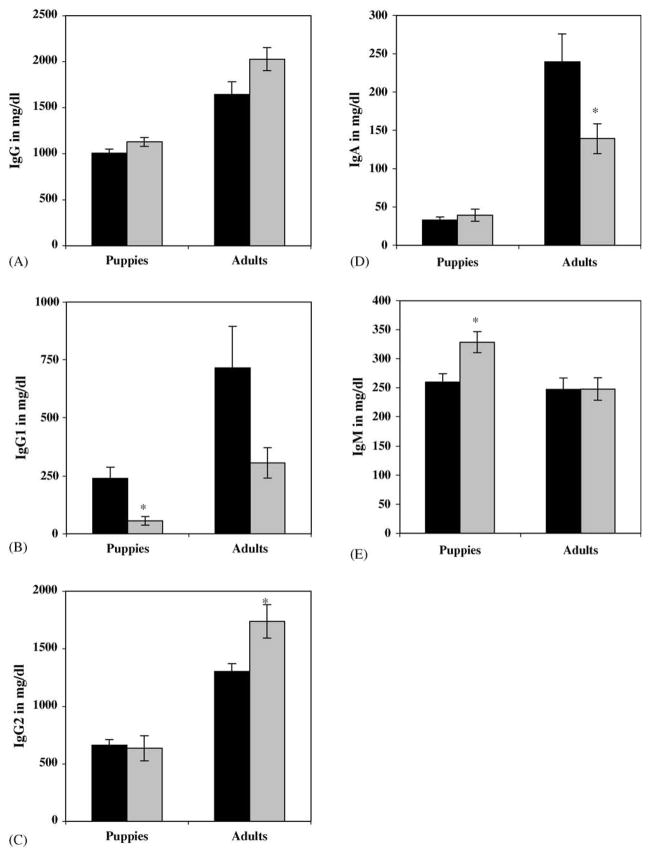
Serum immunoglobulin concentrations were determined in nine affected dogs and nine age-matched normal, control dogs. Results were grouped and statistically evaluated according to age groups, as immunoglobulin concentrations normally differ between adult dogs and those less than 6 months of age. Total serum IgG serum concentrations were slightly higher in both affected puppies and adult dogs than in their normal counterparts. However, there was no statistically significant difference between the values measured in affected versus normal dogs in either age group (Fig. 2A). Serum IgG1 concentrations were significantly lower in affected puppies than in normal puppies. In adults, however, the difference between serum IgG1 levels from affected and normal dogs was not quite significant (p = 0.056; Fig. 2B). Concentrations of IgG2 measured in the serum of affected adult dogs were significantly higher than in that from age-matched normal dogs. In contrast, serum IgG2 concentrations measured in normal puppies were not significantly different from those in affected puppies (Fig. 2C). Similarly, there was no significant difference in serum IgA concentrations between normal and affected puppies, but adult serum IgA levels were significantly lower in the affected dogs (Fig. 2D). Finally, serum IgM concentrations measured in affected puppies were significantly higher than those measured in normal puppies, while they were virtually the same in normal and affected adult dogs (Fig. 2E).
Fig. 2.
Serum concentrations of (A) IgG, (B) IgG1, (C) IgG2, (D) IgA, and (E) IgM in normal (N = 9; black bars) and affected (N = 9; grey bars) dogs grouped according to age (mg/dl ± SEM). Puppies include dogs from 6 to 12 weeks of age and adults are dogs over 1 year of age. *Statistically significant differences between normal and affected dogs.
