Abstract
Objective:
Apart from its well-known deleterious dental and skeletal effects, fluoride excess can have toxic effects on many other tissues. Fluoride, when in excess, is known to interfere with thyroid gland function. Fluoride-induced thyroid disturbances similar to those observed in iodine deficiency state in spite of adequate iodine intake have been documented. Similar thyroid disturbances in individuals with dental fluorosis have not been well studied in populations with endemic fluorosis. This work was undertaken to study the effects of fluoride-induced thyroid disturbances in individuals with dental fluorosis.
Methods:
The study group included 65 subjects with dental fluorosis from endemic fluorosis populations. An additional control group was comprised of 10 subjects without dental fluorosis. The drinking water fluoride levels of the study populations were analyzed. Serum free FT3, FT4, and TSH levels of both groups were assessed.
Results:
All subjects with dental fluorosis had serum levels of thyroid hormones (FT3, FT4, and TSH) within the normal range, with the exception of 1 individual, who had elevated levels of TSH. Statistical significance was found when FT3 and TSH values were compared with different Dean’s index groups by a 1-way ANOVA test: FT3 (F = 3.4572; P=.0377) and TSH (F = 3.2649 and P=.0449).
Conclusions:
Findings of this study did not show any significant alterations in the levels of the thyroid hormones FT3, FT4, and TSH in subjects with dental fluorosis. Our observations suggest that thyroid hormone levels were not altered in subjects with dental fluorosis. Hence, future studies of this kind, along with more detailed investigations are needed.
Keywords: Dental fluorosis, fluorosis and thyroid hormones, fluoride and thyroid
INTRODUCTION
Prevalent in many parts of the world, fluorosis is caused by prolonged ingestion of excessive amounts of fluoride and endangers the health of both humans and animals.1 The excessive intake of fluoride (F−) via drinking water is an endemic problem in a number of countries, including China, India, and Mexico.2 In addition to its well-known deleterious dental and skeletal effects, excess fluoride can have toxic effects on many other tissues.1 Soft tissue involvement in fluoride toxicity is well established. In the past, the toxic effects of fluoride on the thyroid gland were noticed based on clinical experience gained through the treatment of Grave’s disease.3 It has been documented for many years, most recently in India, that excessive fluoride might cause thyroid disturbances similar to those observed in iodine deficiency, even in individuals with adequate iodine intake.1
It is well-established that dental fluorosis can occur as a result of excessive fluoride exposure during crucial developmental stages; and is marked by events related to timing.4 Thus, apart from the structural anomalies, it is also associated with delayed tooth eruption, delayed removal of enamel matrix proteins and delayed enamel maturation, indicating that the tissue-specific differentiation program is disturbed. Endocrinology has firmly established thyroid hormone (TH) as the crucial regulator of all tissue-specific differentiation programs during development. Appropriate TH levels, expressed at precise developmental times, are critically important for the coordination of developmental processes.4
The findings of Susheela et al5 indicate that the thyroid hormone metabolism is disturbed in the peripheral tissues of children with dental fluorosis and the resultant disturbances in TH levels are similar to those seen in iodine deficiency disorders (IDD). Because any alterations in TH during developmental stages may also influence the neurological development in children, enamel defects, such as dental fluorosis in deciduous teeth and permanent incisors, can serve as a warning that may aid in the diagnosis of neurological and iodine deficiency disorders.4
Fluoride, when in excess, is known to interfere with thyroid gland function and to cause degenerative changes in the central nervous system, impairment of brain function and developmental impairment in children; further investigation of fluorosis is warranted, even in populations with adequate iodine intakes.5
As dental fluorosis is prevalent in the Bagalkot district of Karnataka, India, this study was undertaken to investigate the relationship between dental fluorosis and thyroid hormone status in the region, and to gain a better understanding of the fluorosis problem in general.
