The rotation of the earth exposes its inhabitants to a daily rhythm of changes in temperature and light intensity. All species are well adapted to this phenomenon, as they have endogenous clocks that generate internal rhythms with a periodicity of approximately 24 hours. These circadian oscillators govern a plethora of behavioural and physiological functions such as sleeping and the consumption of food and water.
…clock genes seem to be active almost everywhere in the body, governing many biological functions according to the time of the day
Similarly, alcohol consumption follows a circadian rhythm: a study in rodents demonstrated that the animals' internal clock modulates behaviour so that they drink more in the dark or active phase (Spanagel et al, 2005b). Behavioural phenomena induced by drugs of abuse also differ according to the time of day. Thus, the typical increase of activity owing to the repeated administration of a drug—so-called behavioural sensitization—has been shown to display a diurnal rhythm, peaking during the first half of the day, when cocaine is administered (Abarca et al, 2002). The rewarding effects of cocaine also differ according to the time of day and may be modulated by internal clocks (Abarca et al, 2002). Moreover, physiological rhythms for body temperature and heart rate are altered by cocaine self-administration (Tornatzky & Miczek, 1999) and, as such, cocaine might act as a Zeitgeber—an event that provides a cue for certain biological rhythms in an organism.
In effect, these observations represent two sides of the same coin: drugs of abuse can influence the circadian programme, which in turn influences the effects of drugs of abuse. This dual phenomenon is caused by the action of genes that create and govern the function of the internal clock. How do these clock genes influence behaviour and physiology in response to a drug of abuse and, vice versa, how does a drug of abuse influence the action of clock genes? As drugs of abuse can transiently or permanently alter certain circadian functions, which consequently might result in a pathological condition, understanding the activity of clock genes could lead to a new understanding of—and possibly novel therapeutic approaches to treat—addiction.
The first molecular component of the endogenous clock was discovered in Drosophila, with the identification of the period (per) gene, and soon after, more clock genes, including Per, Clock, Bmal1 (brain and muscle Arnt-like protein 1) and Cry (cryptochrome), were identified in mammals (Wager-Smith & Kay, 2000). The current model for the gene-driven clock mechanism proposes that clock proteins interact with each other to induce positive and negative transcriptional–translational regulatory feedback loops (Ko & Takahashi, 2006). A heterodimer of CLOCK and BMAL1 proteins activates the expression of several genes such as Per and Cry by interacting with their promoter enhancer elements, thus constituting a positive feedback loop. Once translated, PER and CRY form heterodimers and enter the nucleus, where they inhibit their own transcription by suppressing the activity of the CLOCK–BMAL1 complex, thereby generating a negative feedback loop.
This daily molecular cycle is sustained by the transcriptional inhibition of the Rev-Erbα gene, which leads to the daytime repression of Bmal1. This process occurs not only in the master clock—located in the suprachiasmatic nucleus of the hypothalamus—but also in other brain areas and peripheral tissues. In principle, clock genes seem to be active almost everywhere in the body, governing many biological functions according to the time of the day (Ko & Takahashi, 2006).
…various neuroanatomical and neurochemical substrates that mediate the effects of psychoactive drugs are under the control of clock genes, and the efficacy of such drugs therefore depends on the activity of these genes and the time of day
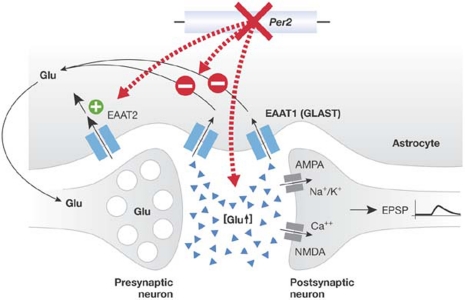
Clock genes also have an important role in regulating the expression of other, so-called clock-controlled genes (Delaunay & Laudet, 2002). They may differentially modulate neurotransmitter systems: the release of dopamine, glutamate and γ-aminobutyric acid in the nucleus accumbens—a brain region in which most psychoactive drugs are active—is governed by circadian rhythms (Castaneda et al, 2004). The modulation of dopamine receptor responsiveness by Per genes (Andretic & Hirsh, 2000) and the regulation of tyrosine hydroxylase activity by the Clock gene (McClung et al, 2005) further show how these systems are under the control of clock genes. In Per2-mutant mice, downregulation of the glutamate transporter GLAST leads to enhanced extracellular levels of glutamate in the nucleus accumbens, demonstrating that the main excitatory transmitter system of the brain is also regulated by a clock gene (Fig 1; Spanagel et al, 2005a). Thus, various neuroanatomical and neurochemical substrates that mediate the effects of psychoactive drugs are under the control of clock genes, and the efficacy of such drugs therefore depends on the activity of these genes and the time of day.
Figure 1.