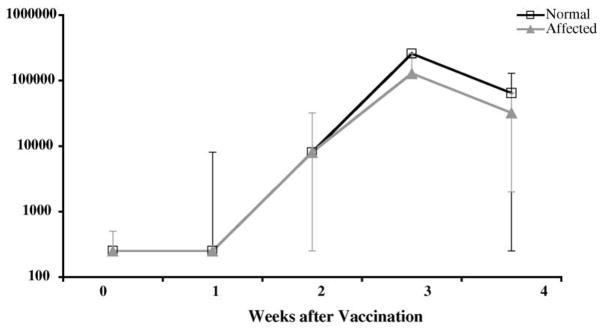
Three normal and three affected 8-week-old dogs were immunized intramuscularly with 0.5 ml tetanus toxoid, which was repeated 2 weeks later. Four normal dogs were used as unvaccinated controls. Blood was drawn at weekly intervals for 4 weeks from all vaccinates and non-vaccinates beginning at the time of vaccination to determine IgG-specific tetanus toxoid antibody production. In all of the vaccinated dogs, the specific antibody concentrations increased slightly at 2 weeks after vaccination, increased dramatically at 3 weeks, and dropped at 4 weeks after vaccination (Fig. 3), while there was no change in titer in the non-vaccinates. Due to the small sample number, statistical analysis was not possible. However, all dogs in the study responded appropriately to the vaccine as has been described elsewhere (Hartnett et al., 2002).
Fig. 3.
Specific antibody response in three normal and three affected 8-week-old dogs after vaccination with tetanus toxoid. The median titers and the ranges are given. Titers measured in four normal dogs used as unvaccinated controls remained at base levels (not shown).
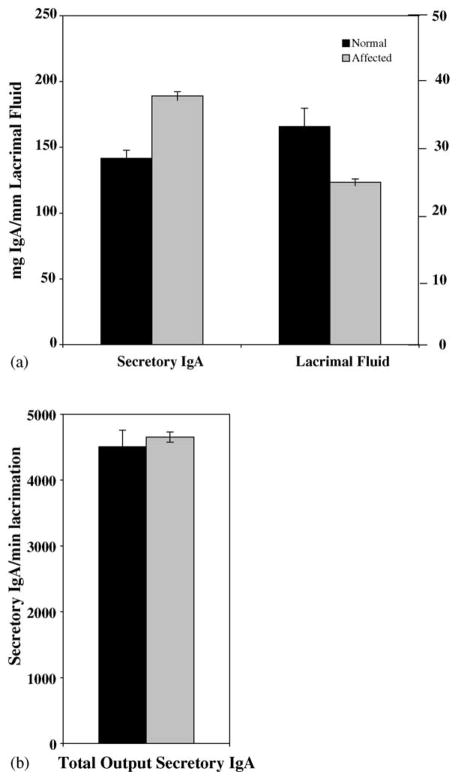
As a measure of local immunity in the respiratory system, secretory IgA (sIgA) was determined in lacrimal fluids from six normal and five affected age-matched pups using an ELISA assay. In affected dogs, tear production per minute was 74.9% of that in normal dogs. However, in normal dogs, sIgA production per ml of lacrimal fluid was 74.3% of that in affected dogs (Fig. 4A). Both differences were significantly different at p ≤ 0.05 but the overall output of sIgA produced per time unit was the same in both affected and normal dogs (Fig. 4B).
Fig. 4.
Secretory IgA and lacrimal fluid production in XHED dogs. Six normal and five affected 12-week-old dogs were assessed for secretory IgA production using an ELISA assay for IgA (mg/mm lacrimal fluid ± SEM) and the Schirmer tear test (mm fluid/min ± SEM). Both differences are statistically significant at p < 0.05 (Mann–Whitney test).
Serum complement (C3) concentrations were measured by radial immunodiffusion in three affecteds and compared to those measured in eight normal dogs. The affected dogs produced significantly more C3 than normal age-matched controls (p = 0.01; Table 4).
Table 4.
Serum complement 3 (C3) concentrations in affected and normal dogs
| Normal dogs (N = 8) | XHED dogs (N = 3) | |
|---|---|---|
| Serum C3 | 100 ± 0.6 | 114.1 ± 4.5* |
Concentrations are expressed in percentage of normal ± SEM.
Statistically significant difference (p = 0.01; Mann–Whitney test).
TNF-alpha production was determined both in macrophages obtained from tracheal washes and in neutrophils from peripheral blood from five affected and five normal age-matched controls. The results were expressed in titers before and after stimulation with LPS. A 128-fold increase in titer was measured in pulmonary alveolar macrophages from all affected and normal dogs except for one normal dog that had only a 16-fold titer increase. All dogs showed a four-to eight-fold increase in titers obtained from blood neutrophils after stimulation. There was no statistical difference between the two groups at p ≤ 0.05 (data not shown).