MATERIAL AND METHODS
The study included total sample size of 75 subjects. The study group comprised of 65 dental fluorosis subjects, who were selected through school dental camps. Selected study areas included those areas where iodized salt was typically accessible and consumed. Subjects who were residents of known endemic fluorosis regions and who consumed fluoride-containing water were selected for the study group. The criterion of inclusion was that of subjects of age range 7–18 years. Premolars and second molars, followed by the upper incisors, are most frequently affected by fluorosis. Therefore, the minimum age of inclusion was set at 7 years, an age point that typically coincides with central incisor eruption. The maximum age was set at 18 years. Subjects of this age group were easily accessible at schools. A control group was selected from a non-endemic fluorosis region in which the fluoride level of drinking water was assessed and considered to be within a safe limit (i.e. below 1 ppm). The control group comprised 10 subjects of the same age range without dental fluorosis. Written consent from the patients and parents (in the case of primary school children) as well as approval from school authorities was obtained. Thorough oral examinations were done under natural light. Dental fluorosis was recorded using Dean's index (1942), according to World Health Organization (1999) criteria. Recording was made on the basis of the two teeth that were most affected. If the two teeth were not equally affected, the score of the less affected of the two was recorded. Details pertaining to dietary and drinking habits were recorded. Thorough clinical examination was done to assess any obvious enlargement of the thyroid gland. 3mL of blood was collected, from which serum was separated and sent to the Thyrocare center, Mumbai, for TH analysis. The serum levels of free tri-iodothyronine (FT3) and free thyroxine (FT4) were analyzed using competitive chemiluminescent immunoassays; thyroid-stimulating hormone (TSH) levels were analyzed by the ultra-sensitive sandwich chemiluminescent immunoassay method. Samples of drinking water from endemic dental fluorosis areas were collected and sent for fluoride analysis to the Civil Engineering Department, Basaveshwar Engineering College, Bagalkot. The analysis was carried out using the fluoride ion selective electrode method. The obtained results were statistically analyzed by the most appropriate methods.
RESULTS
Out of the 65 subjects in the study group, 27 were boys and 38 were girls. The control group comprised of 10 subjects (5 boys and 5 girls). Age range in the study group was 10–18 years, while that in the control group was 12–14 years (there were no subjects aged 7–9). None of the subjects in either group exhibited any obvious clinical manifestations of thyroid enlargement. Water fluoride levels from the areas of the study group ranged from 0.5 ppm to 4 ppm. Using Dean’s fluorosis index, the distribution of dental fluorosis included 4 subjects with mild, 33 subjects with moderate and 28 cases with a severe degree of dental fluorosis. Water fluoride levels from areas corresponding to subjects with mild fluorosis ranged from 0.5 to 0.6 ppm, and corresponding to subjects with moderate and severe fluorosis ranged from 0.5 to 4 ppm.
Thyroid hormone values
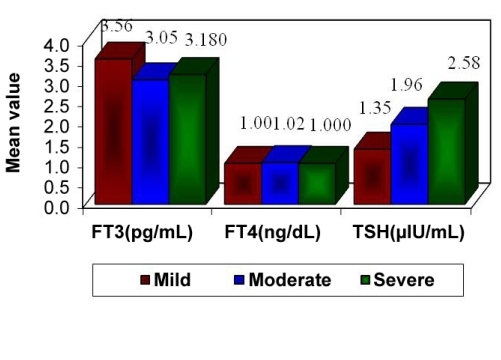
All subjects, except 1 from each of the study and control groups, had normal-range serum levels of thyroid hormones (FT3, FT4, and TSH); 1 subject of each group showed elevated TSH levels. The ranges of serum hormone levels for FT3, FT4, and TSH of both the study and control groups are shown in Table 1. Table 2 shows these serum thyroid hormone levels and Dean’s index of dental fluorosis for all subjects in the study group. Thyroid hormone levels in the control group are shown in Table 3. Comparison of FT3, FT4, and TSH values with mild, moderate, and severe Dean’s index groups by 1-way ANOVA showed a statistical significance FT3 levels (F=3.4572 and P =.0377) and TSH levels (F=3.2649 and P=.0449). There was no significant relationship between fluorosis index and FT4 levels. Pairwise comparison of FT3, FT4, and TSH was performed with Dean’s index groups using student’s t-test. FT3 levels were significantly different between mild and moderate, as well as between mild and severe fluorosis groups, whereas no significant differences in FT3 levels were found between moderate and severe fluorosis groups. On the contrary, FT4 values did not show any significant variation among the 3 fluorosis groups. Pairwise comparison of TSH values between mild and moderate and between mild and severe groups showed that TSH did not significantly vary between these groups, but a significant difference was found between TSH levels of moderate and severe fluorosis groups. Unfortunately, statistical comparison of thyroid hormone levels between the study and control groups was prevented by the small sample size of the control group.
