Abstract
Resin-based materials that release either fluoride or chlorhexidine have been formulated for inhibiting caries activity. It is not known if the two agents, when incorporated into one material, would interact and affect their release potential. We hypothesized that the ratio of fluoride to chlorhexidine incorporated into a resin, and the pH of the storage medium, will affect their releases from the material. The material investigated contained 23 wt% of filler, and the ratios of calcium fluoride to chlorhexidine diacetate were 8/2, 5/5, and 2/8. The release was conducted in pH 4, 5, and 6 acetate buffers. The results showed that release of either agent increased as the pH of the medium decreased. The presence of fluoride salt substantially reduced the chlorhexidine release, while the presence of a specific quantity of chlorhexidine significantly increased fluoride release. This interaction can be utilized to optimize the release of either agent for therapeutic purposes.
Keywords: controlled release, sealants, biomaterial
Introduction
Fluoride inhibits demineralization and enhances remineralization, and chlorhexidine reduces cariogenic bacterial activity. To channel the process toward remineralization, these anticariogenic agents must be delivered at true therapeutic levels over time (Featherstone, 2006). While numerous clinical protocols have been proposed to deliver these agents, patient compliance most often compromises the therapeutic effects of these treatments. To overcome the patient compliance issue, sustained- and controlled-release devices are required in dentistry (Mirth, 1980).
Formulations of sustained-release devices are often polymeric matrices filled with fluoride (Mirth et al., 1982), chlorhexidine (Mirth et al., 1989; Yue et al., 2004), or other antiseptic agents (Friedman and Steinberg, 1990). In fact, a variety of fluoride-releasing dental restorative materials is also available (Eichmiller et al., 2005; Wiegand et al., 2007). Some materials release fluoride from fluoride-containing filler phases, such as those in conventional glass ionomer, resin-modified glass ionomer, compomer, and zinc silicophosphate cement. Other materials, like amalgam, resin composite, fissure sealant, orthodontic bonding material, and base/liner/varnish, require incorporation of other fluoride salts. Similarly, chlorhexidine salts have also been incorporated into conventional and resin-modified glass-ionomer cements to improve their antimicrobial properties (Ribeiro and Ericson, 1991; Sanders et al., 2002; Palmer et al., 2004; Hoszek and Ericson, 2008).
It has been shown that chlorhexidine often precipitates out of solution when chlorhexidine rinses are mixed with fluoride rinses (Barkvoll et al., 1988). Therefore, the effectiveness of combining chlorhexidine with fluoride in either gel or solution form is often challenged. Some evidence suggests that the actions of positively charged chlorhexidine ions and negatively charged fluoride ions do not necessarily negate each other (Anusavice, 2005). In fact, varnishes containing both chlorhexidine and fluoride can improve long-term efficacy in diminishing the cariogenic microbial challenge (van Loveren et al., 1996; Twetman and Petersson, 1997).
As the development of dental adhesives continues, new expanded functions have been suggested and investigated (Tay and Pashley, 2002). Previously, we reported release of fluoride (Anusavice et al., 2005) and chlorhexidine (Anusavice et al., 2006) from a sealant and found that the release rates were significantly influenced by the pH of the storage medium and the loading level of the releasing agents. This study investigated the release of both F− and chlorhexidine from a single filled resin. The aim of this study was to test the hypothesis that the rates of F− and chlorhexidine release from a filled resin are pH-dependent and will be influenced by the ratio of fluoride to chlorhexidine contained in filler particles.
Materials & Methods
CaF2 (F, Fisher Scientific, Pittsburgh, PA, USA) and chlorhexidine diacetate (CHXDA, Sigma, St. Louis, MO, USA) powder were ground to a fine particle size by a M-2 Microsizer (Sturtevant, Hanover, MA, USA) and Mixer Mill MM200 (Retsch, Haan, Germany), respectively. A light-curable resin containing 70 wt% UDMA (Esschem, Linwood, PA, USA), 30 wt% TEGDMA (Aldrich, Milwaukee, WI, USA), and an appropriate amount of light-sensitive initiator and co-catalyst, was produced. Mixtures of CaF2/CHXDA were prepared with weight ratios of 2/8, 5/5, and 8/2. Thirty parts of each CaF2/CHXDA mixture that were added to 100 parts of resin resulted in 3 filled resins with 23 wt% of filler particles. The 3 filled resins were designated as 2Ca/ 8CHX, 5Ca/5CHX, and 8Ca/2CHX, which reflected the calcium fluoride to chlorhexidine diacetate ratios of 2/8, 5/5, and 8/2, respectively. The rheological properties of unfilled and filled resins were measured by means of a Brookfield Digital Rheometer, LVDV III and CP 115 (Brookfield Engineering Laboratories, Middleboro, MA, USA).