3.4. Neutrophil function tests
The capacity of neutrophils to phagocytize opsonized, fluorescent E. coli was assessed using the Phagotest (OrpegenPharma, Germany) and analyzed by flow cytometry. Neutrophils obtained from four affected dogs appeared to phagocytize the bacteria at a lower rate than the five age-matched control dogs. However, there was no statistically significant difference between the two groups (Fig. 5).
Fig. 5.
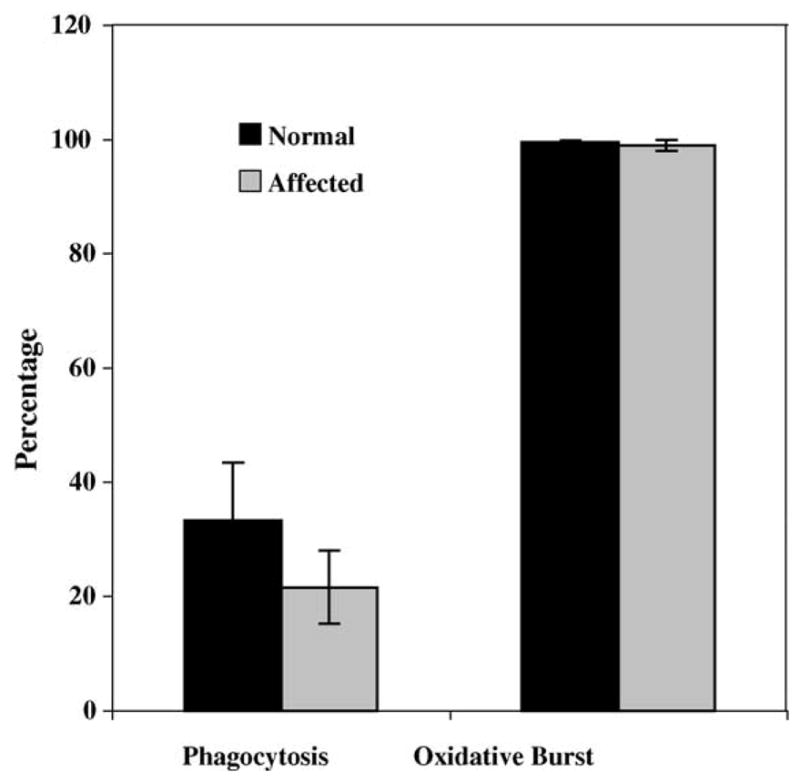
Percentage of neutrophils that engulfed fluorescent bacteria (phagocytosis) and that produced oxygen radicals in the presence of phorbol ester (oxidative burst). There were no statistically significant differences between neutrophils obtained from affected dogs (N = 4) and normal, age-matched controls (N = 5) (p < 0.05; Mann–Whitney test) in either assay. The SEM is indicated on the top of each bar; its absence indicates that the SEM was too small to be visible on the graph.
Neutrophils produce toxic oxygen radicals to assist in the killing of engulfed pathogen by a sudden increase in oxidative metabolism. Neutrophils from five normal and four affected dogs were incubated with non-fluorescent dye and subjected to phorbol ester to produce oxygen radicals, which convert the dye into fluorescent rhodamine that can be visualized by flow cytometry. There was no statistically significant difference in the oxidative burst between neutrophils obtained from affected and normal dogs after stimulation with phorbol ester (Fig. 5).
4. Discussion
Significant morbidity, specifically respiratory tract disease, has often been described in humans with X-linked hypohidrotic ectodermal dysplasia (Reed et al., 1970; Beahrs et al., 1971; Soderholm and Kaitila, 1985; Clarke, 1987; Clarke et al., 1987; Gilgenkrantz et al., 1989; Siegel and Potsic, 1990). All but four of our affected dogs have been housed in a closed, climate-controlled environment and yet the percentage of post-weaning dogs with recurrent respiratory tract infections was around 50%, which is similar to the 44% described in human children (Clarke et al., 1987). Mortality rates in humans and dogs with XHED are also similar at 28% in children under 3 years of age (Clarke et al., 1987) and 30% in puppies less than 3 days of age. Post mortem examination performed in one human patient revealed the absence of bronchial glands in the respiratory tract and non-specific chronic pneumonitis with peribronchiolitis (Reed et al., 1970). However, microscopic evaluation of the respiratory tract in two of our dogs that had died of pulmonary disease revealed not only the expected absence of branched tubuloalveolar mucous (bronchial) glands and chronic interstitial pneumonia, but also a decrease in expected number of macrophages for the severity of disease. Because of the morbidity and mortality associated with XHED and the decreased number of pulmonary macrophages in the face of serious disease in one dog, studies were performed examining the immune system in canine XHED.