Table 1.
Range of the minimum to maximum values for FT3, FT4 and TSH levels in both study and control group.
| Variable | Normal values | Study group | Control group | |||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | Minimum | Maximum | |
| *FT3(pg/ml) | 1,7 | 4,2 | 1,9 | 4,14 | 2,72 | 3,79 |
| *FT4(ng/dl) | 0,7 | 1,8 | 0,83 | 1,25 | 0,83 | 1,36 |
| *TSH(μIU/mL) | 0,3 | 5,5 | 0,43 | 5,91 | 0,94 | 11 |
FT3-free triiodothyronine,
FT4-free thyroxine,
TSH-thyroid stimulating hormone
Table 2.
Serum levels of thyroid hormones (FT3, FT4 and TSH) and Dean’s Index of dental fluorosis in study subjects.
| Nos | Age | Sex | Dean’s Index | *FT3 (1.7–4.2pg/mL) | *FT4 (0.7–1.8ng/dL) | *TSH ( 0.3–5.5uIU/mL) |
|---|---|---|---|---|---|---|
| 1 | 13 | F | moderate | 3,16 | 1,01 | 2,38 |
| 2 | 13 | F | severe | 3,32 | 0,89 | 3,34 |
| 3 | 17 | F | moderate | 2,55 | 0,96 | 1,04 |
| 4 | 18 | M | moderate | 3,23 | 0,94 | 0,56 |
| 5 | 17 | m | moderate | 1,9 | 1,11 | 0,86 |
| 6 | 18 | M | moderate | 3,47 | 1,05 | 0,49 |
| 7 | 18 | F | moderate | 2,3 | 1,07 | 0,95 |
| 8 | 17 | F | moderate | 3,08 | 1,07 | 0,88 |
| 9 | 14 | M | moderate | 3,07 | 1,17 | 2,03 |
| 10 | 17 | M | severe | 3,21 | 0,94 | 1,43 |
| 11 | 14 | M | mild | 3 | 0,98 | 0,72 |
| 12 | 14 | M | mild | 4,08 | 1,16 | 1,6 |
| 13 | 14 | M | mild | 3,52 | 0,86 | 1,67 |
| 14 | 17 | M | moderate | 2,89 | 0,83 | 1,75 |
| 15 | 18 | M | moderate | 2,55 | 0,9 | 1,55 |
| 16 | 16 | M | moderate | 3,14 | 0,9 | 2,2 |
| 17 | 17 | M | moderate | 2,75 | 1,21 | 0,94 |
| 18 | 15 | F | severe | 3,18 | 0,96 | 2,5 |
| 19 | 16 | M | moderate | 3,16 | 0,93 | 1,78 |
| 20 | 11 | M | moderate | 3,37 | 1,11 | 5,91 |
| 21 | 12 | F | mild | 3,65 | 0,99 | 1,41 |
| 22 | 15 | F | moderate | 2,46 | 0,84 | 2,12 |
| 23 | 14 | F | severe | 2,68 | 1,11 | 1,58 |
| 24 | 16 | M | moderate | 3,3 | 1,18 | 2,01 |
| 25 | 15 | F | modeate | 2,98 | 0,89 | 2,88 |
| 26 | 14 | F | severe | 3,39 | 0,91 | 4,66 |
| 27 | 11 | F | severe | 3,55 | 0,88 | 1,81 |
| 28 | 13 | F | severe | 2,72 | 0,93 | 2,21 |
| 29 | 12 | F | severe | 3,42 | 1,13 | 4,36 |
| 30 | 13 | M | severe | 3,45 | 0,96 | 5,39 |
| 31 | 13 | M | severe | 3,79 | 0,87 | 2,64 |
| 32 | 13 | F | severe | 3,71 | 1,02 | 4,75 |
| 33 | 13 | F | modeate | 3,03 | 0,89 | 3 |
| 34 | 15 | F | severe | 3,43 | 1,05 | 2,88 |
| 35 | 15 | F | moderate | 3,01 | 0,89 | 2,3 |
| 36 | 13 | F | severe | 3,07 | 0,98 | 2,59 |
| 37 | 13 | F | severe | 3,35 | 0,98 | 3,9 |
| 38 | 15 | M | severe | 2,62 | 0,88 | 2,33 |
| 39 | 14 | F | severe | 3,01 | 1,05 | 5,11 |
| 40 | 14 | F | severe | 2,98 | 0,91 | 1,46 |
| 41 | 14 | F | moderate | 3,44 | 1 | 2,32 |