Extracellular levels of glutamate in the brain are modulated by the clock gene Per2. Per2-mutant mice have augmented extracellular glutamate (Glu) levels owing to downregulated expression (indicated by −) of the glutamate transporter EAAT1 (also called GLAST). Uptake of glutamate into astrocytes is reduced, which leads to an enhanced concentration of glutamate in the synaptic cleft despite the counteracting, but insufficient, upregulation of EAAT2 (indicated by +). This hyperglutamatergic system can in turn influence the behavioural and physiological effects of psychoactive drugs. EAAT, excitatory amino-acid transporter; EPSP, excitatory post-synaptic potential; NMDA, N-methyl-D-aspartate; Per2, Period 2.
Further support for this hypothesis comes from various studies of clock-gene mutants and systematic dose–response studies of drugs of abuse. Jay Hirsh and co-workers first established a link between the behavioural effects of drugs of abuse and Per gene activity by studying behavioural sensitization—a phenomenon implicated in drug craving—in fruit flies (Andretic et al, 1999). They repeatedly exposed the flies to volatilized freebase cocaine, which produced, in the wild-type flies, typical sensitized behaviours similar to those found in rodents, such as enhanced grooming and locomotor activity, but failed to induce any sensitized response in flies with a mutated Per gene. Similarly, mutant mice lacking a functional Per1 gene showed no sensitized behavioural response to repeated cocaine injections, whereas Per2-mutant mice demonstrated hypersensitization (Abarca et al, 2002). The same study revealed similar results for conditioned place preference, a test used to measure the rewarding properties of a drug: Per1-mutant mice showed a complete lack of cocaine reward, whereas Per2 mutants displayed a strong cocaine-induced place preference. Another illustration of the role of clock genes comes from mice that lack Clock. These knockouts demonstrate an increase in cocaine reward and in the excitability of midbrain dopamine neurons (McClung et al, 2005).
The responsiveness to other drugs of abuse also seems to be under the control of clock genes. A knockdown of Per1 gene expression leads to a loss of morphine-induced place preference in mice (Liu et al, 2005). Although Per1 has an important role in modulating cocaine and opiate reinforcement processes, it does not seem to be involved in alcohol reinforcement processes (Zghoul et al, 2007). However, Per2-mutant mice consume significantly higher levels of alcohol than wild-type mice (Spanagel et al, 2005a). Caloric value, taste differences or variations in alcohol content did not account for this enhanced intake; instead, alterations in the brain reinforcement system of the mutant mice are thought to drive an enhanced motivation to consume alcohol. This also seems true for humans: a sample of patients with high alcohol intake showed an association with genetic variations of human PER2 (Spanagel et al, 2005a).
The fact that psychoactive drugs and severe stressors seem to influence the activity of clock genes could have important implications for the understanding and treatment of psychiatric disorders such as addiction. Several animal studies revealed that drugs of abuse differentially affect the expression of clock genes in various brain regions. Repeated injections of methamphetamine in mice cause a sensitized increase in Per1 gene expression in the striatum, without affecting Per1 or Per2 gene expression in the master clock (Nikaido et al, 2001). Microarray analysis also showed that repeated morphine and cocaine administration leads to a long-lasting upregulation of Per2 expression in the frontal cortex and striatum of rats (Ammon-Treiber & Höllt, 2005; Yuferov et al, 2005). Chronic ethanol administration has a similar effect (Chen et al, 2004). The same researchers showed that adult rats displayed an altered circadian expression of Per genes in the arcuate nucleus after prenatal exposure to alcohol (Chen CP et al, 2006).
Several animal studies revealed that drugs of abuse differentially affect the expression of clock genes in various brain regions
In addition to drugs of abuse, other psychoactive drugs, such as the antidepressant fluoxetine, produce long-lasting effects on the expression of several clock genes (that is, decreasing Per1 and increasing Clock gene expression), with more pronounced changes after repeated treatments (Uz et al, 2005). Finally, Yamamoto et al (2005) found that severe stressors, such as physical restraint, may increase Per1 gene expression in mice, which is essential in the context of any stress-induced disorder such as depression.
Different mechanisms have been suggested as to how drugs of abuse and other psychoactive drugs alter the expression patterns of clock genes. One study showed that the dopaminergic and glutamatergic systems mediate the effects of methamphetamine on Per1 gene expression (Nikaido et al, 2001). The effects of psychostimulants could also be mediated by endogenous melatonin, as this hormone seems to drive the rhythm of PER1 protein expression in the striatum (Akhisaroglu et al, 2004). Corticosterone might represent another key factor, as it is under strong circadian control and has been shown to have a crucial role in responsiveness to psychostimulants and on the action of these drugs when regularly self-administered (Marinelli & Piazza, 2002).
Acute or chronic drug or stressor effects on clock gene expression might transiently or even permanently alter circadian rhythmicity (Fig 2). The latter case would result in a pathological syndrome, which might underlie, at least in part, the symptoms observed in drug addicts or depressed patients. Indeed, there seems to be ample evidence from laboratory animals and humans that chronic drug consumption or stress might lead to circadian alterations that affect sleep and other behaviours. In this context, Rosenwasser et al (2005) showed that, in rats, both chronic ethanol intake and withdrawal affect the period and amplitude of the circadian rhythm of activity. Similarly, rats with unlimited access to heroin or nicotine demonstrated altered circadian parameters of food consumption with increased daytime activity (Chen SA et al, 2006; O'Dell et al, 2007). Moreover, alcoholics and drug addicts often suffer from sleep disturbances (Brower, 2001), eating problems and altered cortisol levels (Walter et al, 2006), which are also some of the hallmarks of depression. In conclusion, the fact that drug addicts and depressed patients suffer from pronounced circadian alterations in physiological, hormonal and behavioural levels supports the hypothesis that both drugs of abuse and chronic stressors have the potential to alter clock gene activity and consequently circadian rhythms.