As in clinically healthy humans with XHED (Clarke et al., 1987), complete blood cell counts were normal in XHED dogs in the absence of other diseases. However, serum C3 concentrations were significantly higher in the affected dogs than the normal controls, whereas they are normal in humans with XHED (Clarke et al., 1987). The importance of this finding is unclear, but the increase in C3 concentrations in the affected dogs may be due to chronic stimulation of the pulmonary system, which shows diminished mucociliary clearance because of the absence of the bronchial glands (Casal et al., 2005). While some serum immunoglobulin concentrations differed significantly between normal dogs and dogs affected with XHED, all of the serum immunoglobulin concentrations in the affected dogs were within the normal limits given by the manufacturer of the kit. The mild elevations in serum IgG, IgA, and IgM in the affected puppies are likely due to chronic antigenic stimulation as described above. The XHED dogs responded appropriately to vaccination with tetanus toxoid suggesting normal systemic B and plasma cell function. Quantitative examination of B cells from blood and lymphoid organs from XHED dogs was also within normal limits when compared to normal dogs. These results are similar to those in humans with XHED, where serum immunoglobulins have been measured and found to be generally normal (Beahrs et al., 1971; Soderholm and Kaitila, 1985; Clarke et al., 1987). In two human cases, increased IgE was found, which was thought to be due to allergies present in these patients (Soderholm and Kaitila, 1985). Serum alpha- and gamma-interferon, serum protein electrophoresis, and alpha1-globulins were evaluated and found to be normal in humans with XHED (Beahrs et al., 1971; Clarke et al., 1987). While we did not measure the same parameters, we did evaluate TNF-alpha production both in peripheral blood and in pulmonary alveolar macrophages of affected dogs and found this to be normal.
Total CD3+ and the percentage of CD8+ cells were lower in affected dogs’ tonsils and peripheral lymph node, respectively, than in those of normal dogs. However, all thymic parameters were within normal limits suggesting normal development of T cells. Except for decreased CD45RA positive cells in the affected dogs, characterization of peripheral blood cells was also normal, suggesting normal development and distribution. The decrease in CD45 RA population can be interpreted as an increase in conversion to competent T cells because of the chronic antigenic stimulation in the lungs of the XHED dogs. Interestingly, two of eight human patients had slight increases in their CD8+ cells, which were attributed to mild viral infections (Clarke et al., 1987). Functional assays examining the neutrophil function in humans with XHED (Clarke et al., 1987) yielded normal results just as they did in affected dogs. The percentage of pulmonary alveolar macrophages was increased in clinically healthy, affected dogs suggesting increased antigenic stimulation, which can be explained by retention of pathogens by increased production of thick mucous from goblet cells and the absence of bronchial glands to facilitate outward transport of particles (Casal et al., 2005). However, this does not explain the paucity of pulmonary macrophages found in lung tissue from two dogs that died of pneumonia. It is possible that the peracute nature of the pneumonia did not allow for adequate time for production of macrophages, sufficient transit time for the macrophages from the bone marrow to the alveoli, or that there is a defect in the inflammatory response that we were not able to detect with the methods used for this study. However, if an immune defect was suspected as the underlying cause of the increased morbidity in humans and dogs with XHED, this would imply that ectodysplasin plays a role in immunity. In our dog model, only the isotypes EDA-A1 and EDA-A2 are missing, which would normally bind to the ectodysplasin receptor (EDAR) and the X-linked ectodysplasin receptor (XEDAR). To date, no studies have shown binding of EDA-A1 or -A2 to immune cells, which are derived from the mesenchyme. In fact, both receptors, EDAR and XEDAR, have been shown to be expressed only in the ectoderm during a very specific time during fetal development and during the early postnatal period in mice and binding to other receptors have not been shown (Yan et al., 2000).