We created specimens by pouring the resin into a Teflon mold (10 mm diameter x 2 mm thick), and light-curing through a Mylar® matrix for 30 sec on each side. Thirty discs were made from each filled resin. Sodium acetate-acetic acid buffer was prepared and adjusted to pH 4.0, 5.0, and 6.0, which are noted as pH4, pH5, and pH6 throughout this article. Ten discs per pH group were stored individually in a 10-mL vial, to which a 5-mL quantity of buffer solution was added, and the vial was immersed in a 37°C water bath. The storage media were replaced at prescribed time intervals up to 2880 hrs. The replaced solutions were stored for analyses of the F− and chlorhexidine concentrations that were released over each of the time periods.
A series of F− and chlorhexidine reference solutions was prepared for each buffer solution. A linear relationship between the logarithm of F− concentration and relative voltage was established for each reference solution by means of a fluoride-ion-specific electrode and a digital voltmeter (Mettler-Toledo, Schwerzenbach, Switzerland). Total Ionic Strength Adjustment Buffer (TISAB) was used as a decomplexing agent for fluorine ion concentration measurement in the replaced solution (Anusavice et al., 2005). Linear relationship between absorbance peak heights at 255 nm via a UV-Visible Spectrophotometer (UV160U, Shimadzu, Kyoto, Japan) and the chlorhexidine concentrations was established for each buffered reference solution. The absorbance peak heights of the replaced solutions at 255 nm were converted to the quantities of chlorhexidine release by the respective linear relationships (Anusavice et al., 2006).
The cumulative release per unit area (Y, µg/cm2) of individual disks was calculated for each prescribed time (t, hr) at which the solution was replaced. Non-linear regression (SAS, SAS Institute, Ver. 9.1, Cary, NC, USA) was used to fit the data, Y and t, to the following equation (De Moor et al., 1996) to estimate the values of a, t½, and b for each disk:
The value of a is the quantity of short-term release, t½ is the time at which one-half of a has been released, and b is the coefficient for long-term Fickian release. The regression analyses showed that the values of t½ were near zero and the values of a were very small (< 1% of the total release at the conclusion of the experiment) for all specimens tested. Therefore, only the values of b and cumulative releases at 2880 hrs were analyzed for the effects of pH and CHXDA loading by two-way ANOVA (SAS).
To depict the effect of combining CaF2 and CHXDA in one resin on the release characteristics of either agent, we compared the mean b values obtained in this study with those from previous studies for either CaF2 (Anusavice et al., 2005) or CHXDA (Anusavice et al., 2006).
After completion of the release study, 2 specimens were randomly selected from each group and cut into halves. Cross-section surfaces were polished through 0.05-µm alumina, coated with carbon, and examined by scanning electron microscopy (JSM 6400, JEOL USA, Peabody, MA, USA).
Results
The mean apparent viscosities (SD) at 1 rpm were 292 (30), 1813 (185), 1595 (115), and 1198 (55) mPa·s for pure resin, 2Ca/8CHX, 5Ca/5CHX, and 8Ca/2CHX groups, respectively.
The mean b values and total cumulative releases (Table) summarize the release characteristics of each group of specimens. Of the 180 regressions performed, the degree of fit (DOF) was better than 99% for 162 of these, and the lowest DOF was 96.5% for one analysis. The ranges of standard error (SE) of b were less than 2% for 156 analyses, and the highest SEs were less than 4% for 7 analyses.
Table.