…there seems to be ample evidence from laboratory animals and humans that chronic drug consumption or stress might lead to circadian alterations that affect sleep and other behaviours
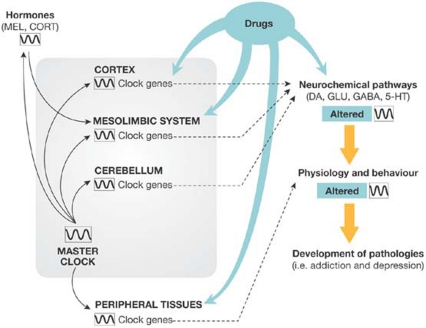
Figure 2.
The interplay between drugs of abuse and clock genes. The expression of clock genes modulates the state of several neurochemical systems in the mesolimbic pathway and other brain areas, thereby facilitating or reducing the effects of drugs of abuse or antidepressants. However, these and other psychoactive drugs also influence the activity of clock genes. Once the activity of clock genes is altered, certain neurochemical pathways are affected, as are the physiological and behavioural functions driven by these pathways. Drugs of abuse induce neuroadaptations and thereby alter these biological outputs both directly and indirectly through their action on clock genes (blue).
The link between drugs or stressors and the activity of clock genes has important clinical implications because it creates a pathological condition resembling drug addiction and depression. The findings described herein shed light on the widespread importance of the circadian programme and could aid in defining further downstream elements affected by psychoactive drugs. Importantly, they could also help improve treatment with psychotherapeutics by emphasizing the importance of a sound understanding of chronopharmacology.

Stéphanie Perreau-Lenz

Tarek Zghoul

Rainer Spanagel
References
- Abarca C, Albrecht U, Spanagel R (2002) Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA 99: 9026–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T (2004) Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol Biochem Behav 79: 37–42 [DOI] [PubMed] [Google Scholar]
- Ammon-Treiber S, Höllt V (2005) Morphine-induced changes of gene expression in the brain. Addict Biol 10: 81–89 [DOI] [PubMed] [Google Scholar]
- Andretic R, Hirsh J (2000) Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci USA 97: 1873–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J (1999) Requirement of circadian genes for cocaine sensitization in Drosophila. Science 285: 1066–1068 [DOI] [PubMed] [Google Scholar]
- Brower KJ (2001) Alcohol's effects on sleep in alcoholics. Alcohol Res Health 25: 110–125 [PMC free article] [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F (2004) Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res 36: 177–185 [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK (2004) Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem 88: 1547–1554 [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK (2006) Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. J Neurochem 97: 1026–1033 [DOI] [PubMed] [Google Scholar]
- Chen SA, O'Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF (2006) Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31: 2692–2707 [DOI] [PubMed] [Google Scholar]
- Delaunay F, Laudet V (2002) Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet 18: 595–597 [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15 (Spec No 2): R271–R277 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Wan C, Zhou W, Peng T, Liu Y, Wang Z, Li G, Cornelisson G, Halberg F (2005) The role of mPer1 in morphine dependence in mice. Neuroscience 130: 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV (2002) Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci 16: 387–394 [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ (2005) Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA 102: 9377–9381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S (2001) Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol 59: 894–900 [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF (2007) Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther 320: 180–193 [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW (2005) Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav 84: 537–542 [DOI] [PubMed] [Google Scholar]
- Spanagel R et al. (2005a) The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11: 35–42 [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK (2005b) Alcohol consumption and the body's biological clock. Alcohol Clin Exp Res 29: 1550–1557 [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA (1999) Repeated limited access to i.v. cocaine self-administration: conditioned autonomic rhythmicity illustrating “predictive homeostasis”. Psychopharmacology (Berl) 145: 144–152 [DOI] [PubMed] [Google Scholar]
- Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Dirim Arslan A, Manev H (2005) Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 134: 1309–1316 [DOI] [PubMed] [Google Scholar]
- Wager-Smith K, Kay SA (2000) Circadian rhythm genetics: from flies to mice to humans. Nat Genet 26: 23–27 [DOI] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Gerlach M, Weijers HG, Boening J, Wiesbeck GA (2006) Cortisol concentrations, stress-coping styles after withdrawal and long-term abstinence in alcohol dependence. Addict Biol 11: 157–162 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T (2005) Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid responsive element. J Biol Chem 280: 42036–42043 [DOI] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek MJ (2005) Microarray studies of psychostimulant-induced changes in gene expression. Addict Biol 10: 101–118 [DOI] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R (2007) Ethanol self-administration and reinstatement of ethanol-seeking behaviour in Per1Brdm1 mutant mice. Psychopharmacology 190: 13–19 [DOI] [PubMed] [Google Scholar]