| 42 | 13 | M | moderate | 3,18 | 0,85 | 2,33 |
| 43 | 13 | M | severe | 3,2 | 0,96 | 1,53 |
| 44 | 10 | F | moderate | 3,33 | 1,09 | 2 |
| 45 | 15 | F | severe | 3,08 | 1,12 | 0,97 |
| 46 | 15 | F | severe | 3,28 | 1,03 | 2,85 |
| 47 | 14 | F | moderate | 3,08 | 1,19 | 1,86 |
| 48 | 15 | F | moderate | 3,36 | 0,91 | 1,22 |
| 49 | 11 | M | severe | 3,21 | 1,08 | 1,91 |
| 50 | 14 | F | moderate | 3,51 | 1,02 | 3,1 |
| 51 | 15 | F | severe | 3,31 | 0,88 | 2,11 |
| 52 | 13 | F | moderate | 4,14 | 0,96 | 2,16 |
| 53 | 15 | F | moderate | 2,94 | 1,11 | 3,05 |
| 54 | 15 | F | moderate | 3,08 | 1,25 | 0,9 |
| 55 | 15 | F | severe | 2,66 | 1,16 | 0,43 |
| 56 | 10 | F | severe | 3,17 | 1,1 | 1,81 |
| 57 | 10 | F | severe | 3,04 | 1,11 | 1,37 |
| 58 | 13 | F | severe | 3,54 | 1,2 | 1,02 |
| 59 | 13 | M | moderate | 3,7 | 1 | 1,48 |
| 60 | 11 | M | moderate | 3,15 | 1,18 | 1,56 |
| 61 | 17 | F | severe | 2,98 | 0,91 | 1,46 |
| 62 | 14 | M | severe | 2,62 | 1,05 | 3,9 |
| 63 | 16 | M | moderate | 2,72 | 0,88 | 2,33 |
| 64 | 15 | M | moderate | 2,68 | 1,21 | 1,78 |
| 65 | 14 | M | moderate | 3,01 | 0,98 | 3 |
FT3-free triiodothyronine,
FT4-free thyroxine,
TSH-thyroid stimulating hormone
Table 3.
Serum levels of the thyroid hormones FT3, FT4 and TSH in the control subjects.
| Nos | Age | Sex | *FT3 (1.7–4.2pg/mL) | *FT4 (0.7–1.8ng/dL) | *TSH (0.3–5.5uIU/mL) |
|---|---|---|---|---|---|
| 1 | 13 | M | 3,79 | 1,04 | 4,74 |
| 2 | 12 | M | 3,33 | 0,91 | 3,53 |
| 3 | 12 | M | 3,44 | 1,05 | 2,97 |
| 4 | 12 | M | 3,41 | 0,84 | 1,14 |
| 5 | 13 | F | 3,46 | 1,17 | 0,94 |
| 6 | 13 | F | 2,9 | 0,83 | 11 |
| 7 | 13 | F | 3,38 | 1,02 | 1,07 |
| 8 | 13 | F | 2,94 | 1,23 | 3,49 |
| 9 | 12 | M | 3,15 | 1,36 | 4,41 |
| 10 | 14 | F | 2,72 | 0,88 | 1,46 |
FT3-free triiodothyronine,
FT4-free thyroxine,
TSH-thyroid stimulating hormone
DISCUSSION
Fluorosis is a major public health problem resulting from the long-term consumption of water with high fluoride (F−) levels.6 Fluorosis is endemic in 20 states of India,7 while goitre is endemic in 12 states; the problems overlap significantly in some regions.3
In India, dental fluorosis has previously been described in humans ingesting 0.5–1 ppm F− in drinking water, while at concentrations of 3.4 to 3.8 ppm, 100% dental fluorosis has been reported. Consistent with this; our study also found dental fluorosis in subjects whose drinking water contained 0.5–0.6 ppm fluoride which is considered to be within safe limit (1ppm). There were total 42 dental fluorosis subjects who consumed water with fluoride levels of 0.5–0.6 ppm in our study.