Because of the lack of eccrine glands in XHED, there is decreased production of lacrimal fluids in both humans and dogs (Clarke et al., 1987; Gilgenkrantz et al., 1989; Casal et al., 1997). Here we demonstrate that the affected dogs consistently produced lacrimal fluid at about 75% of normal. Interestingly, secretory IgA concentrations found in the affected dogs were much higher than in normal dogs. This could be due either to chronic antigenic stimulation or more likely, a compensatory mechanism for the deficient eccrine secretions as the overall production of sIgA was the same as in normal dogs. In humans with XHED, submandibular and/or parotid salivary glands have been shown to produce significantly less fluid than in normal humans (Soderholm and Kaitila, 1985; Clarke et al., 1987; Gilgenkrantz et al., 1989; Nordgarden et al., 2003). However, proteins measured in the saliva were shown to be increased when compared to normal individuals resulting in overall normal output (Nordgarden et al., 2003).
To date, there is only one thorough study that examines systemic immunity in humans with XHED (Clarke et al., 1987), and the results indicated normal immune functions in the few patients examined. In our study, we were able to examine the immune system in a large number of dogs. Our results obtained thus far indicate that systemic and local immunity is normal in XHED dogs as it is in humans. In addition, we were able to follow XHED dogs over long periods of time to examine their disease. The clinical course of XHED is individually variable in both dogs and humans, but the overall picture is the same in both. This further strengthens the XHED dog as a model to understand the pathological basis of disease and ultimately provide a large animal model for therapy trials.
Acknowledgments
The authors wish to thank Margaret Weil, Patty O’Donnell, and the veterinary students of The University of Pennsylvania School of Veterinary Medicine for their excellent XHED colony husbandry and Dr. Mark Haskins for critical advice. This work was supported by NIH grants AR049817, and RR02512 and the National Foundation for Ectodermal Dysplasias.
References
- Beahrs JO, Lillington GA, Rosan RC, Russin L, Lindgren JA, Rowley PT. Anhidrotic ectodermal dysplasia: predisposition to bronchial disease. Ann Int Med. 1971;74:92–96. doi: 10.7326/0003-4819-74-1-92. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Magee D, Kennedy A, Rowlands BJ, Halliday MI. An ELISA for measuring tumour necrosis factor alpha in rat plasma. Eur Cytokine Netw. 1997;8:97–102. [PubMed] [Google Scholar]
- Casal ML, Jezyk PF, Greek JM, Goldschmidt MH, Patterson DF. X-linked ectodermal dysplasia in a German Shepherd dog. J Hered. 1997;88:513–517. doi: 10.1093/oxfordjournals.jhered.a023146. [DOI] [PubMed] [Google Scholar]
- Casal ML, Mauldin EA, Gaide O, Scheidt JL, Rhodes JL. Canine X-linked Ectodermal Dysplasia as a Model for the Human Disease: Mutational Analysis and Further Characterization of Disease. The American Society of Human Genetics; Toronto, Ont., Canada: 2004. [Google Scholar]
- Caswell JL, Yager JA, Parker WM, Moore PF. A prospective study of the immunophenotype and temporal changes in the histologic lesions of canine demodicosis. Vet Pathol. 1997;34:279–287. doi: 10.1177/030098589703400403. [DOI] [PubMed] [Google Scholar]
- Casal ML, Mauldin EA, Scheidt JL, Gaide O. Of mice, dogs, and men: clinical and pathological findings in X-linked ectodermal dysplasia. 2005. in preparation. [Google Scholar]