Mean and Standard Deviation (in parentheses) of Cumulative Release (n = 10) after 2880 hrs in Storage Media, and b Values (n = 10) Calculated from Regression Analyses of Each of the F−- and Chlorhexidine-releasing Specimen Groups
| F− Release |
Chlorhexidine Release |
||||||
|---|---|---|---|---|---|---|---|
| Group | pH | CaF2 Content, wt% | Cumulative Release, µg/cm2 | b, µg/h½ cm2 | CHXDA Content, wt% | Cumulative Release, µg/cm2 | b, µg/h½ cm2 |
| 2Ca/8CHX | 4 | 4.6 | 120 (11) | 2.4 (0.3) | 18.5 | 1291 (185) | 23.8 (4.1) |
| 5Ca/5CHX | 4 | 11.5 | 272 (44) | 5.2 (0.8) | 11.5 | 572 (56) | 10.9 (1.4) |
| 8Ca/2CHX | 4 | 18.5 | 252 (33) | 4.8 (0.6) | 4.6 | 259 (22) | 4.8 (0.5) |
| 2Ca/8CHX | 5 | 4.6 | 79 (9) | 1.6 (0.2) | 18.5 | 646 (72) | 11.2 (0.9) |
| 5Ca/5CHX | 5 | 11.5 | 243 (53) | 4.8 (0.9) | 11.5 | 365 (73) | 7.0 (1.6) |
| 8Ca/2CHX | 5 | 18.5 | 241 (73) | 4.2 (0.5) | 4.6 | 142 (27) | 2.7 (0.6) |
| 2Ca/8CHX | 6 | 4.6 | 73 (13) | 1.5 (0.3) | 18.5 | 497 (86) | 9.0 (1.8) |
| 5Ca/5CHX | 6 | 11.5 | 208 (25) | 4.8 (1.4) | 11.5 | 287 (43) | 5.6 (0.8) |
| 8Ca/2CHX | 6 | 18.5 | 213 (28) | 4.2 (0.5) | 4.6 | 130 (14) | 2.8 (0.4) |
Two-way ANOVA for the effects of pH and filler ratio on the mean b values showed that the influence by either variable was statistically significant (p < 0.001). There was no interaction between the 2 variables for F− release (p = 0.053), but there was a significant interaction with CHX release (p < 0.001). Two-way ANOVA for the effects of pH and filler ratio on the cumulative releases yielded similar results. It is expected, for there is a nearly linear relationship between the cumulative release and the value of b when the value of a is small relative to the cumulative release.
For comparison, the mean b values obtained in this study and those previously reported were plotted against the individual filler content in the resins (Fig. 1). The data in the Fig. labeled as w/o CHXDA were from CaF2-filled resin (Anusavice et al., 2005). The data in the Fig. labeled as w/o CaF2 were obtained from CHXDA-filled resin (Anusavice et al., 2006). The plot shows that the release of F− increased when 11.5 wt% or greater of CHXDA was incorporated into the same resin (Fig. 1A). In contrast, the presence of CaF2 suppressed the release of CHX (Fig. 1B).
Figure 1.
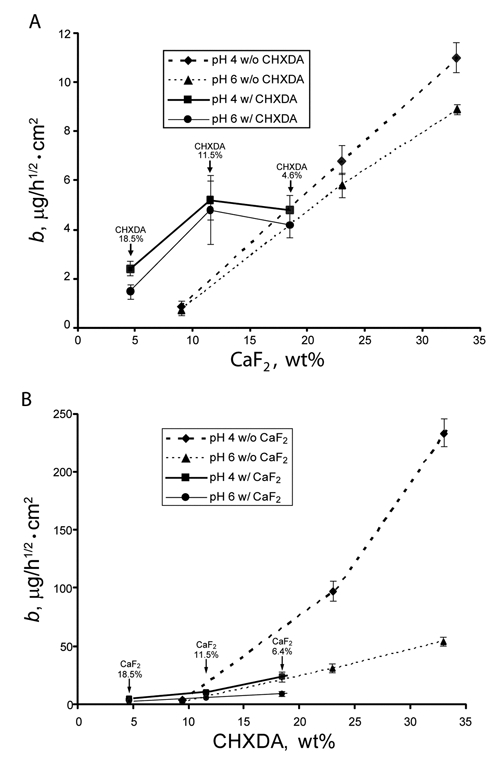
The mean b values (n = 10) of release as function of actual wt% of one type of filler in the filled resin. Data from previous studies are included in this Fig. to depict the effect of interaction between CaF2 and CHXDA on the release of F− and CHX. (A) Release of fluoride. The data points identified as “w/o CHXDA” and connected with dashed lines represent the release of F− from disks with CaF2 only (Anusavice et al., 2005). The datapoints identified as “w/CHXDA” and connected with solid lines represent results from this study. The values of % CHXDA above datapoints are the actual wt% of CHXDA incorporated into the filled resin used in this study. (B) Release of CHX. The datapoints identified as “w/o CaF2” and connected with dashed lines represent the release of CHX from disks with CHXDA only (Anusavice et al., 2006). The datapoints identified as w/CaF2 and connected with solid lines represent results from this study. The values of % CaF2 above datapoints are the actual wt% of CaF2 incorporated into the filled resin used in this study. The error bar represents one standard deviation of the group. For lower b values, the ranges of error bars are often so small that they are overshadowed by the symbol.