Occurrence rates of fluorosis can vary widely among different locations having almost the same drinking water F− concentrations, and are likely affected by a number of other factors, such as nutritional status, climate, individual susceptibility, biological response, duration of F− exposure, and dissolved salts in drinking water.8 It is possible that factors like local environment, individual susceptibility, frequency of water consumption, duration of F− exposure, and use of fluoride-containing dentifrices, might have been external sources of variation in the individuals with dental fluorosis considered in our study.
The thyroid gland appears to be the most sensitive tissue in the body to fluoride, which is able to increase the concentration of thyroid stimulating hormone (TSH) and decrease the concentration of triiodothyronine (T3) and thyroxine (T4) hormones, thereby producing hypothyroidism in some populations. Consequently, prolonged consumption of high F− water has the potential to suppress the function of the thyroid gland.2
It has been shown that fluoride might cause thyroid disturbances, such as those observed in iodine deficiency.1 Fluoride is a well-established TSH analogue. Not only fluoride may act like TSH in its absence, but it can also enhance TSH effects and alter the expression of certain G-proteins, thereby influencing all aspects of iodine uptake, transport, and T4 to T3 conversion.1 There is evidence that fluoride interferes with enzymes such as deiodinases that are required for metabolizing thyroxine (T4) into its various derivative forms.9 It has also been suggested that fluoride interferes with iodide transport, causing damage to iodide pump binding sites, and also potentially displacing iodide and accumulating in the thyroid gland.9 In addition, fluoride also has been shown to influence the balance between reverse T3 (rT3) and T3.9
The relationship between fluoride intoxication and thyroid function is highly debatable.10 Numerous studies have been conducted regarding the effects of fluoride on thyroid function in humans, cattle, calves, and adult rats.11 Investigators have reported that fluoride does induce changes in thyroid function. Association of dental fluorosis and endemic goiter has also been established. Finally, alterations of various physiological and biochemical parameters in the thyroid gland have also been reported in experimental studies of fluorosis.10
Yu found decreased levels of serum T4 and increased TSH levels in residents of endemic fluorosis area with adequate iodine intake.12 Cinar et al13 found a decrease in the serum levels of T3 and T4 in fluorotic cows. In fluoride-exposed pigs, Xiu-An Zhan et al found significantly decreased serum T4,free T4 levels, no significant differences in serum T3, freeT3 levels, and significantly increased levels of serum TSH.1 However, Leone et al and Baum et al reported no significant differences in thyroid status between populations with low and high F− concentrations in their drinking water.14 Interestingly, in a review published by the WHO, Demole stated that fluoride does not accumulate in the thyroid gland, does not decrease iodine up-take, and has no effect on the synthesis of thyroxine.1
A recent study by Susheela et al5 on children with dental fluorosis living in National Capital Territory of Delhi has shown that these children have well-defined thyroid hormone derangements, similar to those observed in subjects with iodine deficiency disorders. Even children without dental fluorosis, but with elevated serum fluoride levels, exhibited similar hormonal derangements. However, this study showed that all subjects with dental fluorosis had serum levels of thyroid hormones (FT3, FT4, and TSH) within the normal range, with the exception of 1 individual, who had elevated levels of TSH. These findings are in contradiction with the findings of Susheela et al. Also in our study, 1 individual in the control (non-fluorosis) group showed an elevated TSH level, which seems to have been a chance finding. Thus, it appears that thyroid hormone levels were not affected in dental fluorosis patients.
Serum fluoride levels may be the best indicator of fluoride toxicity. However, due to practical difficulties, it was not possible for us to analyze serum F− levels of study or control individuals. This could be considered a major limitation of our study. Hence, future studies involving serum fluoride levels will strengthen our understanding of the true relationship between dental fluorosis/ excessive fluoride exposure, and thyroid gland function.
Susheela et al5 study was likely the first to investigate dental fluorosis in relation to freeT3, FT4, and TSH thyroid hormones in human beings. It is also important to note that most of the available data regarding variations in thyroid hormone levels as a result of fluoride exposure are based on animal studies; a cautious approach should be taken when comparing human population studies to those animal studies. Hence, more studies similar to our present work are needed to provide a thorough understanding of the effects of fluoride exposure in the future.