- Clarke A. Hypohidrotic ectodermal dysplasia. J Med Genet. 1987;24:659–663. doi: 10.1136/jmg.24.11.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Phillips DI, Brown R, Harper PS. Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child. 1987;62:989–996. doi: 10.1136/adc.62.10.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogemuller C, Distl O, Leeb T. Partial deletion of the bovine ED1 gene causes anhidrotic ectodermal dysplasia in cattle. Genome Res. 2001;11:1699–1705. doi: 10.1101/gr.182501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogemuller C, Peters M, Pohlenz J, Distl O, Leeb T. A single point mutation within the ED1 gene disrupts correct splicing at two different splice sites and leads to anhidrotic ectodermal dysplasia in cattle. J Mol Med. 2002;80:319–323. doi: 10.1007/s00109-002-0320-z. [DOI] [PubMed] [Google Scholar]
- Drogemuller C, Distl O, Leeb T. X-linked anhidrotic ectodermal dysplasia (ED1) in men, mice, and cattle. Genet Sel Evol. 2003;35 (Suppl 1):S137–S145. doi: 10.1186/1297-9686-35-S1-S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsburg PJ, Somberg RL, Hartnett BJ, Henthorn PS, Carding SR. Canine X-linked severe combined immunodeficiency A model for investigating the requirement for the common gamma chain (gamma c) in human lymphocyte development and function. Immunol Res. 1998;17:63–73. doi: 10.1007/BF02786431. [DOI] [PubMed] [Google Scholar]
- Gilgenkrantz S, Blanchet-Bardon C, Nazzaro V, Formiga L, Mujica P, Alembik Y. Hypohidrotic ectodermal dysplasia. Clinical study of a family of 30 over three generations. Hum Genet. 1989;81:120–122. doi: 10.1007/BF00293886. [DOI] [PubMed] [Google Scholar]
- Hartnett BJ, Yao D, Suter SE, Ellinwood NM, Henthorn PS, Moore PE, McSweeney PA, Nash RA, Brown JD, Weinberg KI, et al. Transplantation of X-linked severe combined immunodeficient dogs with CD34+ bone marrow cells. Biol Blood Marrow Transpl. 2002;8:188–197. doi: 10.1053/bbmt.2002.v8.pm12014808. [DOI] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, et al. X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet. 1996;13:409–416. doi: 10.1038/ng0895-409. [DOI] [PubMed] [Google Scholar]
- Monreal AW, Zonana J, Ferguson B. Identification of a new splice form of the EDA1 gene permits detection of nearly all X-linked hypohidrotic ectodermal dysplasia mutations. Am J Hum Genet. 1998;63:380–389. doi: 10.1086/301984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura E, Cirio SM. Clinical and genetic aspects of X-linked ectodermal dysplasia in the dog — a review including three new spontaneous cases. Vet Dermatol. 2004;15:269–277. doi: 10.1111/j.1365-3164.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- Nordgarden H, Storhaug K, Lyngstadaas SP, Jensen JL. Salivary gland function in persons with ectodermal dysplasias. Eur J Oral Sci. 2003;111:371–376. doi: 10.1034/j.1600-0722.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Reed WB, Lopez DA, Landing B. Clinical spectrum of anhidrotic ectodermal dysplasia. Arch Dermatol. 1970;102:134–143. [PubMed] [Google Scholar]
- Siegel MB, Potsic WP. Ectodermal dysplasia: the otola-yngologic manifestations and management. Int J Pediatr Otorhinol. 1990;19:265–271. doi: 10.1016/0165-5876(90)90006-d. [DOI] [PubMed] [Google Scholar]
- Soderholm AL, Kaitila I. Expression of X-linked hypohidrotic ectodermal dysplasia in six males and in their mothers. Clin Genet. 1985;28:136–144. doi: 10.1111/j.1399-0004.1985.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Yan M, Wang LC, Hymowitz SG, Schilbach S, Lee J, Goddard A, de Vos AM, Gao WQ, Dixit VM. Two-amino acid molecular switch in an epithelial morphogen that regulated binding to two distinct receptors. Science. 2000;290:523–527. doi: 10.1126/science.290.5491.523. [DOI] [PubMed] [Google Scholar]
- Zonana J, Gault J, Davies KJ, Jones M, Browne D, Litt M, Brockdorff N, Rastan S, Clarke A, Thomas NS. Detection of a molecular deletion at the DXS732 locus in a patient with X-linked hypohidrotic ectodermal dysplasia (EDA), with the identification of a unique junctional fragment. Am J Hum Genet. 1993;52:78–84. [PMC free article] [PubMed] [Google Scholar]