SEM examination of disk cross-sections including the outer edge of the specimens revealed microstructural changes in the disks. SEM images of the cross-sections of filled-resin discs with filler ratios of 2Ca/8CHX, 5Ca/5CHX, and 8Ca/2CHX after exposure for 4 mos in ambient air (control), and in pH 4, pH 5, and pH 6 buffer solutions, are shown in Fig. 2. The top edge of each image is the surface exposed to storage media. The bar represents 50 µm. The fine light-colored speckles are CaF2 particles, the large patches are CHXDA agglomerates, and depression areas are likely caused by the loss of CHXDA during polishing of the surface.
Figure 2.
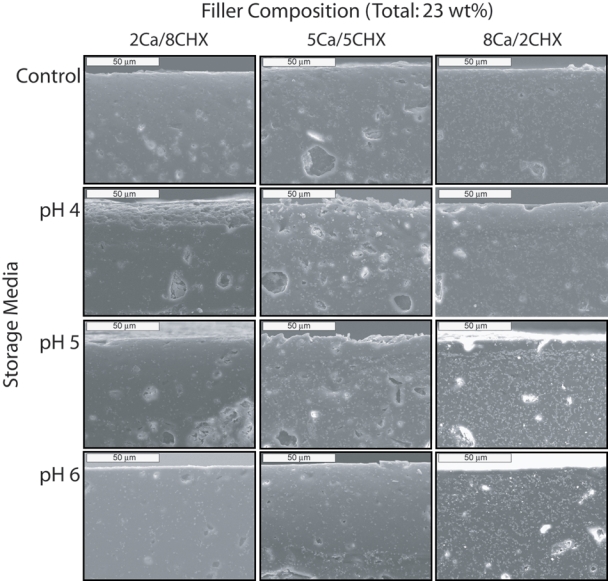
SEM images of cross-sections of the outer edges of filled resin discs containing 23 wt% of combined CaF2 and CHXDA filler particles. The weight ratios of CaF2 to CHXDA filler are 2Ca/8CHX (left column), 5Ca/5CHX (center column), and 8Ca/2CHX (right column). Fine light-colored speckles are CaF2 particles, and large patches are CHXDA agglomerates. Voids within the bulk of the specimens are likely caused by the loss of CHXDA during polishing of the surface. Controls are specimens that have been stored in ambient air.
Discussion
A study (Barkvoll et al., 1988) on the compatibility of chlorhexidine digluconate (CHXDG) and sodium monofluorophosphate (MFP) in a clinically relevant concentration showed that when equal volumes of 0.8 wt% MFP solution were added to 0.2 wt% CHXDG solution, precipitation was observed, and the content of dissolved CHXDG in the combined solution was reduced by 96%. In contrast, there was a slight reduction in F− concentration in 0.8 wt% MFP solution when the CHXDG concentration was increased from 0.0125 wt% to 2.0 wt%. A recent study (Zeng et al., 2009) on the amount of CHX in solution as a function of counter-ion concentration, such as chloride, acetate, or gluconate, reaffirmed that the observed CHX concentration in solution was highly dependent on the types and concentrations of counter-ions present. Our measured solubility also confirmed the reduction of CHX solubility in the presence of acetate ions. The precipitate found in the mixture of CHXDA and MFP (Barkvoll et al., 1988) represents a complex between CHX and counter-ions from MFP.