Figure 1.
Mean values of groups.
Acknowledgments
Thanks are given here to all the school children (Shree Kalmeshwar school, Holehalur, ACO-School, Ilkal, Govt. School- Rampur, and Govt. Kannada Boys and Girls School, Jamkhandi) and their parents for their co-operation. The management and staff members of the schools.
Dr. Veeresh DJ. Professor -Department of Preventive & Community Dentistry
Shri. G.M. Hiremath- Asst. professor, Shri. C. B. Shivayogimath. Prof and HOD. Dept. of Civil Engineering, Basaveshwar Engineering College, Bagalkot, for their help in water fluoride analysis.
CONCLUSIONS
Fluoride toxicity remains a controversial subject, because several animal and human studies have shown conflicting results. Although studies have shown the relationship between thyroid hormone levels and fluorosis/fluoride exposure, the observations of this study suggest that thyroid hormone levels are unaltered in subjects with dental fluorosis. Hence, more studies of this kind are needed, along with detailed investigations of water fluoride content, accurate estimations of dietary iodine and fluoride intake, and serum/urine fluoride and thyroid hormone quantification. These improvements will allow further investigation of the mode of action of fluoride in the thyroid gland, and its consequences on human health.
REFERENCES
- 1.Zhan X-A, Li J-X, Wang M, Xu Z-R. Effects of fluoride on growth and thyroid function in young pigs. Fluoride. 2006;39:95–100. [Google Scholar]
- 2.Ge Y, Ning H, Wang S, Wang J. DNA damage in thyroid gland cells of rats exposed to long term intake of high fluoride and low iodine. Fluoride. 2005;38:318–323. [Google Scholar]
- 3.Desai VK, Solanki DM, Bansal RK. Epidemiological study of Goiter in endemic fluorosis district of Gujarat. Fluoride. 1993;26:187–190. [Google Scholar]
- 4.Schuld A. Is dental fluorosis caused by thyroid hormone disturbances? Fluoride. 2005;38:91–94. [Google Scholar]
- 5.Susheela AK, Bhatnagar M, Vig K, Mondal NK. Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India. Fluoride. 2005;38:98–108. [Google Scholar]
- 6.Shivashankara AR, Shivaraja Shankara YM, Hanumanth Rao S, Gopalkrishna Bhat P. A clinical and biochemical study of chronic fluoride toxicity in children of Kheru Thanda of Gulbarga district, Karnataka, India. Fluoride. 2000;33:66–73. [Google Scholar]
- 7.Kundu Mc, Mandal B. Fluoride concentration in ground-water in the North 24-paraganas district of West Bengal, India. Fluoride. 2010;43:160–164. [Google Scholar]
- 8.Choubisa SL. Endemic fluorosis in southern Rajasthan, India. Fluoride. 2001;34:61–70. [Google Scholar]
- 9.Clinch C. Fluoride interactions with iodine and iodide: implications for breast health. Fluoride. 2009;42:75–87. [Google Scholar]
- 10.Shashi A. Nucleic acids levels in thyroid gland in acute and chronic fluoride intoxication. Fluoride. 1993;26:191–196. [Google Scholar]
- 11.Bouaziz H, Soussia L, Guermazi F, Zeghal N. Fluoride-induced thyroid proliferative changes and their reversal in female mice and their pups. Fluoride. 2005;38:185–192. [Google Scholar]
- 12.Trabelsi M, Guermazi F, Zeghal Effect of fluoride on thyroid function and cerebellar development in mice. Fluoride. 2001;34:165–173. “Yu YN. Effects of chronic fluorosis in the thyroid gland. Chinese Med J 1985;65:747–749” in. [Google Scholar]
- 13.Cinar A, Selcuk M. Effect of chronic fluorosis in thyroxine, triiodothyronine and protein bound iodine in cows. Fluoride. 2005;38:65–68. [Google Scholar]
- 14.Committee on Fluoride in drinking water, Board Environmental studies and Toxicology, Division on Earth and Life Studies, National Research Council. Fluoride in drinking water: A scientific Review of EPA’s standards. National Acadamic Press; Washinton D.C: 2006. Of National Academics. Effects on the endocrine system; p. 231. [Google Scholar]