A study of CHX release (Nerurkar et al., 1995) from CHXDA embedded into methyl methacrylate and 2-hydroxyethyl methacrylate copolymer devices showed that the release rates in an inorganic saliva simulant were significantly lower than those observed from similar devices in water. Release rates were decreased by 36% and 85% in 2.2 mM and 10 mM sodium chloride solutions, respectively. It was hypothesized that the external chloride in the saliva stimulant had diffused into the core of the device, and converted the acetate salt of chlorhexidine (solubility ≈ 23 g/L at 37ºC) to the chloride salt (solubility ≈ 1 g/L at 37ºC). The conversion reduced the quantity of CHX available for the release. Incorporation of sodium chloride into the core of the device yielded a similar reduction in the release rate of CHX.
These results would lead one to expect that fluoride particles provide counter-ions in the resin that alter the chemistry of CHXDA and, subsequently, changes in the release characteristics from filled resins without fluoride particles. The most likely chemical change is the conversion of CHXDA to chlorhexidine difluoride (CHXDF) salt induced by water absorption. The reason is that the solubility of CHXDF in distilled water is about 6.5 g/L, which is about one-third that of CHXDA. In the acetate-based buffer solutions, the saturated concentration of CHX should be lower than those reported for the buffer solutions without fluorides. The lower rate of CHX release observed in this study is consistent with the assumption that CHXDF is present in the filled resin. The formation of CHXDF in the filled resin did not compromise the pH sensitivity of CHX release. For example, when the CHXDA content had been reduced to 4.6 wt% in the 8Ca/2CHX group, the CHX release rate appeared to be similar to that from filled resin with 9.1 wt% of CHXDA (Anusavice et al., 2006). It is important to note that this group of specimens also consists of 18.4 wt% of CaF2. These fluoride particles apparently provide more pathways for the release of CHX.
The presence of CHXDA exhibited a different effect on the release of fluoride. It facilitated the release of F− when its content was 11.5 wt% or greater. A previous study showed that there was a small reduction in the F− concentration of a MFP solution when the CHXDG concentration was increased from 0.0125 wt% to 2.0 wt% (Barkvoll et al., 1988). Therefore, one should not expect to observe noticeable changes in the behavior of F− release. The increase in release may have been caused by the physical presence of CHXDA, which might soften (Riggs et al., 2000) the resin matrix pathway for the release. As the CHXDA content decreased to 4.6 wt%, this advantage disappeared.
SEM micrographs showed that fine CaF2 particles were dispersed throughout the entire specimen, although agglomerates of CHXDA were also present. Surface erosion was more prevalent with specimens stored at lower pH, e.g., 2Ca/8CHX in pH 4, than in those in storage media of higher pH. This erosion is likely associated with the loss of filler particles near the surface. However, the effect of CHXDA agglomeration on release was not observed in the present study.
CHXDA, being a lighter compound, occupies more volume for the same weight as CaF2. Therefore, as the CHXDA content increases by replacing the CaF2 filler, the viscosity of the filled resin increases. Nonetheless, the apparent viscosities of the filled resin used in this study are comparable with the published values for commercial sealants.
The hypothesis of the study is accepted. The study also showed that the presence of fluoride salt in the filled resin must have promoted the formation of the less-soluble CHXDF salt that resulted in a lower release rate. In contrast, the presence of CHX yielded a softer resin matrix that facilitated a greater release of F−. From the controlled release point of view, this interaction can be utilized to optimize the release of either agent for therapeutic purposes.
Acknowledgments
The study was supported by the National Institute of Dental and Craniofacial Research [Grant DE13412], NIH, Bethesda, MD, USA.
References
- Anusavice KJ. (2005). Present and future approaches for the control of caries. J Dent Educ 69:538-554 [PubMed] [Google Scholar]
- Anusavice KJ, Zhang NZ, Shen C. (2005). Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res 84:440-444 [DOI] [PubMed] [Google Scholar]
- Anusavice KJ, Zhang NZ, Shen C. (2006). Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res 85:950-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkvoll P, Rølla G, Bellagamba S. (1988). Interaction between chlorhexidine digluconate and sodium monofluorophosphate in vitro. Scand J Dent Res 96:30-33 [DOI] [PubMed] [Google Scholar]
- De Moor RJ, Verbeeck RM, De Maeyer EA. (1996). Fluoride release profiles of restorative glass ionomer formulations. Dent Mater 12:88-95 [DOI] [PubMed] [Google Scholar]
- Eichmiller FC, Eidelman N, Carey CM. (2005). Controlling the fluoride dosage in a patient with compromised salivary function. J Am Dent Assoc 136:67-70 [DOI] [PubMed] [Google Scholar]
- Featherstone JD. (2006). Delivery challenges for fluoride, chlorhexidine and xylitol. BMC Oral Health 6(Suppl 1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M, Steinberg D. (1990). Sustained-release delivery systems for treatment of dental diseases. Pharm Res 7:313-317 [DOI] [PubMed] [Google Scholar]
- Hoszek A, Ericson D. (2008). In vitro fluoride release and the antibacterial effect of glass ionomers containing chlorhexidine gluconate. Oper Dent 33:696-701 [DOI] [PubMed] [Google Scholar]
- Mirth DB. (1980). The use of controlled and sustained release agents in dentistry: a review of applications for the control of dental caries. Pharmacol Ther Dent 5:59-67 [PubMed] [Google Scholar]
- Mirth DB, Shern RJ, Emilson CG, Adderly DD, Li SH, Gomez IM, et al. (1982). Clinical evaluation of an intraoral device for the controlled release of fluoride. J Am Dent Assoc 105:791-797 [DOI] [PubMed] [Google Scholar]
- Mirth DB, Bartkiewicz A, Shern RJ, Little WA. (1989). Development and in vitro evaluation of an intra-oral controlled-release delivery system for chlorhexidine. J Dent Res 68:1285-1288 [DOI] [PubMed] [Google Scholar]
- Nerurkar MJ, Zentner GM, Rytting JH. (1995). Effect of chloride on the release of chlorhexidine salts from methyl-methacrylate-2-hydroxyethyl methacrylate copolymer reservoir devices. J Control Release 33:357-363 [Google Scholar]
- Palmer G, Jones FH, Billington RW, Pearson GJ. (2004). Chlorhexidine release from an experimental glass ionomer cement. Biomaterials 25:5423-5431 [DOI] [PubMed] [Google Scholar]
- Ribeiro J, Ericson D. (1991). In vitro antibacterial effect of chlorhexidine added to glass-ionomer cements. Scand J Dent Res 99:533-540 [DOI] [PubMed] [Google Scholar]
- Riggs PD, Braden M, Patel M. (2000). Chlorhexidine release from room temperature polymerising methacrylate systems. Biomaterials 21:345-351 [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Gregory RL, Moore K, Avery DR. (2002). Antibacterial and physical properties of resin modified glass-ionomers combined with chlorhexidine. J Oral Rehabil 29:553-558 [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2002). Dental adhesives of the future. J Adhes Dent 4:91-103 [PubMed] [Google Scholar]
- Twetman S, Petersson LG. (1997). Efficacy of a chlorhexidine and a chlorhexidine-fluoride varnish mixture to decrease interdental levels of mutans streptococci. Caries Res 31:361-365 [DOI] [PubMed] [Google Scholar]
- van Loveren C, Buijs JF, Buijs MJ, ten Cate JM. (1996). Protection of bovine enamel and dentine by chlorhexidine and fluoride varnishes in a bacterial demineralization model. Caries Res 30:45-51 [DOI] [PubMed] [Google Scholar]
- Wiegand A, Buchalla W, Attin T. (2007). Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater 23:343-362 [DOI] [PubMed] [Google Scholar]
- Yue IC, Poff J, Cortes ME, Sinisterra RD, Faris CB, Hildgen P, et al. (2004). A novel polymeric chlorhexidine delivery device for the treatment of periodontal disease. Biomaterials 25:3743-3750 [DOI] [PubMed] [Google Scholar]
- Zeng P, Zhang G, Rao A, Bowles W, Wiedmann TS. (2009). Concentration dependent aggregation properties of chlorhexidine salts. Int J Pharm 367:73-78 [DOI] [PubMed] [Google Scholar]


